Abstract
EDP208 pili are encoded by a derepressed derivative of a naturally occurring lac plasmid, F0lac (incompatibility group FV), originally isolated from Salmonella typhi. EDP208 pili are serologically unrelated to F pili and do not promote infection by F-specific ribonucleic acid bacteriophages. However, they do confer sensitivity to the F-specific filamentous deoxyribonucleic acid phages. EDP208-containing cells are multi-piliated and contain approximately 20 pili per cell. These pili contain a single polypeptide subunit of 11,500 daltons. EDP208-specific RNA phages were readily isolated from local sewage. These phages were somewhat smaller in diameter than the F-specific ribonucleic acid phages and absorbed relatively weakly to EDP208 pili. Comparing EDP208 pilin to F, it was found that both contain the equivalent of two to three hexose units per subunit as well as blocked N-termini. EDP208 pilin contains one covalently linked phosphate residue per subunit, whereas the F pilin subunit contains two such residues. Although notable differences were found in the case of three or four amino acids, the overall amino acid compositions of F and EDP208 were very similar. Moreover, the tryptic peptide maps of the two proteins contained seven peptides with similar mobilities, suggesting considerable homology in their amino acid sequences. Substantial similarities were also noted in the secondary structures of F and EDP208 pilin on the basis of circular dichroism studies. The α-helix content of both proteins was calculated to be 65 to 70%. X-ray fiber diffraction studies have indicated that the arrangements of subunits in F and EDP208 pili are also similar. It was concluded that F and EDP208 pili are closely related structures.
Full text
PDF

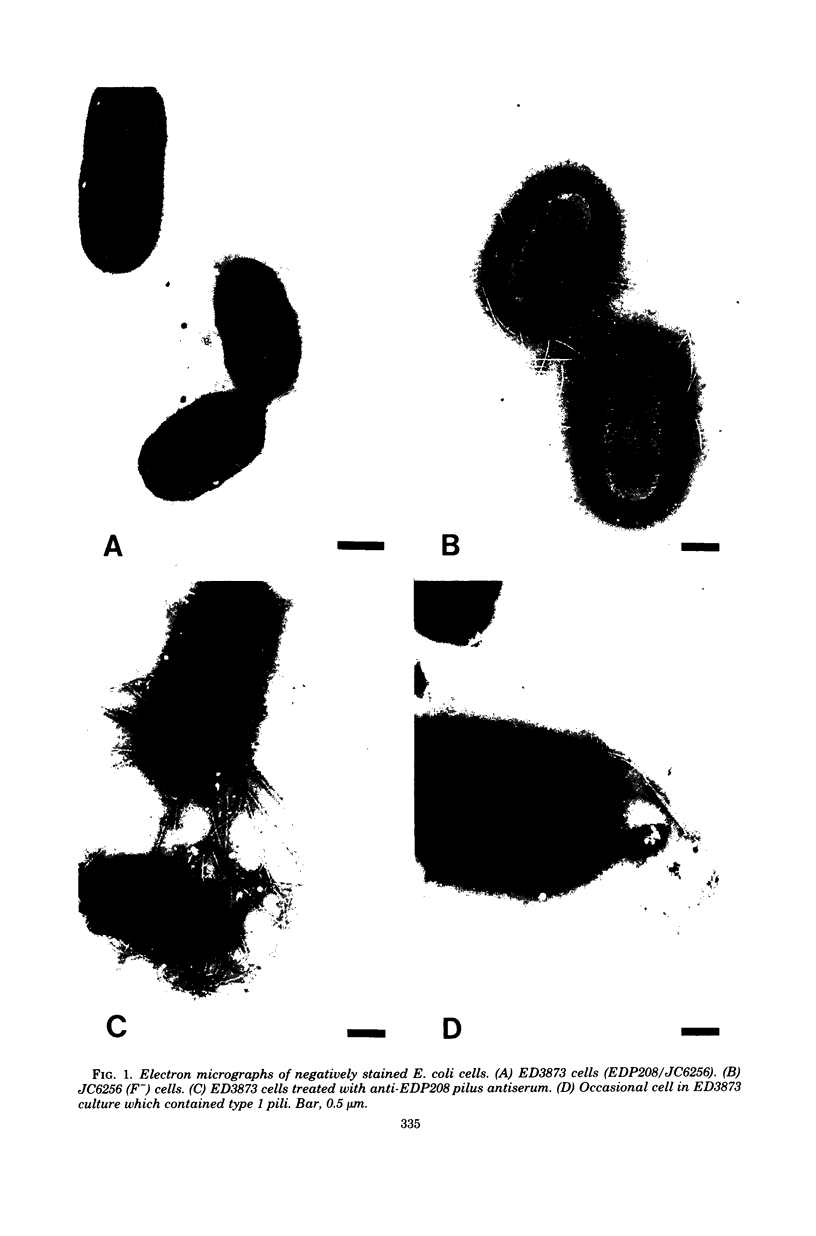

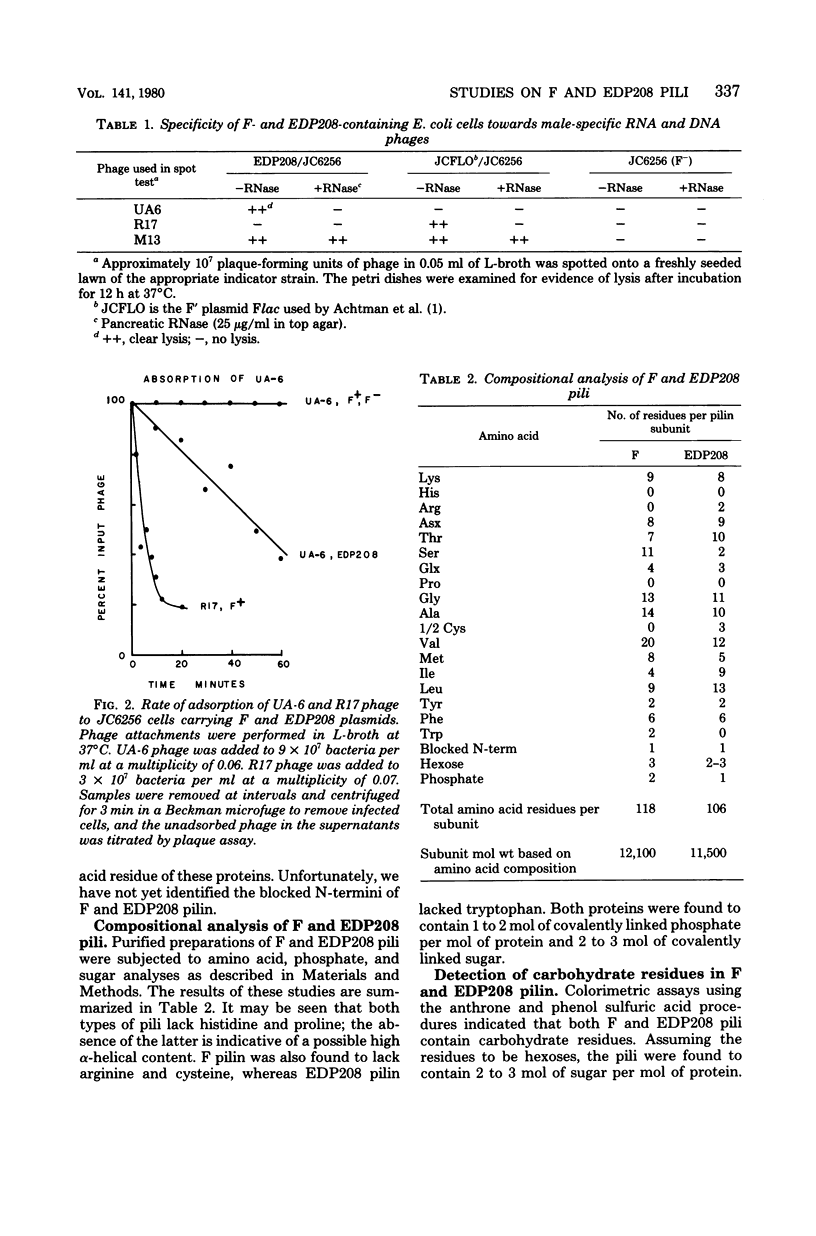
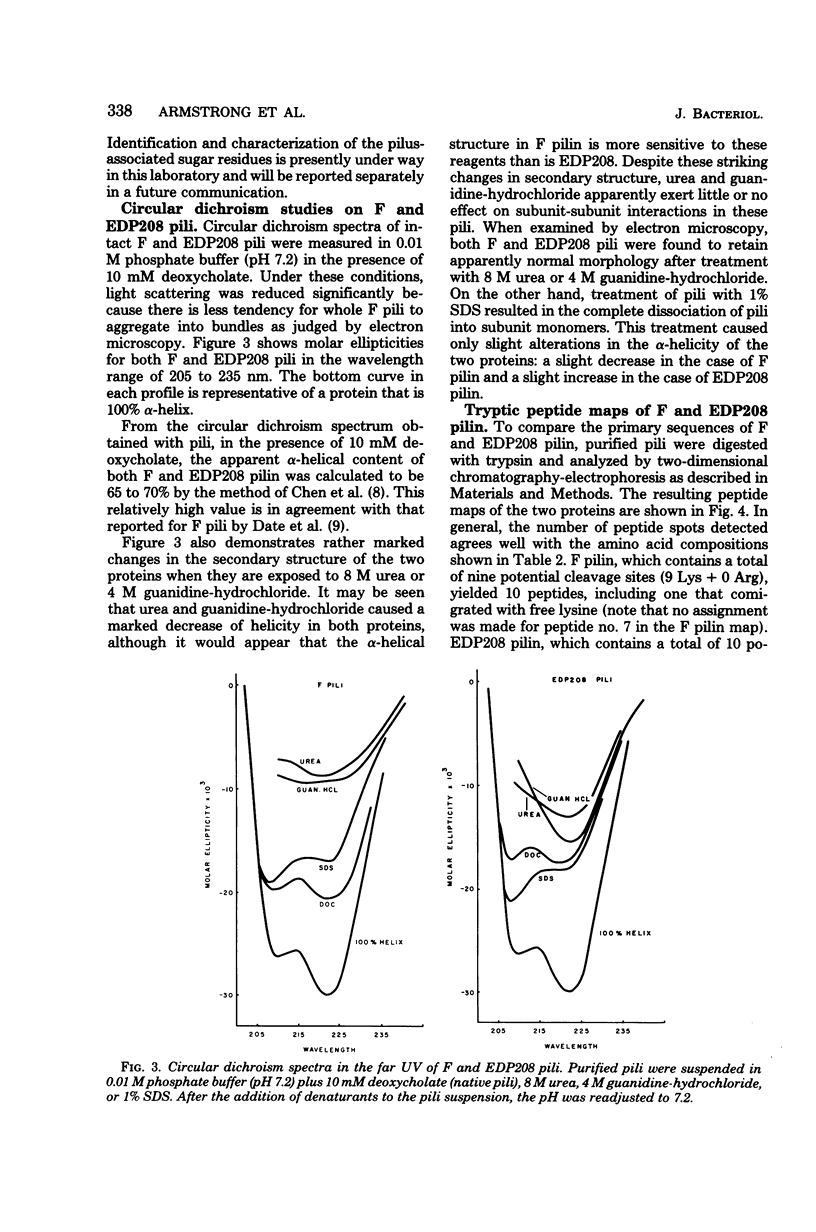


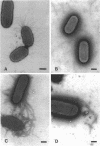
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achtman M., Willetts N., Clark A. J. Beginning a genetic analysis of conjugational transfer determined by the F factor in Escherichia coli by isolation and characterization of transfer-deficient mutants. J Bacteriol. 1971 May;106(2):529–538. doi: 10.1128/jb.106.2.529-538.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bovre K., Froholm L. O. Correlation between the fimbriated state and competence of genetic transformation in Moraxella nonliquefaciens strains. Acta Pathol Microbiol Scand B Microbiol Immunol. 1970;78(4):526–528. doi: 10.1111/j.1699-0463.1970.tb04337.x. [DOI] [PubMed] [Google Scholar]
- Bradley D. E., Meynell E. Serological characteristics of pili determined by the plasmids R711b and F0lac. J Gen Microbiol. 1978 Sep;108(1):141–149. doi: 10.1099/00221287-108-1-141. [DOI] [PubMed] [Google Scholar]
- Bradley D. E., Pitt T. L. Pilus-dependence of four Pseudomonas aeruginosa bacteriophages with non-contractile tails. J Gen Virol. 1974 Jul;24(1):1–15. doi: 10.1099/0022-1317-24-1-1. [DOI] [PubMed] [Google Scholar]
- Brinton C. C., Jr The properties of sex pili, the viral nature of "conjugal" genetic transfer systems, and some possible approaches to the control of bacterial drug resistance. CRC Crit Rev Microbiol. 1971 May;1(1):105–160. doi: 10.3109/10408417109104479. [DOI] [PubMed] [Google Scholar]
- Brinton C. C., Jr The structure, function, synthesis and genetic control of bacterial pili and a molecular model for DNA and RNA transport in gram negative bacteria. Trans N Y Acad Sci. 1965 Jun;27(8):1003–1054. doi: 10.1111/j.2164-0947.1965.tb02342.x. [DOI] [PubMed] [Google Scholar]
- Chen Y. H., Yang J. T., Chau K. H. Determination of the helix and beta form of proteins in aqueous solution by circular dichroism. Biochemistry. 1974 Jul 30;13(16):3350–3359. doi: 10.1021/bi00713a027. [DOI] [PubMed] [Google Scholar]
- Date T., Inuzuka M., Tomoeda M. Purification and characterization of F pili from Escherichia coli. Biochemistry. 1977 Dec 13;16(25):5579–5585. doi: 10.1021/bi00644a030. [DOI] [PubMed] [Google Scholar]
- Edman P., Begg G. A protein sequenator. Eur J Biochem. 1967 Mar;1(1):80–91. doi: 10.1007/978-3-662-25813-2_14. [DOI] [PubMed] [Google Scholar]
- Folkhard W., Leonard K. R., Malsey S., Marvin D. A., Dubochet J., Engel A., Achtman M., Helmuth R. X-ray diffraction and electron microscope studies on the structure of bacterial F pili. J Mol Biol. 1979 May 15;130(2):145–160. doi: 10.1016/0022-2836(79)90423-6. [DOI] [PubMed] [Google Scholar]
- Froholm L. O., Sletten K. Purification and N-terminal sequence of a fimbrial protein from Moraxella nonliquefaciens. FEBS Lett. 1977 Jan 15;73(1):29–32. doi: 10.1016/0014-5793(77)80008-2. [DOI] [PubMed] [Google Scholar]
- Frost L. S., Carpenter M., Paranchych W. N-methylphenylalanine at the N-terminus of pilin isolated from Pseudomonas aeruginosa K. Nature. 1978 Jan 5;271(5640):87–89. doi: 10.1038/271087a0. [DOI] [PubMed] [Google Scholar]
- Frost L. S., Paranchych W. Composition and molecular weight of pili purified from Pseudomonas aeruginosa K. J Bacteriol. 1977 Jul;131(1):259–269. doi: 10.1128/jb.131.1.259-269.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmuth R., Achtman M. Cell-cell interactions in conjugating Escherichia coli: purification of F pili with biological activity. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1237–1241. doi: 10.1073/pnas.75.3.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrichsen J., Froholm L. O., Bovre K. Studies on bacterial surface translocation. 2. Correlation of twitching motility and fimbriation in colony variants of Moraxella nonliquefaciens, M. bovis, and M. kingii. Acta Pathol Microbiol Scand B Microbiol Immunol. 1972;80(3):445–452. [PubMed] [Google Scholar]
- Hermodson M. A., Chen K. C., Buchanan T. M. Neisseria pili proteins: amino-terminal amino acid sequences and identification of an unusual amino acid. Biochemistry. 1978 Feb 7;17(3):442–445. doi: 10.1021/bi00596a010. [DOI] [PubMed] [Google Scholar]
- Liu T. Y., Chang Y. H. Hydrolysis of proteins with p-toluenesulfonic acid. Determination of tryptophan. J Biol Chem. 1971 May 10;246(9):2842–2848. [PubMed] [Google Scholar]
- McMichael J. C., Ou J. T. Structure of common pili from Escherichia coli. J Bacteriol. 1979 Jun;138(3):969–975. doi: 10.1128/jb.138.3.969-975.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novotny C., Carnahan J., Brinton C. C., Jr Mechanical removal of F pili, type I pili, and flagella from Hfr and RTF donor cells and the kinetics of their reappearance. J Bacteriol. 1969 Jun;98(3):1294–1306. doi: 10.1128/jb.98.3.1294-1306.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARANCHYCH W., GRAHAM A. F. Isolation and properties of an RNA-containing bacteriophage. J Cell Comp Physiol. 1962 Dec;60:199–208. doi: 10.1002/jcp.1030600303. [DOI] [PubMed] [Google Scholar]
- Paranchych W., Frost L. S., Carpenter M. N-Terminal amino acid sequence of pilin isolated from Pseudomonas aeruginosa. J Bacteriol. 1978 Jun;134(3):1179–1180. doi: 10.1128/jb.134.3.1179-1180.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punsalang A. P., Jr, Sawyer W. D. Role of pili in the virulence of Neisseria gonorrhoeae. Infect Immun. 1973 Aug;8(2):255–263. doi: 10.1128/iai.8.2.255-263.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randerath K. An evaluation of film detection methods for weak beta-emitters, particularly tritium. Anal Biochem. 1970 Mar;34:188–205. doi: 10.1016/0003-2697(70)90100-4. [DOI] [PubMed] [Google Scholar]
- Robertson J. N., Vincent P., Ward M. E. The preparation and properties of gonococcal pili. J Gen Microbiol. 1977 Sep;102(1):169–177. doi: 10.1099/00221287-102-1-169. [DOI] [PubMed] [Google Scholar]
- Wendt L. W., Ippen K. A., Valentine R. General properties of F-pili. Biochem Biophys Res Commun. 1966 May 25;23(4):375–380. doi: 10.1016/0006-291x(66)90736-4. [DOI] [PubMed] [Google Scholar]