Abstract
Background
Childhood asthma is most often characterized by recurrent wheezing, airway hyper-reactivity, and atopy; however, our understanding of these relationships from early in life remains unclear. Respiratory illnesses and atopic sensitization early in life may produce an interaction between innate and acquired immune responses leading to airway inflammation and heightened airway reactivity.
Objective
We hypothesized that pre-morbid airway reactivity and immunologic characteristics of infants without prior episodes of wheezing would be associated with subsequent wheezing during 1-year follow-up.
Methods
116 infants with chronic dermatitis were enrolled prior to episodes of wheezing. Airway reactivity, allergen-specific IgE, cytokine production by stimulated peripheral blood mononuclear cells (PBMCs), and percentages of dendritic cells were measured upon entry and airway reactivity was reassessed at 1-year follow-up. Linear regression models were used to evaluate predictor’s effect on continuous outcomes.
Results
milk and/or egg sensitization was associated with heightened airway reactivity prior to wheezing and after the onset of wheezing; however, these factors were not associated with an increased risk of wheezing. There was an interaction between initial airway reactivity and wheezing as a determinant of airway reactivity at follow-up. In addition, cytokine production by stimulated PBMCs was a risk factor for wheezing, while increased percentages of conventional dendritic cells were protective for wheezing.
Conclusion
Our data in a selected cohort of infants support a model with multiple risk factors for subsequent wheezing that are independent of initial airway reactivity; however, the etiologic factors that produce wheezing very early in life may contribute to heightened airway reactivity.
Keywords: infants, wheezing, airway reactivity, atopy, dermatitis, cytokines, dendritic cells, food allergy
INTRODUCTION
Episodes of wheezing early in life are primarily related to viral respiratory illnesses, which are important risk factors for developing childhood asthma; however, most infants and toddlers who wheeze do not develop asthma1–2. Atopic sensitization early in life, which is most often to egg or milk, is also a risk factor for developing childhood asthma; however, not all atopic infants develop asthma3. Infants and toddlers with atopy and recurrent wheeze early in life are at greater risk for developing childhood asthma than non-atopic subjects who wheeze4–5. In addition, reduced IFNγ production from stimulated mononuclear cells obtained from infants prior to wheezing is a risk factor for subsequent wheezing6–7. This finding suggests that the infant’s immune responsiveness to environmental stimuli, which is promoted by antigen presenting cells, such as dendritic cells, is also an important determinant of wheezing and the development of asthma. Therefore, investigators proposed an hypothesis that requires two-hits early in life for the development of childhood asthma; viral respiratory infections and atopic sensitization produce an interaction between innate and acquired immune responses leading to airway inflammation and persistently altered airway function8. Childhood asthma is most often characterized by recurrent wheezing, airway hyper-reactivity, and atopy; however, our understanding of the development of these relationships from early in life remains unclear.
We previously described that airway reactivity was heightened in infants with eczema (atopic and non-atopic based upon the presence or absence of specific IgE), sensitized to egg and/or milk, and recruited prior to wheezing 9. In our current 1-year follow-up, we hypothesized that the infant’s pre-morbid immunologic characteristics (allergen-specific IgE, cytokine production by stimulated peripheral blood mononuclear cells, and percentages of dendritic cells in the peripheral blood), as well as airway reactivity would be associated with episodes of wheezing during 1-year follow-up. In addition, if major determinants of airway reactivity were present upon entry into the study, airway reactivity would track longitudinal. Lastly, a two-hit hypothesis suggests that wheezing would modify premorbid airway reactivity as a determinant of airway reactivity at 1-year follow-up.
METHODS
Subjects
The infants and toddlers with chronic dermatitis (N=116) recruited for this study have previously been described 9. Subjects were excluded for history of prior wheezing, lower respiratory illness, treatment with asthma medications, or congenital heart disease. The Institutional Review Board approved the study and informed consent was obtained from parents.
Airway Reactivity
Following chloral hydrate sedation (50–100 mg/kg), airway reactivity was quantified by PC30, the methacholine concentration that decreased forced expiratory flows at 75% expired volume (FEF75) by ≥30% 10.
Dermatitis
Severity was quantified using SCORAD (Scoring Atopic Dermatitis)11.
Respiratory History
Maternal history of cigarette smoking during pregnancy (any versus none), and family history of asthma or allergy (parents or siblings) was obtained at initial and 1-year follow-up visits. Wheezy illnesses and medication usage were updated by monthly telephone contact.
Immune Characteristics
Atopic Status
Total IgE and allergen-specific IgE levels (egg white, milk, wheat, cat, house dust mite, timothy grass, Bermuda grass, ragweed, alternaria, and cedar) were measured from blood samples (Immune Tech, Cal.). Subjects were characterized as atopic if there was any specific IgE > 0.35 IU/ml.
Peripheral blood mononuclear cells (PBMC)
Techniques for isolation, culture and stimulation (Phorbol myristilacetate (PMA) + ionomycin (IONO); egg or milk antigens), as well as measurement of cytokine production, and staining for flow cytometric identification of cell populations are summarized in on-line repository.
Statistical Analysis
For association studies using regression models, PC30, and dendritic cell variables were log-transformed due to the right-skewed distribution. The ratio of TH2/TH1 cytokine amounts was log transformed for normality. Analyses were adjusted for enrollment age, gender, race, and maternal smoking during pregnancy. PC30 at entry to study, sensitization to egg and/or milk, and the number of wheezing episodes, as predictors of PC30 at 1-year follow-up were evaluated using linear regression models. A Linear regression model including interaction between PC30 at entry and wheezing episodes was fitted to evaluate the modification of wheezing episode on the effect of PC30 at entry on 1-year PC30. To evaluate dendritic cells and cytokine production by PBMC as predictors of the number of wheezing episodes during first year follow up, a Poisson regression model was used for each variable. Forward stepwise regression was to further select predictors among all predictors; the cutoff p-value for inclusion was 0.15. Models for number of wheezing episode adjusting for interaction between smoking during pregnancy and each cytokine production variable were also fitted to evaluate the interaction effect. Cytokine production following egg or milk antigen stimulation by PBMC was compared between the subjects with and without specific IgE to egg or milk antigen using two-sample t tests.
RESULTS
Subjects
Of 116 subjects recruited at a median age of 10.7 months (range, 2.6–19.1 months), 98 remained at 1-year follow-up (Table 1). Infants that dropped out had a higher percentage with maternal history of smoking compared to the group that completed the 1-year follow-up evaluation. At follow-up, subjects were evenly divided by sex and race. In addition, 77% of the subjects had an immediate family history of asthma or allergy, while 11% had a history of maternal smoking during pregnancy. Atopic subjects had higher total IgE (log) at baseline (1.08 vs. 0.68; p < 0.006) and follow-up (1.58 vs. 1.04; p < 0.001).
Table 1.
Demographics for Subjects
| 1-Year Follow-up | Initial (Dropped-out) | P value* | |
|---|---|---|---|
| Number of Subjects | 98 | 18 | |
| Gender (% Males) | 49 (50%) | 7 (39%) | 0.386 |
| Race (% Caucasian) | 48 (49%) | 5 (28%) | 0.097 |
| Family History - Asthma/Allergy† (%Yes) | 82 (85%) | 11 (61%) | 0.120 |
| Maternal Smoking during Pregnancy (% Yes) | 8 (8%) | 5 (28%) | 0.030 |
The p-values are for comparison of baseline characteristics between the dropped and non-dropped subjects using two-sample t-tests for continuous variables and Chi-square test for binary variables.
Smoking during pregnancy was different between dropped-out and non-dropped-out groups.
Family history not available for 1 subject
Missing data for airway reactivity resulted for 8 subjects who did not sleep for the measurement at entry to the study and 2 subjects at follow-up, Subjects did not undergo bronchial challenge secondary to baseline FEF75< 2SD below predicted (n=11 at initial evaluation and n=1 at follow-up). These 11 subjects did not differ from remaining subjects with respect to any other measurements other than there were fewer males (18% vs. 51%, p=0.04). Veni-puncture was not successful for 3 subjects.
Frequency of Wheezing during 1-year Follow-up
Forty-eight percent of infants had no reported wheezing, 31% had 1 or 2 episodes, and 21% had ≥3 episodes of wheezing during the 1-year follow-up. Among subjects with reported wheezing, 75% had wheeze only with colds, while 25% had wheezing with and without colds.
Predictors of Wheezing (Table 2A)
Table 2A.
Predictors of Wheezing
| Age, Race, Gender, Maternal Smoking during Pregnancy |
| Airway Reactivity Upon Entry to Study |
| Specific IgE |
| Cytokine Production by stimulated PBMCs |
| Dendritic Cells |
Table 2A and Figure 1 summarize the predictors of wheeze that were evaluated. An increased odds ratio for risk of wheezing was associated with maternal smoking during pregnancy (1.6; p < 0.034), there was a very small decreased risk with increased age at enrollment (0.96; p < 0.019), and a tendency for decreased risk of wheezing for females (0.76; p=0.090), while Race was not significant (1.18; p=0.296).
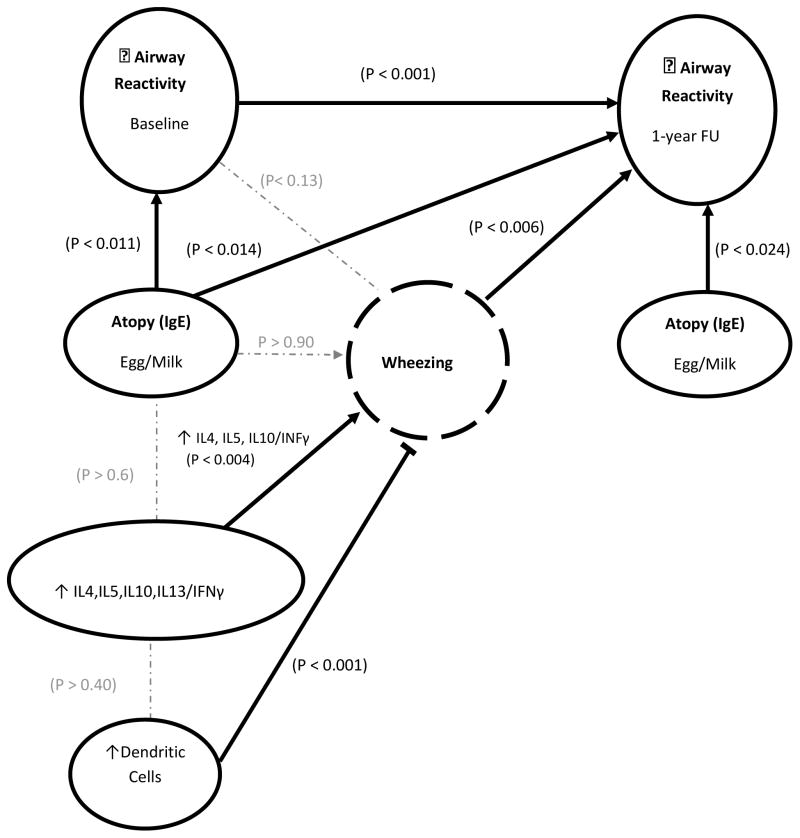
Figure 1.
Illustration summarizing associations of airway reactivity, immune characteristics, and wheezing.
➞ a significant positive association;
 a significant negative association;
a significant negative association;
 no significant association;
no significant association;
Airway Reactivity
Among the subjects with airway reactivity assessed upon entry to the study (N=89), the median (range) for PC30 was 0.418 (0.046 – 10.000) mg/ml. A lower PC30 (greater reactivity) was not a significant risk factor for increased number of wheezing episodes (p>0.12).
Allergen-specific IgE
Atopy defined as specific IgE to egg and/or milk antigens or as specific IgE to any of the 10 antigens was not associated with an increased risk of wheezing.
Cytokine Production
We observed increased ratios of IL4/IFNγ, IL5/IFNγ, and IL10/IFNγ, were associated with an increased risk for wheezing (Table 3; Figure 1). In addition to maternal smoking being a significant predictor of increased risk of wheezing (odds ratio 1.6; p < 0.041) independent of the cytokines, there was a significant interaction between maternal smoking during pregnancy and cytokine production as risk for increased episodes of wheezing. Increasing cytokine production (IL4/IFNγ, IL5/IFNγ) was associated with increasing number of wheezing episodes of (p < 0.027) for subjects with maternal smoking during pregnancy, but not for subjects without maternal smoking during pregnancy.
Table 3.
Cytokine production by PBMC and Dendritic Cells at Entry to Study as Predictors of Wheezing
| Outcome | Covariate | Rates Ratio | P value |
|---|---|---|---|
| Episodes of Wheezing | IL4/IFNγ ln | 1.33 (1.11, 1.6) | 0.002 |
| IL5/IFNγ ln | 1.33 (1.1, 1.62) | 0.004 | |
| IL10/IFNγ ln | 1.35 (1.14, 1.59) | <0.001 | |
| IL13/IFNγ ln | 1.11 (0.93, 1.34) | 0.258 | |
| cDC ln | 0.64 (0.52, 0.79) | <0.001 |
Cytokines and cDC natural log transformed (ln)
At each row, a Poisson regression model was fit and adjusted Age at entry to study, Gender, Race, and Maternal smoking during pregnancy
There were no significant associations between ratios of cytokines produced by PBMCs following PMA/Ionomycin and atopic status defined as specific IgE to egg or milk antigens (p>0.6; Figure 1). Despite the lack of an association of polyclonal cytokine production with specific IgE levels, there was an associated allergen-specific T cell response. In a limited number of subjects with an adequate number of PBMCs available, we stimulated PBMCs from subjects that were egg positive (N=8) or milk protein positive (N=9), and similar numbers of subjects who were negative to all antigens. Production of IL4, IL5, IL10, and IL13, but not IFNγ, by stimulated PBMCs were significantly greater for subjects with IgE specific to egg or milk compared to subjects negative for specific IgE to all 10 antigens (data not shown). There were no significant differences in demographics or total serum IgE among subjects with and without availability of additional PBMCs. These findings confirm the development of an allergen-specific TH2 response.
Dendritic Cells
There was a significant inverse relationship between the percentages of cDC in peripheral blood upon entry into the study and the subsequent number of episodes of wheezing; greater percentages of dendritic cells were associated with fewer episodes of wheezing (Table 3; Figure 1). The percentages of cDC were not associated with cytokine production. There were no significant associations between TH2, Treg, or NKT cells and wheezing. When all 5 covariates in Table 3 were included in a step-wise model as predictors of number of episodes of wheezing, cDCs remained significant (p < 0.001) and IL10/IFNγ approached significance (p < 0.061).
Predictors of Airway Reactivity at 1-year Follow-Up (Table 2B)
Table 2B.
Predictors of Airway Reactivity at 1-year Follow-Up
| Age, Race, Gender, Maternal Smoking during Pregnancy |
| Specific IgE |
| Airway Reactivity Upon Entry to Study |
| Episodes of Wheezing |
| Cytokine Production by stimulated PBMCs |
| Dendritic Cells |
Allergen Specific IgE
Subjects with specific IgE to egg and/or milk antigens upon entry into the study had lower PC30 upon entry into the study, as well as at 1-year follow-up compared to subjects negative for milk and egg (Table 4). In addition, egg/milk sensitization at follow-up was still associated with heightened reactivity at follow-up (Table 4; Figure 1). These relationships were not present when atopy was defined as specific IgE to any of the 10 antigens.
Table 4.
Airway Reactivity at 1-year Follow-up
| Variable | Parameter | Estimate | Std Err | P value |
|---|---|---|---|---|
| PC30 ln at 1-year Follow-up | PC30 ln At Entry to Study | 0.578 | 0.097 | < 0.001 |
| Number of Wheezing Episodes | −0.144 | 0.051 | 0.006 | |
| Egg or Milk + At entry to Study | −0.601 | 0.239 | 0.014 | |
| Egg or Milk + At 1-yr FU | −0.541 | 0.234 | 0.024 |
PC30 natural log transformed (ln)
At each row, a linear regression model was fit and adjusted for Age at entry to study, Gender, Race, and Maternal smoking during pregnancy
Airway Reactivity at Entry to Study
Airway reactivity upon entry into the study was highly correlated with airway reactivity at 1-year follow-up (N=82); infants with lower PC30 at the initial evaluation were more likely to have lower PC30 at follow-up (Table 4; Figure 1). There was also a significant relationship between episodes of wheezing and PC30 at follow-up; the greater the number of episodes of wheezing the lower PC30. When all 4 covariates in Table 4 were included in a step-wise model as predictors of PC30 at follow-up, PC30 upon entry into the study (p < 0.001) and egg-milk sensitization at follow-up (p < 0.012) remained as significant covariates adjusting for Age, Gender, Race, and Maternal smoking during pregnancy. When initial airway reactivity and subsequent episodes of wheezing were included in the model as predictors of airway reactivity at follow-up, adjusting for age at entry into study, gender, race and maternal smoking, there was a significant interaction term between initial airway reactivity and episodes of wheezing (p<0.006). Among infants with lower PC30 upon entry into the study, those with ≥3 episodes of wheezing maintained lower PC30 at follow-up; those infants with fewer than 3 episodes of wheezing had higher PC30 at follow-up.
Cytokine Production
Cytokine production was not associated with PC30 upon entry into the study; however, increased IL10/IFNγ was associated with decreased PC30 at follow-up (p < 0.037).
Dendritic Cells
The percentages of cDC were not associated with PC30.
DISCUSSION
Our study has demonstrated that increased ratios of TH2/TH1 cytokine production by stimulated PBMC prior to wheezing was a risk factor for subsequent wheezing, while increased dendritic cells in the blood prior to wheezing was protective for subsequent wheezing. Although allergen-specific IgE and airway reactivity upon entry into the study were not associated with an increased risk for subsequent wheezing, specific IgE to egg or milk was not only associated with heightened airway reactivity prior to episodes of wheezing, but this relationship persisted even after the onset of wheezing early in life. We also found that heightened airway reactivity prior to wheezing, subsequent episodes of wheezing, and an interaction between these parameters were associated with heightened airway reactivity at 1-year follow-up. These later findings suggest that airway reactivity may be modified by etiologic factors contributing to wheezing early in life. Cumulatively, our data support a model wherein there are multiple risk factors for subsequent wheezing that are independent of initial airway reactivity; however, wheezing very early in life may contribute to the persistence of heightened premorbid airway reactivity.
We did find that the ratio of TH2/IFNγ cytokine production from stimulated PBMCs obtained upon entry into the study was a significant risk factor for subsequent wheezing in the 1-year follow-up. The ratio was employed to assess the balance between these components of immune response. In the Tucson cohort study, investigators have reported that lower levels of IFNγ were associated with an increased risk for wheezing early in life6–7 and we found similar results in our data when analyzed for absolute amounts of IFNγ. However, we found that using absolute values for TH2 cytokines, increased levels were associated with decreased, rather than increased, wheezing. This suggests that the risk of wheezing may be associated with an overall decreased cytokine response by stimulated PBMCs (TH2 and TH1). Despite this, an increased TH2/TH1 cytokine ratio is still associated with an increased risk for wheezing. This may reflect that pathology depends not only on the absolute amount of cytokine in the system, but also on the balance of signals provided by cytokines that have opposing effects on cells. Zhang and coworkers recently reported that an increased ratio of IL10/IL5 following PHA stimulation of cord blood mononuclear cells was associated with a decreased risk of wheezing during childhood16. In our study, we found that increased IL10/IL5 production by PBMCs following PMA/ionomycin stimulation was associated with an increased risk of wheezing. The results of these studies may differ secondary to populations of subjects evaluated, the source of the mononuclear cell stimulated, the stimulation, and the length of follow-up.
A previous report identified a protective effect of pDC numbers on subsequent wheezing episodes and the development of asthma17, although they did not observe an association with cDCs (termed mDCs in their study). We observed a similar protective effect of pDCs on subsequent wheezing in our study (data not shown), but we also observed that increased numbers of cDCs in peripheral blood obtained from infants prior to wheezing were protective for subsequent episodes of wheezing. One likely explanation for this difference is in how the DC subsets were defined. The similar results for pDCs, which Upham et al defined as CD11c-intermediate and CD23-positive, and we defined as BDCA-2-positive, but negative for CD19 and CD14, suggest that these populations overlap. However, the distinct results for cDCs, defined by Upham et al as CD11c and HLA-DR-positive and defined by us as BDCA-1-positive but negative for CD19 and CD14, suggest that the precise populations of cells being identified may be different. Since many early episodes of wheezing are triggered by viral infections, Upham et al proposed that increased pDC numbers might promote better clearing of viral infections by production of type I IFNs. Similarly, increased numbers of cDCs might promote increased anti-viral immune responses. Indeed, the percentages of cDCs, as well as the absolute amount of IFNγ produced in culture, were inversely correlated with subsequent wheezing, suggesting a potential pathway. Thus, the negative impact of cDCs on wheezing may be indirect.
We found that airway reactivity upon entry into the study, prior to any episodes of wheezing was highly correlated with airway reactivity at 1-year follow-up. This longitudinal finding has not previously been reported in this age group. In addition, infants and toddlers with specific IgE to egg and/or milk at follow-up had heightened airway reactivity at follow-up compared to subjects negative for all IgE specific antigens evaluated. This current finding on the relationship between sensitization to egg and/or milk early in life and airway reactivity is consistent with our previous report relating egg/milk sensitizations to airway reactivity upon entry into the study, and indicates that this relationship is not transient9. Illi and coworkers reported that food sensitization prior to 2 years of age is a risk factor for the development of asthma as an older child, while inhalant sensitization prior to 2 years of age without concurrent food sensitization does not increase the risk for asthma3. There are multiple determinants of airway reactivity; however, our findings suggest that significant components of airway reactivity are present very early in life. The persistence of a relationship between egg/milk sensitization and airway reactivity may identify subjects who will maintain heightened airway reactivity and develop asthma as an older child. The absence of an association between sensitization to egg or milk and wheezing early in life may reflect that wheezing at this young age is not primarily related to atopy or airway reactivity.
We had previously reported that infants and toddlers had heightened airway reactivity several months following a wheezy respiratory illness, which was still present 8 months following the acute illness12. Similarly, Saga and coworkers reported that heightened airway reactivity among wheezy infants was associated with asthma as an older child13. However, in the absence of a premorbid assessment of airway reactivity, it is difficult to interpret the relationships between airway reactivity and wheezing. In our current study, we did not find that premorbid airway reactivity was a risk factor for subsequent wheezing. This finding differs from the results of Clarke and coworkers14, who assessed the relationship between airway reactivity at 1-month of age and the risk of wheezing during 1-year follow-up. In their population of neonates selected for family asthma, these investigators did not find an association between airway reactivity and a single wheezy illness; however, infants that developed ≥3 episodes of wheeze had heightened premorbid airway reactivity compared to subjects that developed ≤2 episodes of wheeze. The results of these two studies may have differed secondary to the selected populations. Our subjects were older (10 vs. 1 month) and selected by the criteria of eczema, rather than family history of asthma; therefore, we may have excluded subjects with heightened airway reactivity that wheezed at a younger age.
In our current study, we measured premorbid airway reactivity and at 1-year follow-up; therefore, we were also able to evaluate the relationship that premorbid airway reactivity and subsequent episodes of wheezing with airway reactivity at follow-up. Although premorbid airway reactivity was not directly associated with risk of wheezing, the number of episodes of wheezing were associated with airway reactivity at 1-year follow-up; subjects with more episodes of wheezing had a lower PC30 or heightened airway reactivity. There was also a significant interaction between premorbid airway reactivity and wheezing as predictors of airway reactivity at follow-up. Among infants with heightened premorbid airway reactivity, those with an increased number of episodes of wheezing were more likely to maintain heightened airway reactivity at follow-up. The only other longitudinal study of airway reactivity with premorbid measurements during infancy is from the Perth cohort15. Our findings in a selected cohort are consistent with their recently reported long term follow-up of an unselected cohort that heightened airway during infancy was associated with heightened airway reactivity in childhood for subjects with a lower respiratory illness prior to 6 months of age. Although these investigators have not reported the relationship between wheezy illness and airway reactivity early in life, they have reported that heightened airway reactivity at 12 months of age was more consistently associated with asthma outcomes as an older child than airway reactivity at 1 or 6 months of age. Their long term and our short term follow-up are consistent with the hypothesis that respiratory illnesses early in life contribute to the persistence of heightened airway reactivity.
There are several limitations to our study. We enrolled only infants with chronic dermatitis (atopic and non-atopic); there were no subjects without any dermatitis. There is support for two variants of eczema18, the non-atopic eczema has a lower risk for developing asthma than subjects with atopic eczema19–20. In addition, as our subjects were not a birth cohort, but recruited with eczema, those subjects that were older upon entry had gone slightly longer without wheezing, which may provide a small selection bias as the prevalence of wheezing decreases with increasing age. This could contribute to our finding that increasing age upon entry into the study was associated with a very small, but statistically significant decrease in the risk for subsequent wheezing. We did not specifically evaluate whether wheezing illnesses were associated with viral infections; however, most families reported that wheezy illnesses occurred with colds, which is consistent with the importance of viral agents, such as RSV and rhinovirus, as etiologic factors for wheezy in this age group2. Lastly, episodes of wheezing were determined by parents and not a physician; however, information was obtained from monthly telephone contact, which should minimize errors related to recall.
In summary, in our selected cohort of infants, we found a relationship between milk/egg sensitization and heightened airway reactivity that begins early in life prior to episodes of wheezing and persists after the onset of wheezing; however, these two factors were not associated with an increased risk of wheezing. In addition, we found an interaction between premorbid airway reactivity and episodes of wheezing, which suggest airway reactivity at follow-up may be modified by factors contributing to wheezing early in life. These factors may be related to TH2/TH1 cytokine production by stimulated PBMC, which were a risk factor for wheezing, as well as increased dendritic cells in the blood, which was protective for wheezing. Our data support a model with multiple risk factors for subsequent wheezing that are independent of initial airway reactivity; however, the etiologic factors that produce wheezing very early in life may contribute to the persistence of heightened airway reactivity.
Abbreviation
- IFNγ
Interferon gamma
- IL4
Interleukin 4
- IL5
Interleukin 5
- IL10
Interleukin 10
- PBMC
Peripheral blood mononuclear cell
- cDC
Conventional dendritic cells
- TH2
T helper 2 cell
- TH1
T helper 1 cell
- Treg
T regulatory cell
- NKT
Natural Killer T cell
- PMA
Phorbol myristilacetate
- FEF75
Forced expiratory flow at 75% expired volume
- MCh
Methacholine
- PC30
Provocative concentration of methacholine to reduce FEF75 by 30%
Footnotes
Clinical Implications
Airway reactivity, atopy, cytokine production by stimulated peripheral blood mononuclear cells, and the number of dendritic cells contribute to the risk for recurrent wheezing and airway hyper-reactivity early in life.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen, Morgan WJ. Asthma and wheezing in the first six years of life. The Group Health Medical Associates. New England Journal of Medicine. 1995;332(3):133–38. doi: 10.1056/NEJM199501193320301. [DOI] [PubMed] [Google Scholar]
- 2.Jackson DJ, Gangnon RE, Evans MD, Roberg KA, Anderson EL, Pappas TE, et al. Wheezing Rhinovirus Illnesses in Early Life Predict Asthma Development in High-Risk Children. American journal of respiratory and critical care medicine. 2008;178(7):667–72. doi: 10.1164/rccm.200802-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Illi S, von ME, Lau S, Nickel R, Niggemann B, Sommerfeld C, et al. The pattern of atopic sensitization is associated with the development of asthma in childhood. Journal of Allergy & Clinical Immunology. 2001;108(5):709–14. doi: 10.1067/mai.2001.118786. [DOI] [PubMed] [Google Scholar]
- 4.Oddy WH, de Klerk NH, Sly PD, Holt PG. The effects of respiratory infections, atopy, and breastfeeding on childhood asthma. European Respiratory Journal. 2002;19(5):899–905. doi: 10.1183/09031936.02.00103602. [DOI] [PubMed] [Google Scholar]
- 5.Sherrill D, Stein R, Kurzius-Spencer M, Martinez F. On early sensitization to allergens and development of respiratory symptoms. Clin Exp Allergy. 1999;29(7):905–11. doi: 10.1046/j.1365-2222.1999.00631.x. [DOI] [PubMed] [Google Scholar]
- 6.Guerra S, Lohman IC, Halonen M, Martinez FD, Wright AL. Reduced Interferon {gamma} Production and Soluble CD14 Levels in Early Life Predict Recurrent Wheezing by 1 Year of Age. American journal of respiratory and critical care medicine. 2004;169(1):70–76. doi: 10.1164/rccm.200304-499OC. [DOI] [PubMed] [Google Scholar]
- 7.Stern DA, Guerra S, Halonen M, Wright AL, Martinez FD. Low IFN-gamma production in the first year of life as a predictor of wheeze during childhood. J Allergy Clin Immunol. 2007;120(4):835–41. doi: 10.1016/j.jaci.2007.05.050. [DOI] [PubMed] [Google Scholar]
- 8.Sly PD, Boner AL, Bjorksten B, Bush A, Custovic A, Eigenmann PA, et al. Early identification of atopy in the prediction of persistent asthma in children. Lancet. 2008;372(9643):1100–06. doi: 10.1016/S0140-6736(08)61451-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tepper RS, Llapur CJ, Jones MH, Tiller C, Coates C, Kimmel R, et al. Expired nitric oxide and airway reactivity in infants at risk for asthma. Journal of Allergy & Clinical Immunology. 2008;122(4):760–65. doi: 10.1016/j.jaci.2008.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tepper RS, Williams-Nkomo T, Martinez T, Kisling J, Coates C, Daggy J. Parental smoking and airway reactivity in healthy infants. American Journal of Respiratory & Critical Care Medicine. 2005;171(1):78–82. doi: 10.1164/rccm.200406-711OC. [DOI] [PubMed] [Google Scholar]
- 11.Pucci N, Novembre E, Cammarata MG, Bernardini R, Monaco MG, Calogero C, et al. Scoring atopic dermatitis in infants and young children: distinctive features of the SCORAD index. Allergy. 2005;60(1):113–16. doi: 10.1111/j.1398-9995.2004.00622.x. [DOI] [PubMed] [Google Scholar]
- 12.Tepper RS, Rosenberg D, Eigen H. Airway responsiveness in infants following bronchiolitis. Pediatric Pulmonology. 1992;13(1):6–10. doi: 10.1002/ppul.1950130104. [DOI] [PubMed] [Google Scholar]
- 13.Saga R, Mochizuki H, Tokuyama K, Morikawa A. Relationship between bronchial hyperresponsiveness and development of asthma in wheezy infants. Chest. 2001;119(3):685–90. doi: 10.1378/chest.119.3.685. [DOI] [PubMed] [Google Scholar]
- 14.Clarke J, Salmon B, Silverman M. Bronchial responsiveness in the neonatal period as a risk factor for wheezing in infancy. American journal of respiratory and critical care medicine. 1995;151:1434–40. doi: 10.1164/ajrccm.151.5.7735597. [DOI] [PubMed] [Google Scholar]
- 15.Turner SW, Young S, Goldblatt J, Landau LI, Le Souef PN. Childhood Asthma and Increased Airway Responsiveness: A Relationship That Begins in Infancy. American journal of respiratory and critical care medicine. 2009;179(2):98–104. doi: 10.1164/rccm.200805-804OC. [DOI] [PubMed] [Google Scholar]
- 16.Zhang G, Rowe J, Kusel M, Bosco A, McKenna K, de Klerk N, et al. Interleukin-10/interleukin-5 responses at birth predict risk for respiratory infections in children with atopic family history. American journal of respiratory and critical care medicine. 2009;179(3):205–11. doi: 10.1164/rccm.200803-438OC. [DOI] [PubMed] [Google Scholar]
- 17.Upham JW, Zhang G, Rate A, Yerkovich ST, Kusel M, Sly PD, et al. Plasmacytoid dendritic cells during infancy are inversely associated with childhood respiratory tract infections and wheezing. Journal of Allergy and Clinical Immunology. 2009;124(4):707–13.e2. doi: 10.1016/j.jaci.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 18.Kusel MM, Holt PG, de Klerk N, Sly PD. Support for 2 variants of eczema. Journal of Allergy and Clinical Immunology. 2005;116(5):1067–72. doi: 10.1016/j.jaci.2005.06.038. [DOI] [PubMed] [Google Scholar]
- 19.Bohme M, Wickman M, Lennart NS, Svartengren M, Wahlgren CF. Family history and risk of atopic dermatitis in children up to 4 years. Clinical & Experimental Allergy. 2003;33(9):1226–31. doi: 10.1046/j.1365-2222.2003.01749.x. [DOI] [PubMed] [Google Scholar]
- 20.Wuthrich B, Schmid-Grendelmeier P. Natural course of AEDS. Allergy. 2002;57(3):267–68. doi: 10.1034/j.1398-9995.2002.1n3572.x. [DOI] [PubMed] [Google Scholar]