Abstract
Thyroid hormones have widespread cellular effects; however it is unclear whether their effects on the central nervous system (CNS) contribute to global energy balance. Here, we demonstrate that either whole body hyperthyroidism or central administration of triiodothyronine (T3) decreases the activity of hypothalamic AMP-activated protein kinase (AMPK), increases sympathetic nervous system (SNS) activity and upregulates thermogenic markers in brown adipose tissue (BAT). Inhibition of the lipogenic pathway in the ventromedial nucleus of the hypothalamus (VMH) prevents CNS-mediated activation of BAT by thyroid hormone and reverses the weight loss associated with hyperthyroidism. Similarly inhibition of thyroid hormone receptors (TRs) in the VMH reverses the weight loss associated with hyperthyroidism. This regulatory mechanism depends on AMPK inactivation as genetic ablation of this enzyme in the VMH of euthyroid rats induces feeding-independent weight loss and increases expression of thermogenic markers in BAT. These effects are reversed by pharmacological blockade of the SNS. Thus, thyroid-hormone-induced modulation of AMPK activity and lipid metabolism in the hypothalamus is an important regulator of energy homeostasis.
The thyroid axis is an important modulator of both energy balance and lipid metabolism 1-3. Hyperthyroidism is a clinical disorder characterized by excessive production of thyroid hormones (T3 and T4), which causes a hypermetabolic state characterized by increased energy expenditure and weight loss, despite marked hyperphagia 3, 4. Although it is generally assumed that most effects of thyroid hormones on energy homeostasis are exerted peripherally 1, 3, 5 recent evidence indicates that hypothalamic neurons sense nutritional deficit through a mechanism that involves local generation of T3 and leading to the induction of uncoupling protein 2 (UCP2) 2.
Excess thyroid hormone elicits substantial changes in lipid metabolism. Specifically, hyperthyroidism leads to increased fatty acid synthesis in liver, kidney, heart, BAT and white adipose tissue (WAT), through increased expression and activation of key lipogenic enzymes such as acetyl-CoA carboxylase (ACC) and fatty acid synthase (FAS) 6-9. AMPK activity is stimulated in muscle in hyperthyroid states 10-13. In contrast, it is generally assumed that thyroid status does not alter fatty acid biosynthesis in the whole brain 6, 8, 14, 15. However, thyroid hormones might selectively modulate lipid metabolism in discrete areas of the hypothalamus that contribute to energy balance; a theory supported by the recent finding that hypothalamic de novo lipogenesis is involved in the physiological control of feeding 16-20. Furthermore, evidence indicates that fatty acid metabolism is regulated differently in the hypothalamus compared to other CNS regions, in addition to pharmacological studies and genetic models, showing that impaired hypothalamic lipid metabolism impacts on the control of feeding 19-28. However, despite such evidence there is no data linking alterations of these homeostatic mechanisms to a specific disease state.
Here, we show that thyroid hormones regulate lipid metabolism in the hypothalamus and investigate whether this effect accounts for the changes in energy balance typically associated with hyperthyroidism. Our data demonstrate that de novo lipogenesis increases specifically in the hypothalamus of hyperthyroid rats and that this effect is directly mediated by T3. This enhanced lipogenesis leads to activation of the SNS and induction of BAT. Overall, these data indicate that fatty acid metabolism in the hypothalamus mediates the physiological and pathophysiological effects of thyroid hormone on energy balance.
RESULTS
Hyperthyroid rats lose weight despite marked hyperphagia
Confirming their hyperthyroid status T4-treated rats exhibited increased plasma T4 and T3, reduced thyrotropin (TSH) levels and reduced thyrotropin-releasing hormone (Thr) expression in the paraventricular nucleus of the hypothalamus (PVN) (Supplementary Table 1). T4-treated animals gained considerable less weight (Supplementary Fig. 1a) despite marked hyperphagia (Supplementary Fig. 1b). Analysis of fat-pads showed that T4-treatment decreased WAT and increased BAT mass compared with euthyroid rats (Supplementary Fig. 1c and Supplementary Table 1). Diiodothyronine (T2), an inactive thyroid hormone metabolite, did not change either body weight or food intake (data not shown).
Consistent with their hyperphagic state, expression of agouti-related protein (Agrp) and neuropeptide Y (Npy) increased and levels of proopiomelanocortin (Pomc) decreased in the arcuate nucleus of the hypothalamus (ARC) of hyperthyroid rats (Supplementary Fig. 1d).
Hyperthyroidism inhibits the hypothalamic AMPK pathway
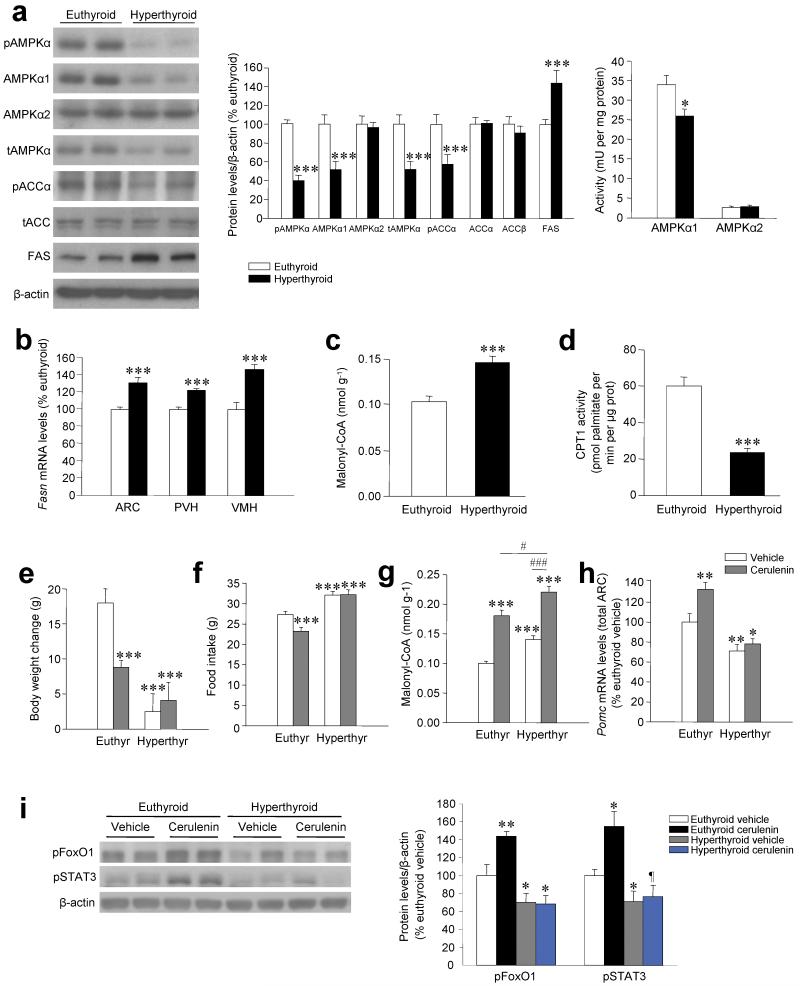
Hyperthyroid rats, but not rats treated with T2 (data not shown), showed a marked decrease in phosphorylation of hypothalamic AMPKα (pAMPKα) and ACCα (pACCα) associated with a decrease in the concentration and activity of AMPKα1 (Fig. 1a). Our data also revealed a marked increase in FAS protein concentration (Fig. 1a), FAS expression (encoded by Fasn) (Fig. 1b and Supplementary Fig. 1e) and the concentration of malonyl-CoA (Fig. 1c) in the hypothalamus of hyperthyroid rats, accompanied by a reduction in carnitine palmitoyltransferase 1 (CPT1) activity (Fig. 1d). Food restriction reduced malonyl-CoA concentration in the hypothalamus of euthyroid rats but had no effect in hyperthyroid rats (Supplementary Fig. 1f). Hyperthyroidism did not alter the AMPK pathway in the cerebral cortex (Supplementary Fig. 2a), indicating that thyroid hormone excess specifically activates the early stages of fatty acid lipogenesis in the hypothalamus. By using a lipidomic approach we also saw increased accumulation of complex lipid species specifically in the hypothalamus of hyperthyroid rats (data not shown). As in the hypothalamus, hyperthyroidism decreased AMPK levels in liver, but increases it in muscle and WAT (Supplementary Fig. 2b–d).
Figure 1. Energy balance, AMPK pathway and POMC expression.
(a) Western blots (left panel) for hypothalamic protein levels (middle panels) of pAMPKα, AMPKα1, AMPKα2, tAMPKα, pACCα, ACCα (lower band in the tAMPK gel), ACCβ (upper band in the tAMPK gel) and FAS and hypothalamic AMPKα1 and AMPKα2 activities (right panel) in euthyroid and hyperthyroid rats. (b–d) Hypothalamic levels of Fasn (b), malonyl-CoA content (c) and CPT1 activity (d) in euthyroid and hyperthyroid rats. (e–i) Body weight change (e), daily food intake (f), hypothalamic malonyl-CoA levels (g), Pomc mRNA levels in the ARC (h) and western blots (left panel) for hypothalamic protein levels (right panel) of pFoxO1 and pSTAT3 (i) of euthyroid and hyperthyroid rats treated ICV with vehicle or cerulenin for 4 d. ¶P = 0.1, *P < 0.05, **P < 0.01, ***P < 0.001 vs. vehicle or euthyroid vehicle; #P < 0.05 euthyroid cerulenin vs. hyperthyroid cerulenin; ###P < 0.001 hyperthyroid vehicle vs. hyperthyroid cerulenin; all data are expressed as mean ± SEM.
We also analyzed the effect of hypothyroidism induced in rats by 3-week treatment with aminotriazole (AMT) which decreased plasma concentrations of T4 and T3, increased plasma TSH levels and increased Trh expression in the PVH (Supplementary Table 1). Hypothyroidism increased AMPKα1 levels and activity in the hypothalamus (Supplementary Fig. 2e,f) and markedly decreased FAS concentration in the liver (Supplementary Fig. 2g). The AMPK pathway was activated in the muscle and WAT of hypothyroid rats (Supplementary Fig. 2h,i).
Hyperthyroid rats do not respond to central FAS inhibition
Our data showed that hyperthyroid rats were hyperphagic despite a higher concentration of hypothalamic malonyl-CoA than euthyroid rats. We therefore tested whether further increasing malonyl-CoA levels by inhibiting FAS 21, 26, 27 would reverse hyperphagia in hyperthyroid animals. To evaluate this, we centrally treated hyperthyroid rats with the FAS inhibitor cerulenin for 4 d. This treatment did not alter body weight (Fig. 1e) or food intake (Fig. 1f and Supplementary Fig. 3a) in hyperthyroid rats, despite a marked additional increase in hypothalamic malonyl-CoA levels (Fig. 1g). In contrast, in euthyroid rats, the same dose of cerulenin increased malonyl-CoA to levels comparable to those in hyperthyroid rats, accompanied by marked anorexia and weight loss (Fig. 1e–g and Supplementary Fig. 3a).
We observed no change in the expression of either Agrp or Npy in the ARC after cerulenin treatment (Supplementary Fig. 3b,c). However, cerulenin induced a marked increase in Pomc mRNA in euthyroid but not hyperthyroid rats (Fig. 1h and Supplementary Fig. 3d), which might account for the anorexic effects of this drug in euthyroid animals. The increase in Pomc expression was accompanied by parallel changes in the concentration of phospho-forkhead box O1 (pFoxO1) and phospho-signal transducer and activator of transcription 3 (pSTAT3) (Fig. 1i),
Central T3 increase BAT thermogenesis through β3 adrenoceptors
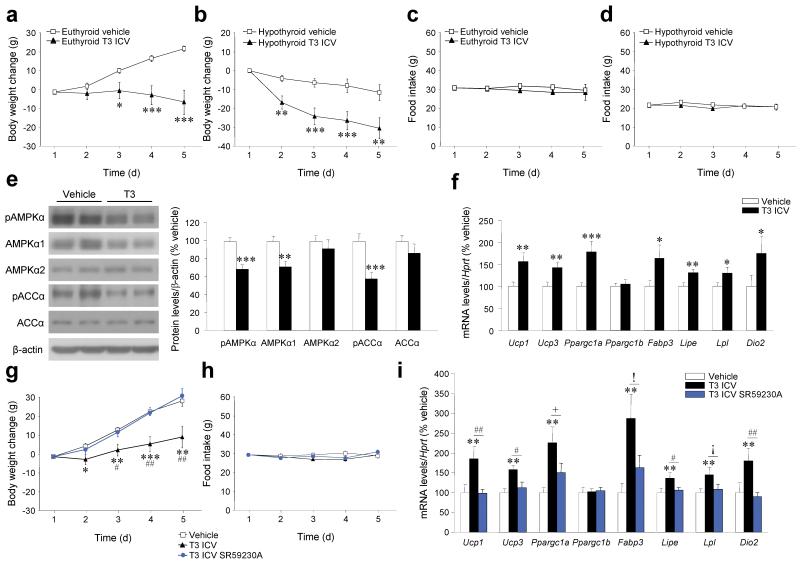
Chronic intracerebroventricular (ICV) treatment with T3 markedly reduced body weight (Fig. 2a,b) without altering food intake (Fig. 2c,d) in either euthyroid or hypothyroid rats. Such treatment decreased phosphorylation of hypothalamic AMPK and ACC (Fig. 2e). It also increased the expression of thermogenic markers, such as uncoupling protein 1 (Ucp1) and 3 (Ucp3), peroxisome-proliferator-activated receptor-gamma co-activator 1 alpha (PGC1α, encoded by Ppargc1a), fatty acid binding protein 3 (Fabp3), hormone sensitive lipase (HSL, encoded by Lipe), lipoprotein lipase (Lpl) and type 2 deiodinase (D2, encoded by Dio2) in the BAT of euthyroid rats (Fig. 2f), but not in other peripheral organs, such as liver and muscle (Supplementary Fig. 4a,b). Chronic ICV treatment with T3 also increased Ucp1, Ucp3, Dio2 and Fabp3 expression in the BAT of hypothyroid rats but did not have major effects in other thermogenic markers either in liver or muscle (Supplementary Fig. 4c-e). ICV administration of T3 did not alter plasma T3 or T4 (data not shown). Furthermore, there were no changes in body weight, food intake, the hypothalamic AMPK pathway or the thermogenic program in BAT following intraperitoneal (IP) administration of T3 at the dose administered ICV (Supplementary Fig. 4f-i).
Figure 2. Effects of chronic central T3 administration.
(a–d) Body weight change (a, b), daily food intake (c, d) of euthyroid and hypothyroid rats ICV-treated with T3 for 4 d. (e–f) Western blots (left panel) for hypothalamic protein levels (right panel) of pAMPKα, AMPKα1, AMPKα2, pACCα and ACCα (e) and mRNA expression profiles in BAT (f) of euthyroid rats ICV-treated with T3 for 4 d. (g–i) Body weight change (g), daily food intake (h) and mRNA expression profiles in BAT (i) of euthyroid rats ICV-treated with T3 and subcutaneously (SC) -treated with the β3-AR specific antagonist SR59230A for 4 d.!P = 0.09, ¡P = 0.08, +P = 0.06, *P < 0.05, **P < 0.01, ***P < 0.001 vs. vehicle; #P < 0.05, ##P < 0.01 T3 ICV vs. T3 ICV SR59230A; all data are expressed as mean ± SEM.
In rodents, SNS stimulation activates beta 3 adrenoceptors (β3-AR, encoded by Adrb3) on brown adipocytes 5, 29. Pharmacological antagonism of β3-AR by subcutaneous administration of the specific antagonist SR59230A 30 prevented the reduction in body weight associated with central administration of T3 (Fig. 2g) without changing food intake (Fig. 2h). In keeping with these observations, SR59230A blocked central T3-induction of the thermogenic program in BAT (Fig. 2i).
Central T3 activates the SNS subserving BAT
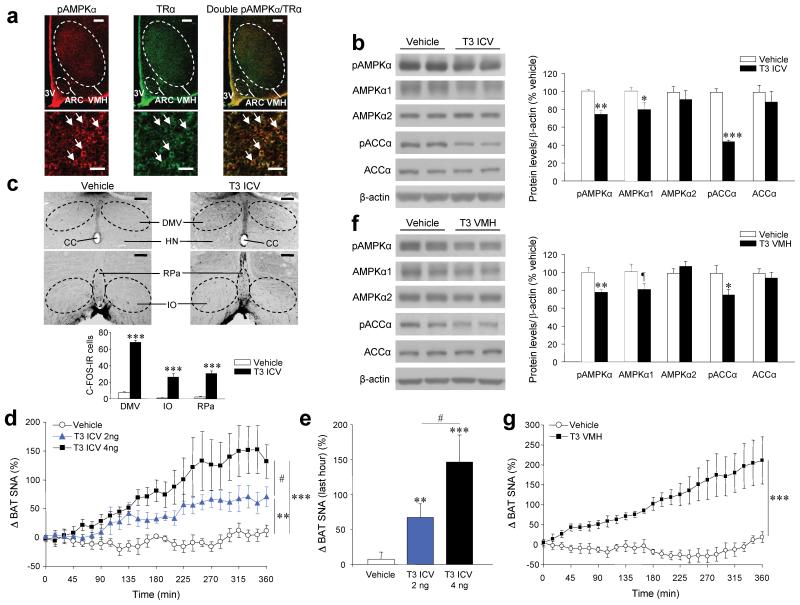
Colocalization studies of pAMPKα and thyroid hormone receptor alpha (TRα) showed that pAMPKα is widely expressed in the hypothalamus, with high levels in the ARC, PVH and VMH (Supplementary Fig. 5a-c) and that there is a high degree of colocalization of pAMPKα and TRα in the VMH (Fig. 3a).
Figure 3. Effects of central T3 on BAT activation via the SNS.
(a) Double immunohistochemistry (upper: 40 ×, scale bar, 200 μm; lower: 200 ×; scale bar, 20 μm) showing pAMPKα and TRα coexpression in the VMH. (b–e) Western blots (left panel) for hypothalamic protein levels (right panel) of pAMPKα, AMPKα1, AMPKα2, pACCα and ACCα (b), immunohistochemistry showing c-FOS immunoreactivity (IR) in the DMV (upper images 40 ×, scale bar, 200 μm) and in the RPa and the IO (lower images 100 ×, scale bar 100 μm) and c-FOS-IR cells in those nuclei (c) and BAT SNA (d, e) of euthyroid rats 1–3 h (protein and c-FOS) or 6 h (SNA) after ICV treatment with T3. (f, g) Western blots (left panel) for hypothalamic protein levels (right panel) of pAMPKα, AMPKα1, AMPKα2, pACCα and ACCα (f) and BAT SNA (g) of euthyroid rats 1 h after VMH microinjection of T3. ¶P = 0.1, *P < 0.05, **P < 0.01, ***P < 0.001 vs. vehicle; #P < 0.05 T3 ICV 2 ng vs. T3 ICV 4 ng; all data are expressed as mean ± SEM. 3V: third ventricle; CC: central canal; HN: hypoglossal nucleus.
ICV injection of T3 exerted a robust, rapid (within 1 h) reduction in pAMPK and pACC levels in the hypothalamus (Fig. 3b) accompanied by a marked increase in c-FOS immunoreactivity (IR) in the raphe pallidus (RPa), the inferior olive (IO) nuclei and in the dorsal motor nucleus of the vagus (DMV). Notably, all these nuclei receive neuronal projections from the VMH 5, 31 (Fig. 3c). This central effect of T3 was associated with a dose-dependent increase in activity of SNS subserving to BAT (Fig. 3d,e). Of note, we detected no changes in feeding after acute ICV treatment with T3 (Supplementary Fig. 5d). Stereotaxic administration of T3 to the VMH (Supplementary Fig. 5e) caused a rapid (within 1 h) effect, decreasing both pAMPK and pACC in this area (Fig. 3f), accompanied by increased BAT sympathetic nerve activity (SNA) (Fig. 3g). Acute VMH treatment with T3 did not induce changes in food intake (Supplementary Fig. 5f).
Central T3 is essential for BAT thermogenic program
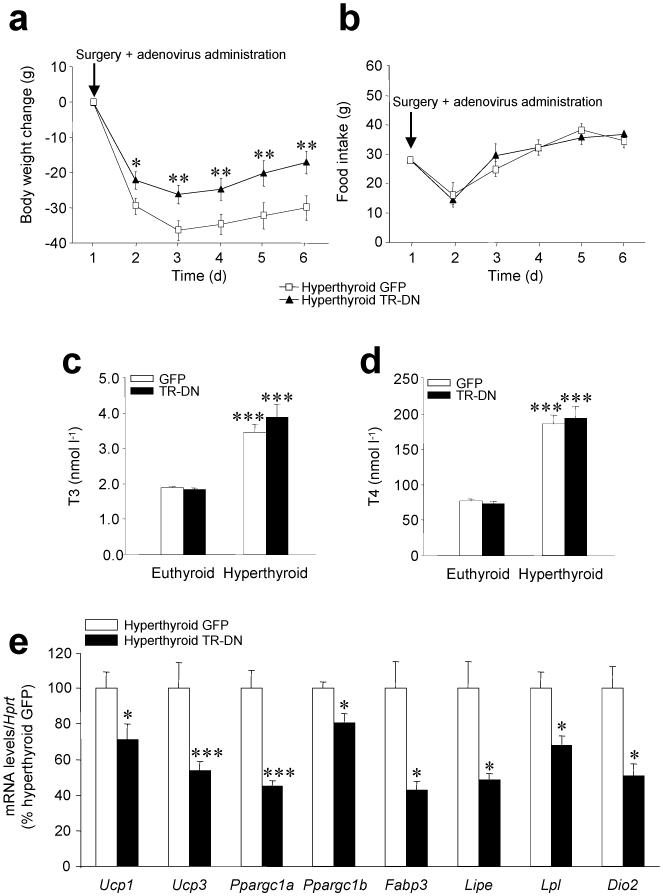
We then ablated TR action in the VMH of hyperthyroid rats using an adenovirus encoding a dominant-negative TR (TR-DN) 32, as previously described 19 (Supplementary Fig. 6a-c). TR-DN adenovirus prevented T3-dependent activation of a thyroid response element-driven reporter gene and inhibited induction of sex hormone binding globulin (Shbg) expression (Supplementary Fig. 6d-f).
Injecting TR-DN into the VMH of hyperthyroid rats blunted weight loss (Fig. 4a), did not alter either feeding (Fig. 4b) or circulating levels of T3 and T4 (Fig. 4c,d) and decreased expression of thermogenic markers in BAT (Fig. 4e), Similar treatment in euthyroid rats was without effect (Supplementary Fig. 6g-j).
Figure 4. Effects of genetic ablation of thyroid hormone receptor in the VMH.
(a–e) Body weight change (a), daily food intake (b), plasma T3 (c) and T4 (d) levels and mRNA expression profiles in BAT (e) of hyperthyroid (and euthyroid when indicated) rats stereotaxically treated with GFP-expressing adenoviruses or GFP plus TR-DN adenoviruses into the VMH. *P < 0.05, **P < 0.01, ***P < 0.001 vs. euthyroid GFP or hyperthyroid GFP; all data are expressed as mean ± SEM.
Central T3 effect on BAT depends on hypothalamic lipogenesis
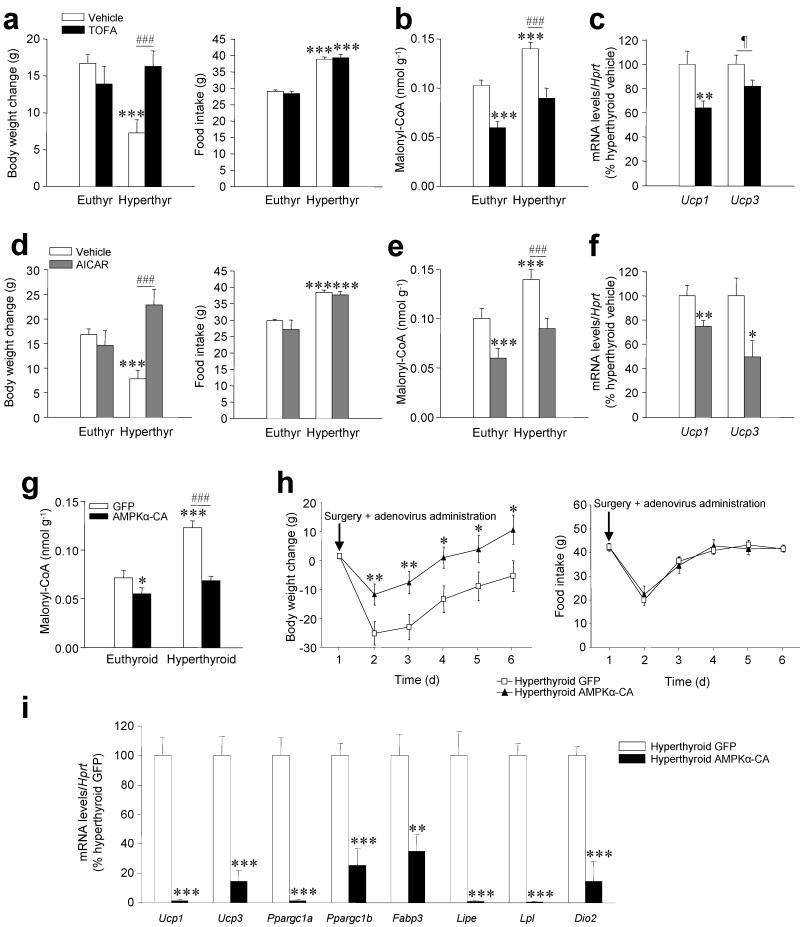
ICV administration of the ACC inhibitor TOFA did not alter body weight in euthyroid rats; however, it reversed weight loss in hyperthyroid rats without changes in feeding (Fig. 5a). The increase in body weight induced by TOFA was associated with decreased levels of hypothalamic malonyl-CoA and decreased mRNA expression of Ucp1 and Ucp3 in BAT (Fig. 5b,c).
Figure 5. Effects of inactivation of hypothalamic de novo lipogenesis.
(a–d) Body weight change (left panel) and daily food intake (right panel) (a), hypothalamic malonyl-CoA levels (b) and Ucp1 and Ucp3 mRNA in the BAT (c) of hyperthyroid (and euthyroid when indicated) rats treated with vehicle or TOFA. (d–f) Body weight change (left panel) daily food intake (right panel) (d), hypothalamic malonyl-CoA levels (e) and Ucp1 and Ucp3 mRNA and in the BAT (f) of hyperthyroid (and euthyroid when indicated) rats treated with vehicle or AICAR. (g–i) Malonyl-CoA levels in the ventral hypothalamus (g) body weight change (left panel), food intake (right panel) (h) and mRNA expression profiles in BAT (i) of hyperthyroid (or euthyroid when indicated) rats stereotaxically treated with a GFP-expressing adenoviruses or GFP plus AMPK constitutively active (AMPKα-CA) adenoviruses into the VMH. *P < 0.05, **P < 0.01, ***P < 0.001 vs. vehicle or GFP; ###P < 0.01 hyperthyroid vehicle vs. hyperthyroid TOFA or AICAR and hyperthyroid GFP vs. hyperthyroid AMPKα-CA; all data are expressed as mean ± SEM.
ICV administration of the AMPK activator AICAR showed similar effects on body weight, food intake (Fig. 5d), malonyl-CoA (Fig. 5e) and Ucp1 and Ucp3 expression in BAT (Fig. 5f).
AMPK in the VMH modulates the thermogenic program in BAT
Adenoviruses encoding constitutively active AMPKα-CA 16, were injected stereotaxically into the VMH of hyperthyroid rats as above described. AMPKα-CA reduced malonyl-CoA levels in the ventral hypothalamus (Fig. 5g), which was associated with weight gain in hyperthyroid but not euthyroid rats (Fig. 5h and Supplementary Fig. 7a), although there was no alteration in food intake (Fig. 5h and Supplementary Fig. 7b).
Overexpression of AMPKα-CA in the VMH was associated with a specific reduction in the expression of BAT thermogenic markers in hyperthyroid (Fig. 5i) but not euthyroid rats (Supplementary Fig. 7c,d).
AMPK in the VMH modulates BAT thermogenesis via the SNS
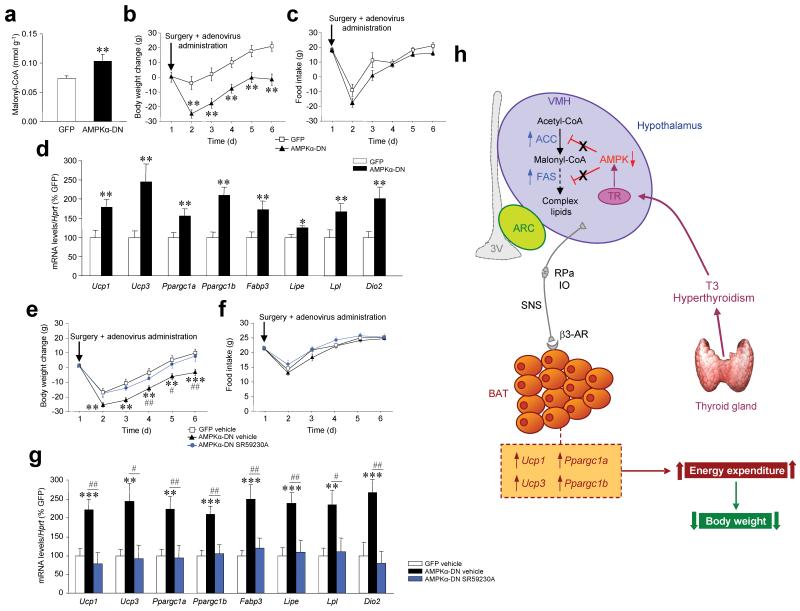
To assess the role of AMPKα in the regulation of energy balance we inactivated hypothalamic AMPK in euthyroid rats. Our data demonstrated that stereotaxic delivery of a dominant-negative AMPKα (AMPKα-DN) 19 into the VMH increased malonyl-CoA levels in the ventral hypothalamus (Fig. 6a) and induced weight lost (Fig. 6b) independently of food intake (Fig. 6c) and neuropeptide expression (Supplementary Fig. 7e).
Figure 6. Effects of selective inactivation of AMPK in the VMH.
(a–d) Malonyl-CoA levels in the ventral hypothalamus (a), body weight change (b), food intake (c), and mRNA expression profiles in BAT (d) of euthyroid rats stereotaxically treated with a GFP-expressing adenoviruses or GFP plus AMPK dominant negative (AMPKα-DN) into the VMH. (e–g) Body weight change (e), food intake (f), and mRNA expression profiles in BAT (g) of rats stereotaxically treated into the VMH with GFP-expressing adenoviruses SC-treated with vehicle, GFP plus AMPKα-DN SC-treated with vehicle and GFP plus AMPKα-DN SC-treated with the β3-AR specific antagonist SR59230A. (h) Proposed model of the effect of thyroid hormones excess on hypothalamic fatty acid metabolism. Hyperthyroidism and T3 upregulate de novo lipogenesis in the hypothalamus which results from decreased activity of AMPK, activation of ACC and increased expression of Fasn. Thyroid hormone-induced changes in hypothalamic lipid biosynthetic pathway increases levels of hypothalamic malonyl-CoA and complex lipids. These changes are associated with the activation of the SNS through the RPa and the IO, resulting in increased expression of BAT markers, such as Ucp1, Upc3, Ppargc1a (which encodes PGC1α) and Ppargc1b (which encodes PGC1β), promoting negative energy balance and weight loss. *: P < 0.05, **P < 0.01, ***P < 0.001 vs. GFP; #P < 0.05, ##P < 0.01 AMPKα-DN vehicle vs. AMPKα-DN SR59230A; all data are expressed as mean ± SEM.
AMPKα-DN promoted expression of thermogenic markers specifically in BAT (Fig. 6d and Supplementary Fig. 7f,g). Inhibition of β3-AR in BAT by subcutaneous administration of SR59230A 30 reversed AMPKα-DN-induced weight loss and blunted the mRNA expression of BAT thermogenic markers without altering food intake (Fig. 6e–g).
DISCUSSION
This study identifies a homeostatic link between the effects of thyroid hormone on hypothalamic AMPK and activation of BAT by the SNS (Fig. 6h). Hyperthyroidism is a metabolic state characterized by negative energy balance and decreased fat mass 1. There is evidence that impaired expression or activity of enzymes involved in fatty acid metabolism in the hypothalamus is associated with changes in neuropeptide expression and corresponding changes in food intake 17, 19, 21, 22, 24, 26, 27, 33. Thyroid hormones also modulate lipid metabolism in peripheral tissues 6, 7, 34, 35 8, 15, 36. Therefore, we hypothesized that hyperthyroidism-induced hyperphagia might be mediated by specific alterations in lipid metabolism in the hypothalamus. 8, 14, 15. Here, we show that hyperthyroidism and T3 increase de novo lipogenesis specifically in the hypothalamus but not in other brain regions. These prolipogenic changes in the hypothalamus are mediated by decreased levels and activity of AMPK, which results in activation of ACC and accumulation of malonyl-CoA 37, 38. The pathophysiological relevance of the T3-mediated increase in hypothalamic lipid biosynthesis is intriguing and we speculate that it represents an adaptive allostatic response that helps maintain appropriate levels of structural and signaling lipids in a hypermetabolic state. Alternatively, it might reflect an imbalance between lipid biosynthetic and pro-oxidative pathways 34-36.
Our results indicate that hyperthyroidism induces a hyperphagic response that is accompanied by corresponding changes in the levels of orexigenic and anorexigenic neuropeptides in the hypothalamus. Of interest, the decrease in Pomc expression was associated with decreased levels of the transcription factors pFoxO1 and pSTAT3, which are known to regulate Pomc transcription 39, 40. One of the more puzzling aspects of our data is the resilience of the hyperthyroidism-induced hyperphagia, which is resistant to the known anorectic effects of malonyl-CoA accumulation and inactivation of CPT1 in the hypothalamus. This is observed even after treatment with cerulenin, which inhibits FAS and potentiates the increase in hypothalamic malonyl-CoA. Although the mechanisms by which this occurs are unclear, we speculate that accumulation of specific lipid species in the hypothalamus of hyperthyroid rats might have a role. Moreover, it is possible that, under conditions of elevated energy demand, specific allostatic responses might overrule the usual inhibition of energy intake by malonyl-CoA. In support of this, recent evidence shows that although hypothalamic fatty acid metabolism is important in mediating adaptive feeding responses to fasting 19, 21-23, 26, 41, 42 and acute administration of hormones 16-20, its role in the long-term control of feeding is less clear 43. One possibility is that changes in lipids species in addition to malonyl-CoA, might interfere with the usual mechanisms controlling feeding.
The main finding of our research is the fundamental role of T3 in the CNS, activating the thermogenic program in BAT. Conventionally, this effect has been ascribed to the direct action of T3 on BAT and muscle 1-3, 5. Given that thyroid hormones also exert important effects in the CNS 2, we hypothesized that central T3 action might contribute to whole-body metabolism. In support of this, we demonstrate that genetic inhibition of TR in the VMH reverses the effects of hyperthyroidism on energy balance and the thermogenic program in BAT. Our results also indicate that hyperthyroidism alters hypothalamic AMPK-dependent activation of de novo lipogenesis which results in weight loss via increased energy dissipation in BAT. Conversely, inactivation of hypothalamic fatty acid metabolism reduces activation of BAT and prevents the weight loss associated with hyperthyroidism. To determine the physiological relevance of these observations, we investigated whether selective alteration of fatty acid metabolism in the VMH is sufficient to modulate BAT function 5, 44-46. We demonstrate that inactivation of AMPK in the VMH activates thermogenesis in BAT through the SNS. Indeed, selective genetic ablation of AMPK in the VMH induced weight loss and increased BAT activation without altering feeding. Moreover, changes in mRNA expression in BAT and the decrease in body weight, were reversed by treatment with the selective β3-AR antagonist SR59230A, indicating an essential role of the SNS in these effects. The precise mechanisms linking hypothalamic AMPK activity with sympathetic tone require further investigation. However, our evidence shows that central administration of T3 elicits a marked increase in neuronal activity in the RPa and the IO, nuclei that receive output from the VMH to the intermediolateral neurons and the sympathetic chain, which ultimately innervates BAT 5.
Although, classically, the VMH is considered to be a regulator of feeding control (“the satiety center”), recent evidence reveals a more complex physiological role. For example, AMPK in the VMH is involved in integrating peripheral signals such as ghrelin with the hypothalamic signaling network 19, 33, and in detecting acute hypoglycemia and mounting a counter regulatory glucose response 47. Overall, these data indicate that AMPK acts in the VMH as a “global energy gauge” that modulates both food intake and the thermogenic program. Our data reveal a pathophysiological relevance of this homeostatic regulatory mechanism in the context of hyperthyroidism. Further studies are needed to determine whether this mechanism is restricted to the hyperthyroid state or if it contributes to other pathological states characterized by negative energy balance (e.g. cachexia, wasting diseases). In this sense, recent evidence highlights the importance of BAT in adult humans 48-51, and that thyroid status affects BAT activity 52. The role of AMPK activity and fatty acid metabolism in the hypothalamus in mediating this effect indicates that selective modulation of AMPK activity in this region, and more specifically in the VMH, might be a useful strategy to treat disorders that involve compromised energy balance, such as hyperthyroidism, cachexia and obesity. Similarly, our study indicates that strategies to modulate either AMPK or lipid levels in the hypothalamus might be of therapeutic benefit in controlling the peripheral effects of thyrotoxicosis and life-threatening conditions, such as thyroid storm, for which current treatments are not satisfactory.
In summary, we show that hyperthyroidism inactivates AMPK, induces a marked upregulation of de novo lipogenesis specifically in the hypothalamus and activates the BAT via the SNS, leading to weight loss. Thus, our data indicate that dysregulation of fatty acid metabolism in the hypothalamus is an important effector of hyperthyroidism-induced negative energy balance through its effects on energy dissipation. This observation provides new insights into the pathogenesis of hyperthyroidism-induced effects on energy balance and suggests potential therapeutic strategies to counteract these effects in the context of this disorder or other catabolic states
Supplementary Material
ACKNOWLEDGEMENTS
We thank to Maria Adams and Andy Whittle (University of Cambridge, UK) for their helpful discussion and editing, Luz Casas and Montserrat Portas (University of Santiago de Compostela, Spain) and Keith Burling (University of Cambridge, UK) for their excellent technical assistance. This work has been supported by grants from the Medical Research Council (A.V-P.: G0802051), Wellcome Trust (K.C.: 080237; A.V-P.: 065326/Z/01/Z), Xunta de Galicia (R.G.: PGIDITPXIB20811PR), Fondo Investigationes Sanitarias (M.L.: PS09/01880), Ministerio de Ciencia e Innovación (C.D.: BFU2008; M.L.: RyC-2007-00211; R.N.: RyC-2008-02219 and SAF2009-07049), European Union (A.V-P. and M.O.: FP7MITIN; A.V-P. and M.O.: LSHM-CT-2005–018734: “Hepadip”, http://www.hepadip.org; C.D., M.L. and R.N.: Health-F2-2008-223713: “Reprobesity”; M.L.: Marie Curie Program QLK6-CT-2002-51671) and the US National Institutes of Health (A.K.S.: K-19514 and DK-67509; K.R.: HL-084207). CIBER de Fisiopatología de la Obesidad y Nutrición is an initiative of ISCIII.
ABBREVIATIONS
- 3V
third ventricle
- ACC
acetyl-CoA carboxylase
- α2A-AR
adrenergic receptor alpha 2a; encoded by Adra2a
- AgRP
agouti-related protein; encoded by Agrp
- AMPK
AMP-activated protein kinase
- AMPKα1
AMP-activated protein kinase α1
- AMPKα2
AMP-activated protein kinase α2
- AMT
aminotriazole
- ARC
arcuate nucleus of the hypothalamus
- β3-AR
adrenergic receptor beta 3; encoded by Adrb3
- BAT
brown adipose tissue
- CART
cocaine and amphetamine-regulated transcript; endoded by Cartpt
- CNS
central nervous system
- CPT1
carnitine palmitoyltransferase 1
- D2
type 2 deiodinase, encoded by Dio2
- DMH
dorsomedial nucleus of the hypothalamus
- DMV
dorsal motor nucleus of the vagus
- FAs
fatty acids
- FABP3
fatty acid binding protein 3 encoded by Fabp3
- FAS
fatty acid synthase, encoded by Fasn
- HPRT
hypoxanthine guanine phosphoribosyl transferase, encoded by Hprt
- HSL
hormone sensitive lipase; encoded by Lipe
- ICV
intracerebroventricular
- IO
inferior olive nucleus
- LPL
lipoprotein lipase; encoded by Lpl
- Malonyl-CoA
malonyl-coenzyme A
- NRF-1
nuclear respiratory factor 1; encoded by Nrf1
- NPY
neuropeptide Y; encoded by Npy
- pACC
phospho-acetyl CoA carboxylase
- pAMPK
phospho- AMP-activated protein kinase
- pFoxO1
phospho-forkhead box O1
- PGC1α
peroxisome-proliferator-activated receptor-gamma co-activator 1 alpha; encoded by Ppargc1a
- PGC1β
peroxisome-proliferator-activated receptor-gamma co-activator 1 beta; encoded by Ppargc1b
- POMC
proopiomelanocortin; encoded by Pomc
- pSTAT3
phospho-signal transducer and activator of transcription 3
- PVH
paraventricular nucleus of the hypothalamus
- RPa
raphe pallidus nucleus
- RPLP0
ribosomal protein, large, P0; encoded by Rplp0
- SC
subcutaneous
- SHBG
sex hormone binding globulin; encoded by Shbg
- SNA
sympathetic nervous activity
- SNS
sympathetic nervous system
- T2
diiodothyronine
- T3
triiodothyronine
- T4
L-thyroxine
- TR
thyroid hormone receptor
- TSH
thyrotropin
- TRH
thyrotropin-releasing hormone; encoded by Trh
- UCP1
uncoupling protein 1; encoded by Ucp1
- UCP2
uncoupling protein 2
- UCP3
uncoupling protein 3; encoded by Ucp3
- VMH
ventromedial nucleus of the hypothalamus
- WAT
white adipose tissue
Appendix
METHODS
Animals
We used adult male Sprague-Dawley rats (9–11 weeks old). For all the experimental groups and analytic methods, we used 9–12 rats per group as a minimum. All the experiments were repeated at least twice (Supplementary Methods). University of Santiago de Compostela and University of Iowa Animal Research Committees approved all procedures. All studies were conducted in accordance with the International Law on Animal Experimentation and National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals.
Induction of hyperthyroidism and hypothyroidism
We induced hyperthyroidism by chronic subcutaneous (SC) administration of T4 (100 μg day−1, dissolved in 200 μl of saline) for a period of three weeks (21 d). We induced hypothyroidism by administration of 0.1% aminotriazole in drinking water for three weeks. All drugs were from Sigma.
ICV treatments
For the chronic experiments, we gave rats either a single ICV daily administration of triiodothyronine (T3; 4 ng during 4 d, dissolved in 5 μl of DMSO) or the drugs acting on fatty acid synthesis pathway, namely cerulenin, AICAR (5-aminoimidazole-4-carboxamide-1-D-ribofuranoside), TOFA (5-(tetradecyloxy)-2-furoic acid) (10 μg, 5 μg and 5 μg per rat, respectively, dissolved in 5 μl of DMSO) or vehicle (5 μl DMSO) at 20:00, just before turning the light off during 4 d. For the acute (1–6 h) experiments rats received a single dose of T3 (4 ng, dissolved in 5 μl of DMSO). All drugs were from Sigma.
Stereotaxic microinjection of adenoviral expression vectors and T3
We targeted the VMH bilaterally as previously reported 19 and administered adenovirus vectors (GFP, TR-DN, AMPKα-CA or AMPKα-DN) or T3 (0.4 ng per 100 nl of DMSO) or vehicle (100 nl of DMSO) as described (Supplementary Methods).
Peripheral treatments
We administered T2 was subcutaneously (SC; 100 μg per day, dissolved in 200 μl of saline; Sigma) and T3 intraperitoneally (IP; 4 ng, dissolved in 200 μl of saline) during 4 d. The beta adrenergic receptor 3 (β3-AR) specific antagonist SR59230A ([3-(2-ethylphenoxy)-1-[(1,S)-1,2,3,4-tetrahydronapth-1-ylamino]-2S-2-propanol oxalate])30 (2 mg kg−1 day−1) was administrated by using osmotic minipumps (model 2001D or 2ML2, Alza Corp) as previously reported 43. We treated animals during 2 d before the T3 or the adenoviral injection. The selected dose of SR59230A was that able to reverse the 24-h-induced Ucp1 mRNA expression in BAT (Ucp1 mRNA/Hprt (% vehicle at room temperature), vehicle at room temperature: 100 ± 12.75 vs. vehicle at 4°C: 550.2 ± 155.5 (P<0.05) vs. SR59230A at 4°C: 127.2 ± 11.1 (P<0.05 vs. vehicle at 4°C)).
Sympathetic nerve activity recording
We obtained multi-fiber recording of SNA from the nerve subserving BAT, as previously described (Supplementary Methods)53, 54.
Analytical methods
Real-time RT-PCR, in situ hybridization, western blotting, immunohistochemistry and enzymatic and malonyl-CoA assays were performed as previously described 19, 26, 27, 33, 54.
Statistical analysis
Data are expressed as mean ± SEM. Statistic significance was determined by t-Student or ANOVA followed of post-hoc two-tailed Bonferroni test. P<0.05 was considered significant.
Footnotes
COMPETING INTERESTS STATEMENT
The authors declare that they have no competing financial interests.
C.L. is an employee of AstraZeneca R&D and holds stock in AstraZeneca R&D.
REFERENCES
- 1.Silva JE. Thyroid hormone control of thermogenesis and energy balance. Thyroid. 1995;5:481–492. doi: 10.1089/thy.1995.5.481. [DOI] [PubMed] [Google Scholar]
- 2.Coppola A, et al. A central thermogenic-like mechanism in feeding regulation: an interplay between arcuate nucleus T3 and UCP2. Cell Metab. 2007;5:21–33. doi: 10.1016/j.cmet.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herwing A, Ross AW, Nilaweera KN, Morgan PJ, Barrett P. Hypothalamic thyroid hormone in energy balance regulation. Obes. Facts. 2008;1:71–79. doi: 10.1159/000123428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pijl H, et al. Food choice in hyperthyroidism: potential influence of the autonomic nervous system and brain serotonin precursor availability. J. Clin. Endocrinol. Metab. 2001;86:5848–5853. doi: 10.1210/jcem.86.12.8112. [DOI] [PubMed] [Google Scholar]
- 5.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 6.Volpe JJ, Kishimoto Y. Fatty acid synthetase of brain: development, influence of nutritional and hormonal factors and comparison with liver enzyme. J Neurochem. 1972;19:737–753. doi: 10.1111/j.1471-4159.1972.tb01389.x. [DOI] [PubMed] [Google Scholar]
- 7.Gnoni GV, Landriscina C, Ruggiero FM, Quagliariello E. Effect of hyperthyroidism on lipogenesis in brown adipose tissue of young rats. Biochim. Biophys. Acta. 1983;751:271–279. doi: 10.1016/0005-2760(83)90284-9. [DOI] [PubMed] [Google Scholar]
- 8.Blennemann B, Leahy P, Kim TS, Freake HC. Tissue-specific regulation of lipogenic mRNAs by thyroid hormone. Mol. Cell Endocrinol. 1995;110:1–8. doi: 10.1016/0303-7207(95)03509-6. [DOI] [PubMed] [Google Scholar]
- 9.Cachefo A, et al. Hepatic lipogenesis and cholesterol synthesis in hyperthyroid patients. J. Clin. Endocrinol. Metab. 2001;86:5353–5357. doi: 10.1210/jcem.86.11.7981. [DOI] [PubMed] [Google Scholar]
- 10.Park SH, et al. Effects of thyroid state on AMP-activated protein kinase and acetyl-CoA carboxylase expression in muscle. J Appl. Physiol. 2002;93:2081–2088. doi: 10.1152/japplphysiol.00504.2002. [DOI] [PubMed] [Google Scholar]
- 11.Winder WW, et al. Long-term regulation of AMP-activated protein kinase and acetyl-CoA carboxylase in skeletal muscle. Biochem. Soc. Trans. 2003;31:182–185. doi: 10.1042/bst0310182. [DOI] [PubMed] [Google Scholar]
- 12.Branvold DJ, et al. Thyroid Hormone effects on LKB1, MO25, phospho-AMPK, phospho-CREB, and PGC-1{alpha} in Rat Muscle. J. Appl. Physiol. 2008;105:1218–1227. doi: 10.1152/japplphysiol.00997.2007. [DOI] [PubMed] [Google Scholar]
- 13.Irrcher I, Walkinshaw DR, Sheehan TE, Hood DA. Thyroid hormone (T3) rapidly activates p38 and AMPK in skeletal muscle in vivo. J. Appl. Physiol. 2008;104:178–185. doi: 10.1152/japplphysiol.00643.2007. [DOI] [PubMed] [Google Scholar]
- 14.Morini P, Conserva AR, Lippolis R, Casalino E, Landriscina C. Differential action of thyroid hormones on the activity of certain enzymes in rat kidney and brain. Biochem. Med. Metab Biol. 1991;46:169–176. doi: 10.1016/0885-4505(91)90064-r. [DOI] [PubMed] [Google Scholar]
- 15.Blennemann B, Moon YK, Freake HC. Tissue-specific regulation of fatty acid synthesis by thyroid hormone. Endocrinology. 1992;130:637–643. doi: 10.1210/endo.130.2.1733712. [DOI] [PubMed] [Google Scholar]
- 16.Minokoshi Y, et al. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature. 2004;428:569–574. doi: 10.1038/nature02440. [DOI] [PubMed] [Google Scholar]
- 17.Gao S, et al. Leptin activates hypothalamic acetyl-CoA carboxylase to inhibit food intake. Proc. Natl. Acad. Sci. U. S. A. 2007;104:17358–17363. doi: 10.1073/pnas.0708385104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kola B, et al. The orexigenic effect of ghrelin is mediated through central activation of the endogenous cannabinoid system. PLoS. ONE. 2008;3:e1797. doi: 10.1371/journal.pone.0001797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.López M, et al. Hypothalamic fatty acid metabolism mediates the orexigenic action of ghrelin. Cell Metab. 2008;7:389–399. doi: 10.1016/j.cmet.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 20.Andrews ZB, et al. UCP2 mediates ghrelin’s action on NPY/AgRP neurons by lowering free radicals. Nature. 2008;454:846–851. doi: 10.1038/nature07181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loftus TM, et al. Reduced food intake and body weight in mice treated with fatty acid synthase inhibitors. Science. 2000;288:2379–2381. doi: 10.1126/science.288.5475.2379. [DOI] [PubMed] [Google Scholar]
- 22.Hu Z, Cha SH, Chohnan S, Lane MD. Hypothalamic malonyl-CoA as a mediator of feeding behavior. Proc. Natl. Acad. Sci. U. S. A. 2003;100:12624–12629. doi: 10.1073/pnas.1834402100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Obici S, Feng Z, Arduini A, Conti R, Rossetti L. Inhibition of hypothalamic carnitine palmitoyltransferase-1 decreases food intake and glucose production. Nat. Med. 2003;9:756–761. doi: 10.1038/nm873. [DOI] [PubMed] [Google Scholar]
- 24.Lam TK, Schwartz GJ, Rossetti L. Hypothalamic sensing of fatty acids. Nat. Neurosci. 2005;8:579–584. doi: 10.1038/nn1456. [DOI] [PubMed] [Google Scholar]
- 25.Wolfgang MJ, et al. The brain-specific carnitine palmitoyltransferase-1c regulates energy homeostasis. Proc. Natl. Acad. Sci. U. S. A. 2006;103:7282–7287. doi: 10.1073/pnas.0602205103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.López M, et al. Tamoxifen-induced anorexia is associated with fatty acid synthase inhibition in the ventromedial nucleus of the hypothalamus and accumulation of malonyl-CoA. Diabetes. 2006;55:1327–1336. doi: 10.2337/db05-1356. [DOI] [PubMed] [Google Scholar]
- 27.Chakravarthy MV, et al. Brain fatty acid synthase activates PPAR-alpha to maintain energy homeostasis. J. Clin. Invest. 2007;117:2539–2552. doi: 10.1172/JCI31183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lam TK. Neuronal regulation of homeostasis by nutrient sensing. Nat. Med. 2010;16:392–395. doi: 10.1038/nm0410-392. [DOI] [PubMed] [Google Scholar]
- 29.Dulloo AG. Biomedicine. A sympathetic defense against obesity. Science. 2002;297:780–781. doi: 10.1126/science.1074923. [DOI] [PubMed] [Google Scholar]
- 30.Commins SP, Watson PM, Levin N, Beiler RJ, Gettys TW. Central leptin regulates the UCP1 and ob genes in brown and white adipose tissue via different beta-adrenoceptor subtypes. J. Biol. Chem. 2000;275:33059–33067. doi: 10.1074/jbc.M006328200. [DOI] [PubMed] [Google Scholar]
- 31.Tong Q, et al. Synaptic glutamate release by ventromedial hypothalamic neurons is part of the neurocircuitry that prevents hypoglycemia. Cell Metab. 2007;5:383–393. doi: 10.1016/j.cmet.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chatterjee VK, et al. Thyroid hormone resistance syndrome. Inhibition of normal receptor function by mutant thyroid hormone receptors. J. Clin. Invest. 1991;87:1977–1984. doi: 10.1172/JCI115225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lage R, et al. Ghrelin effects on neuropeptides in the rat hypothalamus depend on fatty acid metabolism actions on BSX but not on gender. FASEB J. 2010;24:2670–2679. doi: 10.1096/fj.09-150672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hagenfeldt L, Wennlung A, Felig P, Wahren J. Turnover and splanchnic metabolism of free fatty acids in hyperthyroid patients. J. Clin. Invest. 1981;67:1672–1677. doi: 10.1172/JCI110204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beylot M, et al. Lipolytic and ketogenic fluxes in human hyperthyroidism. J. Clin. Endocrinol. Metab. 1991;73:42–49. doi: 10.1210/jcem-73-1-42. [DOI] [PubMed] [Google Scholar]
- 36.Riis AL, et al. Elevated regional lipolysis in hyperthyroidism. J. Clin. Endocrinol. Metab. 2002;87:4747–4753. doi: 10.1210/jc.2002-020174. [DOI] [PubMed] [Google Scholar]
- 37.Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: Ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005;1:15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 38.Lage R, Diéguez C, Vidal-Puig A, López M. AMPK: a metabolic gauge regulating whole-body energy homeostasis. Trends Mol. Med. 2008;14:539–549. doi: 10.1016/j.molmed.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 39.Plum L, et al. The obesity susceptibility gene Cpe links FoxO1 signaling in hypothalamic proopiomelanocortin neurons with regulation of food intake. Nat. Med. 2009;15:1195–1201. doi: 10.1038/nm.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Belgardt BF, et al. PDK1 deficiency in POMC-expressing cells reveals FOXO1-dependent and - independent pathways in control of energy homeostasis and stress response. Cell Metab. 2008;7:291–301. doi: 10.1016/j.cmet.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 41.Pocai A, et al. Restoration of hypothalamic lipid sensing normalizes energy and glucose homeostasis in overfed rats. J. Clin. Invest. 2006;116:1081–1091. doi: 10.1172/JCI26640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.He W, Lam TK, Obici S, Rossetti L. Molecular disruption of hypothalamic nutrient sensing induces obesity. Nat. Neurosci. 2006;9:227–233. doi: 10.1038/nn1626. [DOI] [PubMed] [Google Scholar]
- 43.Sangiao-Alvarellos S, et al. Influence of ghrelin and GH deficiency on AMPK and hypothalamic lipid metabolism. J. Neuroendocrinol. 2010;22:543–556. doi: 10.1111/j.1365-2826.2010.01994.x. [DOI] [PubMed] [Google Scholar]
- 44.Niijima A, Rohner-Jeanrenaud F, Jeanrenaud B. Role of ventromedial hypothalamus on sympathetic efferents of brown adipose tissue. Am. J. Physiol. 1984;247:R650–R654. doi: 10.1152/ajpregu.1984.247.4.R650. [DOI] [PubMed] [Google Scholar]
- 45.Holt SJ, Wheal HV, York DA. Hypothalamic control of brown adipose tissue in Zucker lean and obese rats. Effect of electrical stimulation of the ventromedial nucleus and other hypothalamic centres. Brain Res. 1987;405:227–233. doi: 10.1016/0006-8993(87)90292-7. [DOI] [PubMed] [Google Scholar]
- 46.Halvorson I, Gregor L, Thornhill JA. Brown adipose tissue thermogenesis is activated by electrical and chemical (L-glutamate) stimulation of the ventromedial hypothalamic nucleus in cold-acclimated rats. Brain Res. 1990;522:76–82. doi: 10.1016/0006-8993(90)91579-6. [DOI] [PubMed] [Google Scholar]
- 47.McCrimmon RJ, et al. Key role for AMP-activated protein kinase in the ventromedial hypothalamus in regulating counterregulatory hormone responses to acute hypoglycemia. Diabetes. 2008;57:444–450. doi: 10.2337/db07-0837. [DOI] [PubMed] [Google Scholar]
- 48.Nedergaard J, Bengtsson T, Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am. J. Physiol Endocrinol. Metab. 2007;293:E444–E452. doi: 10.1152/ajpendo.00691.2006. [DOI] [PubMed] [Google Scholar]
- 49.Marken Lichtenbelt WD, et al. Cold-activated brown adipose tissue in healthy men. N. Engl. J. Med. 2009;360:1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 50.Cypess AM, et al. Identification and importance of brown adipose tissue in adult humans. N. Engl. J. Med. 2009;360:1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Virtanen KA, et al. Functional brown adipose tissue in healthy adults. N. Engl. J. Med. 2009;360:1518–1525. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- 52.Skarulis MC, et al. Thyroid hormone induced brown adipose tissue and amelioration of diabetes in a patient with extreme insulin resistance. J. Clin. Endocrinol. Metab. 2010;95:256–262. doi: 10.1210/jc.2009-0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rahmouni K, et al. Hypothalamic PI3K and MAPK differentially mediate regional sympathetic activation to insulin. J. Clin. Invest. 2004;114:652–658. doi: 10.1172/JCI21737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nogueiras R, et al. Direct control of peripheral lipid deposition by CNS GLP-1 receptor signaling is mediated by the sympathetic nervous system and blunted in diet induced obesity. J Neurosci. 2009;29:5916–5925. doi: 10.1523/JNEUROSCI.5977-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.