Abstract
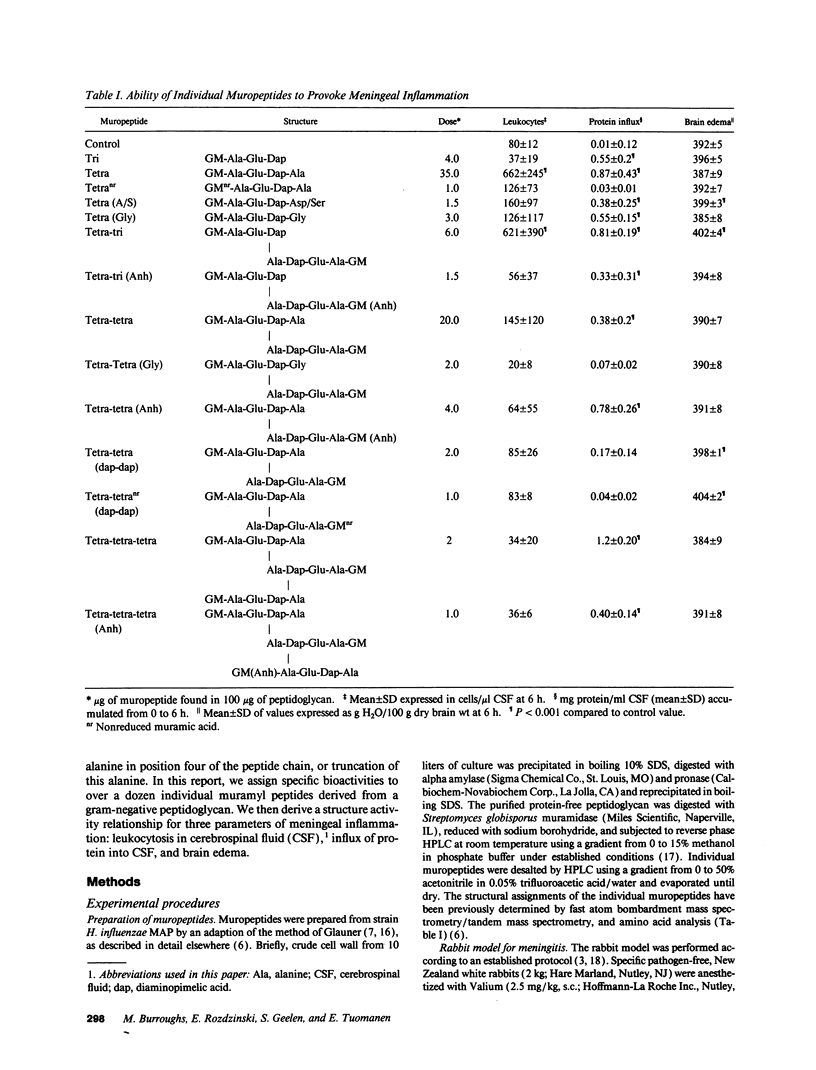
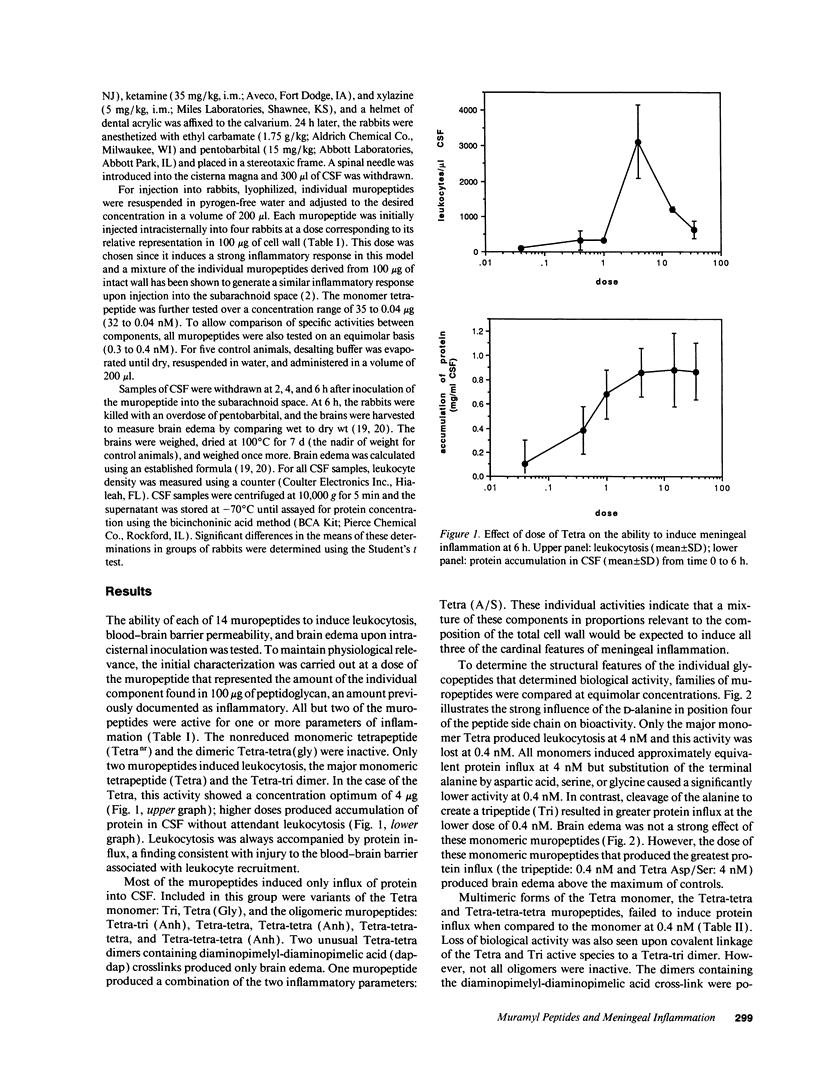
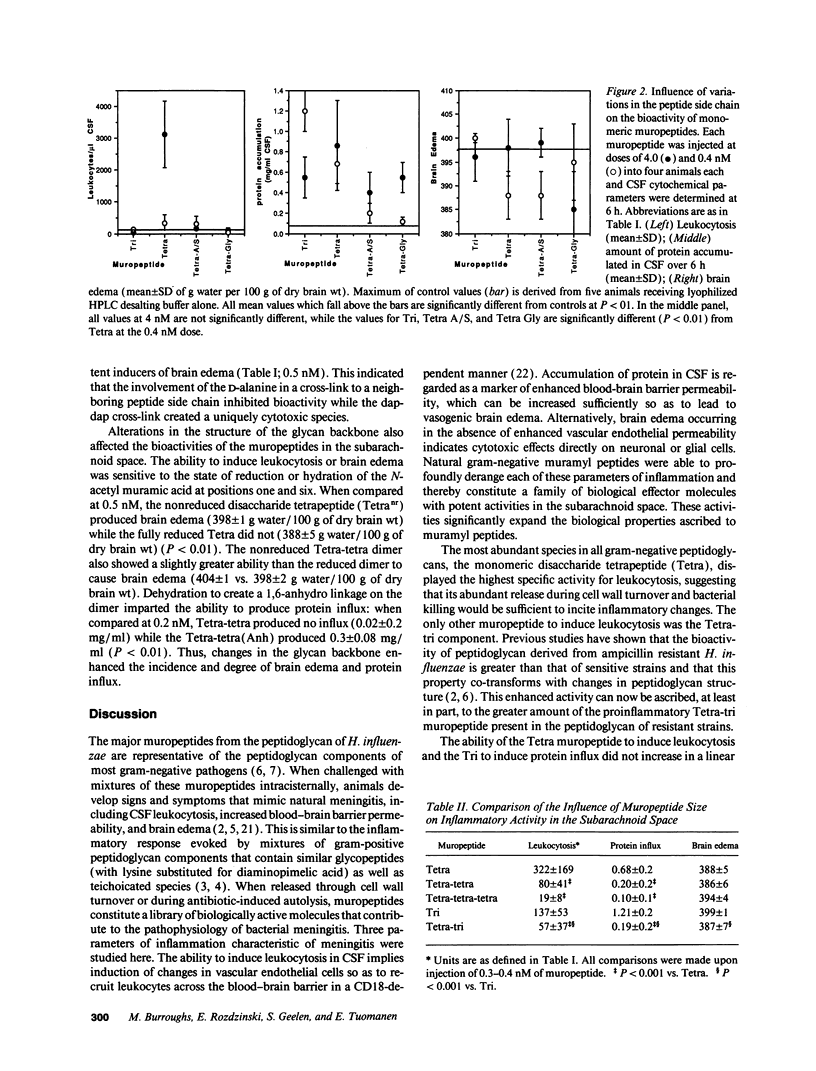
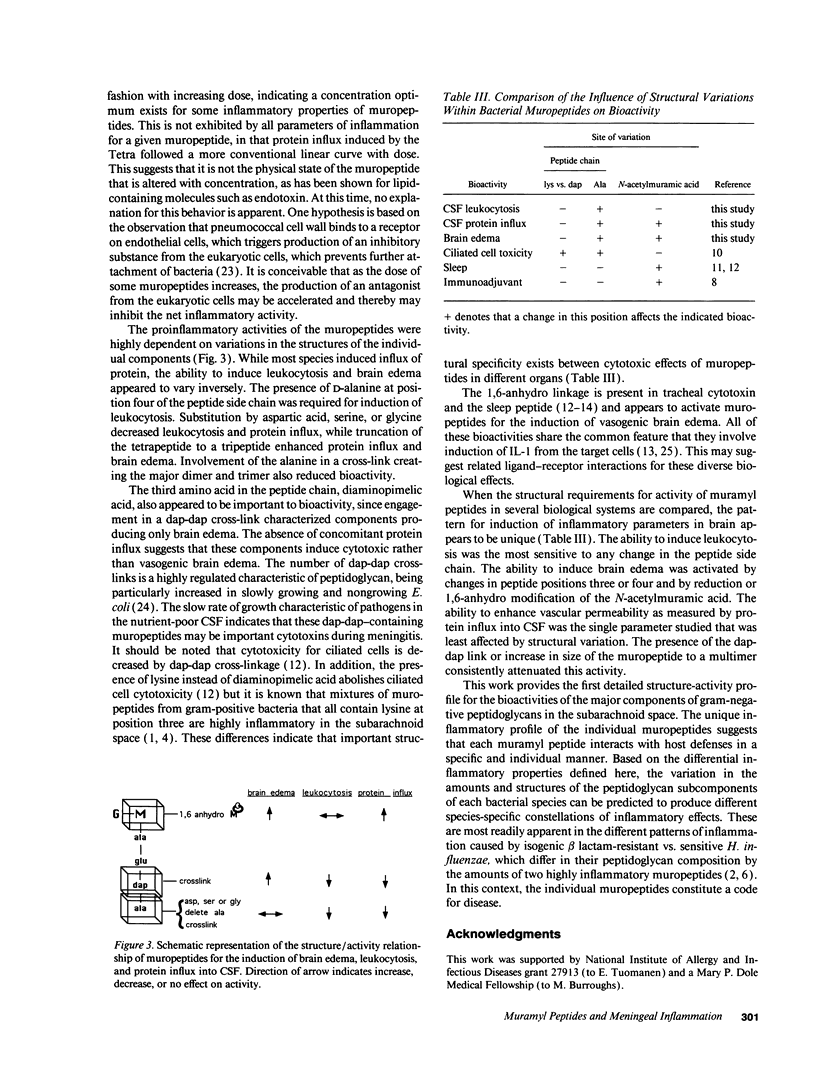
Components of bacterial peptidoglycans have potent biological activities, including adjuvant effects, cytotoxicity, and induction of sleep. Mixtures of peptidoglycan components also induce inflammation in the lung, subarachnoid space, and joint, but the structural requirements for activity are unknown. Using a rabbit model for meningitis, we determined the biological activities of 14 individual muramyl peptides constituting > 90% of the peptidoglycan of the gram-negative pediatric pathogen Haemophilus influenzae. Upon intracisternal inoculation, most of the muropeptides induced leukocytosis in cerebrospinal fluid (CSF), influx of protein into CSF, or brain edema, alone or in combination. The disaccharide-tetrapeptide, the major component of all gram-negative peptidoglycans, induced CSF leukocytosis and protein influx at doses as low as 0.4 microgram (0.42 nM). Modification of the N-acetyl muramic acid or substitution of the alanine at position four in the peptide side chain decreased leukocytosis but enhanced brain edema. As the size of the muropeptide increased, the inflammatory activity decreased. Muropeptide carrying the diaminopimelyl-diaminopimelic acid cross-link specifically induced cytotoxic brain edema. These findings significantly expand the spectrum of biological activities of natural muramyl peptides and provide the basis for a structure-activity relationship for the inflammatory properties of bacterial muropeptides.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burroughs M., Cabellos C., Prasad S., Tuomanen E. Bacterial components and the pathophysiology of injury to the blood-brain barrier: does cell wall add to the effects of endotoxin in gram-negative meningitis? J Infect Dis. 1992 Jun;165 (Suppl 1):S82–S85. doi: 10.1093/infdis/165-supplement_1-s82. [DOI] [PubMed] [Google Scholar]
- Burroughs M., Prasad S., Cabellos C., Mendelman P. M., Tuomanen E. The biologic activities of peptidoglycan in experimental Haemophilus influenzae meningitis. J Infect Dis. 1993 Feb;167(2):464–468. doi: 10.1093/infdis/167.2.464. [DOI] [PubMed] [Google Scholar]
- Dacey R. G., Sande M. A. Effect of probenecid on cerebrospinal fluid concentrations of penicillin and cephalosporin derivatives. Antimicrob Agents Chemother. 1974 Oct;6(4):437–441. doi: 10.1128/aac.6.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellouz F., Adam A., Ciorbaru R., Lederer E. Minimal structural requirements for adjuvant activity of bacterial peptidoglycan derivatives. Biochem Biophys Res Commun. 1974 Aug 19;59(4):1317–1325. doi: 10.1016/0006-291x(74)90458-6. [DOI] [PubMed] [Google Scholar]
- Fleming T. J., Wallsmith D. E., Rosenthal R. S. Arthropathic properties of gonococcal peptidoglycan fragments: implications for the pathogenesis of disseminated gonococcal disease. Infect Immun. 1986 May;52(2):600–608. doi: 10.1128/iai.52.2.600-608.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geelen S., Bhattacharyya C., Tuomanen E. The cell wall mediates pneumococcal attachment to and cytopathology in human endothelial cells. Infect Immun. 1993 Apr;61(4):1538–1543. doi: 10.1128/iai.61.4.1538-1543.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glauner B. Separation and quantification of muropeptides with high-performance liquid chromatography. Anal Biochem. 1988 Aug 1;172(2):451–464. doi: 10.1016/0003-2697(88)90468-x. [DOI] [PubMed] [Google Scholar]
- Goldman W. E., Klapper D. G., Baseman J. B. Detection, isolation, and analysis of a released Bordetella pertussis product toxic to cultured tracheal cells. Infect Immun. 1982 May;36(2):782–794. doi: 10.1128/iai.36.2.782-794.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitano K., Tuomanen E., Tomasz A. Transglycosylase and endopeptidase participate in the degradation of murein during autolysis of Escherichia coli. J Bacteriol. 1986 Sep;167(3):759–765. doi: 10.1128/jb.167.3.759-765.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger J. M., Davenne D., Walter J., Shoham S., Kubillus S. L., Rosenthal R. S., Martin S. A., Biemann K. Bacterial peptidoglycans as modulators of sleep. II. Effects of muramyl peptides on the structure of rabbit sleep. Brain Res. 1987 Feb 17;403(2):258–266. doi: 10.1016/0006-8993(87)90063-1. [DOI] [PubMed] [Google Scholar]
- Pappenheimer J. R., Koski G., Fencl V., Karnovsky M. L., Krueger J. Extraction of sleep-promoting factor S from cerebrospinal fluid and from brains of sleep-deprived animals. J Neurophysiol. 1975 Nov;38(6):1299–1311. doi: 10.1152/jn.1975.38.6.1299. [DOI] [PubMed] [Google Scholar]
- Rosenthal R. S., Nogami W., Cookson B. T., Goldman W. E., Folkening W. J. Major fragment of soluble peptidoglycan released from growing Bordetella pertussis is tracheal cytotoxin. Infect Immun. 1987 Sep;55(9):2117–2120. doi: 10.1128/iai.55.9.2117-2120.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saukkonen K., Sande S., Cioffe C., Wolpe S., Sherry B., Cerami A., Tuomanen E. The role of cytokines in the generation of inflammation and tissue damage in experimental gram-positive meningitis. J Exp Med. 1990 Feb 1;171(2):439–448. doi: 10.1084/jem.171.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A., Saukkonen K. The nature of cell wall-derived inflammatory components of pneumococci. Pediatr Infect Dis J. 1989 Dec;8(12):902–903. doi: 10.1097/00006454-198912000-00034. [DOI] [PubMed] [Google Scholar]
- Tuomanen E. I., Saukkonen K., Sande S., Cioffe C., Wright S. D. Reduction of inflammation, tissue damage, and mortality in bacterial meningitis in rabbits treated with monoclonal antibodies against adhesion-promoting receptors of leukocytes. J Exp Med. 1989 Sep 1;170(3):959–969. doi: 10.1084/jem.170.3.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuomanen E., Cozens R. Changes in peptidoglycan composition and penicillin-binding proteins in slowly growing Escherichia coli. J Bacteriol. 1987 Nov;169(11):5308–5310. doi: 10.1128/jb.169.11.5308-5310.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuomanen E., Hengstler B., Rich R., Bray M. A., Zak O., Tomasz A. Nonsteroidal anti-inflammatory agents in the therapy for experimental pneumococcal meningitis. J Infect Dis. 1987 May;155(5):985–990. doi: 10.1093/infdis/155.5.985. [DOI] [PubMed] [Google Scholar]
- Tuomanen E., Hengstler B., Zak O., Tomasz A. Induction of meningeal inflammation by diverse bacterial cell walls. Eur J Clin Microbiol. 1986 Dec;5(6):682–684. doi: 10.1007/BF02013304. [DOI] [PubMed] [Google Scholar]
- Tuomanen E., Liu H., Hengstler B., Zak O., Tomasz A. The induction of meningeal inflammation by components of the pneumococcal cell wall. J Infect Dis. 1985 May;151(5):859–868. doi: 10.1093/infdis/151.5.859. [DOI] [PubMed] [Google Scholar]
- Tuomanen E., Schwartz J., Sande S., Light K., Gage D. Unusual composition of peptidoglycan in Bordetella pertussis. J Biol Chem. 1989 Jul 5;264(19):11093–11098. [PubMed] [Google Scholar]
- Täuber M. G., Khayam-Bashi H., Sande M. A. Effects of ampicillin and corticosteroids on brain water content, cerebrospinal fluid pressure, and cerebrospinal fluid lactate levels in experimental pneumococcal meningitis. J Infect Dis. 1985 Mar;151(3):528–534. doi: 10.1093/infdis/151.3.528. [DOI] [PubMed] [Google Scholar]
- Täuber M. G., Shibl A. M., Hackbarth C. J., Larrick J. W., Sande M. A. Antibiotic therapy, endotoxin concentration in cerebrospinal fluid, and brain edema in experimental Escherichia coli meningitis in rabbits. J Infect Dis. 1987 Sep;156(3):456–462. doi: 10.1093/infdis/156.3.456. [DOI] [PubMed] [Google Scholar]



