Abstract
The application of nanotechnology in medicine, referred to as nanomedicine, is offering numerous exciting possibilities in healthcare. Herein, we discuss two important aspects of nanomedicine—drug delivery and tissue engineering—highlighting the advances we have recently experienced, the challenges we are currently facing, and what we are likely to witness in the near future.
Keywords: Nanotechnology, nanomedicine, drug delivery, tissue engineering
In pharmaceutical industries, a new molecular entity (NME) that demonstrates potent biological activity but poor water solubility, or a very short circulating half-life, will likely face significant development challenges or be deemed undevelopable. On the other hand, less active but pharmaceutically optimal compounds may become more suitable candidates for development. In either case, there is always a degree of compromise and such trade-offs may inevitably result in the production of less-ideal drugs. However, with the emerging trends and recent advances in nanotechnology, it has become increasingly possible to address some of the shortcomings associated with potential NMEs. By using nanoscale delivery vehicles, the pharmacological properties (e.g. solubility and circulating half-life) of such NMEs can be drastically improved—essentially leading to the discovery of optimally safe and effective drug candidates. This is just one example that demonstrates the degree to which nanotechnology may revolutionize the rules and possibilities of drug discovery and change the landscape of pharmaceutical industries. Indeed, current nanotechnology-based therapeutic products have been validated through the improvement of previously approved drugs, and many novel classes of nanotherapeutics are now underway.1-3
Drug discovery is only one of the many areas in healthcare that nanotechnology is now benefiting. The current and promising applications of nanomedicine include, but are not limited to, drug delivery, in vitro diagnostics, in vivo imaging, therapy techniques, biomaterials, and tissue engineering. Summarized in Box 1 are some important opportunities that nanotechnology may afford in each research area. Some of these opportunities are becoming realities or are actually being used today, while others are generating promise in their early phases of development and are expected to experience vigorous growth in the foreseeable future. As recognition of the importance of this exciting field, it is expected that the global market of nanoscale applications in the medical field could grow to $70 - $160 billion by 2015.4,5 In this perspective, we discuss the applications of nanomedicine with specific focus on drug delivery and tissue engineering.
Box 1. Nanoscale Applications in Medicine.
Drug delivery
Nanoscale delivery vehicles can (1) enhance the therapeutic efficacy and minimize adversities associated with available drugs; (2) enable new classes of therapeutics; and (3) encourage the re-investigation of pharmaceutically suboptimal but biologically active new molecular entities that were previously considered undevelopable.
In vitro diagnostics
Nanotechnology-based sensors (e.g. nanowires, nanotubes, nanoparticles, cantilevers, and micro-/nanoarrays) can enable fast and high throughput detection of disease biomarkers with higher sensitivity and lower sample consumption. Nanotechnology also offers hope for the early detection of viruses, bacteria, and circulating tumor cells, as well as for single cell analysis.
In vivo imaging
Targeted imaging nanoprobes (e.g. magnetic nanoparticles, quantum dots, and carbon nanotubes) could provide a faster, less invasive, and more accurate way to diagnose diseases (e.g. cancer) at their earliest stages and monitor disease progression. Some other possible opportunities include reporting in vivo efficacy of therapeutics, tracking nanocarrier biodistribution in the body, and helping surgeons to locate tumors and their margins, identify important adjacent structures, and map sentinel lymph nodes.
Therapy techniques
Certain nanomaterials have unique therapeutic properties that differ from conventional drugs, and can, therefore, be directly used to treat diseases. For example, hafnium oxide- and gold-based nanoparticles can greatly enhance X-ray therapy; gold nanoshells/nanorods, carbon nanotubes, magnetic nanoparticles can induce hypothermia to kill cancer cells; and nanocrystalline silver is being used as an antimicrobial agent.
Biomaterials
Biocompatible nanomaterials that have optimal mechanical properties can be used as medical implants (e.g. dental restoratives and bone substitutes)†. Nanocoatings or nanostructured surfaces can also improve the biocompatibility and adhesion of biomaterials.
Tissue engineering
Nanotechnology can enable the design and fabrication of biocompatible scaffolds at the nanoscale and control the spatiotemporal release of biological factors—resembling native extracellular matrix—to direct cell behaviors, and eventually lead to the creation of implantable tissues.
(†It can also be categorized as hard-tissue engineering.)
Drug Delivery
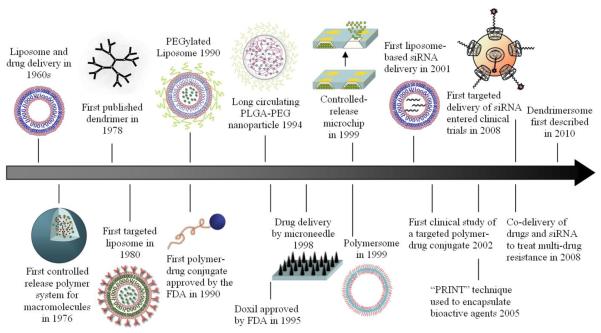
Since liposomes were first described in the 1960s and proposed as carriers of proteins and drugs for disease treatment,6 nanotechnology has made a significant impact on the development of drug delivery systems. A variety of organic/inorganic nanomaterials and devices have been used as delivery vehicles to develop effective therapeutic modalities (Figure 1). So far, there are over two dozen nanotechnology-based therapeutic products approved by Food and Drug Administration (FDA) for clinical use, and more are in clinical trials.1-3 Of these products, the majority are composed of a non-targeted delivery system (e.g. liposomes and polymers) and a drug, and are therefore considered first generation nanotherapeutics.7
Figure 1.
Timeline of nanotechnology-based drug delivery. Here, we highlight some nanoscale delivery systems that serve as important milestones throughout the history of drug delivery.
Compared to conventional drug delivery, the first generation nanosystems provide a number of advantages. In particular, they can enhance the therapeutic activity by prolonging drug half-life, improving solubility of hydrophobic drugs, reducing potential immunogenicity, and/or releasing drugs in a sustained or stimuli-triggered fashion. Thus, the toxic side effects of drugs can be reduced, as well as the administration frequency. In addition, nanoscale particles can passively accumulate in specific tissues (e.g. tumors) through the enhanced permeability and retention (EPR) effect.8 Beyond these clinically efficacious nanosystems, nanotechnology has been utilized to enable new therapies and to develop next generation nanosystems for “smart” drug delivery. Below are several areas, from our perspective, that will generate medical breakthroughs in the near future, and some of them could inspire the derivation of the next “Killer Applications”.
New therapeutics delivery
Gene therapy and RNA interference (RNAi), the two most recent and notable therapies, hold great promise as treatment and prevention methods for various diseases.9,10 However, the systemic administration of new therapeutics (e.g. DNA and siRNA) is affected by several barriers such as enzymatic degradation, uptake by reticuloendothelial system, kidney filtration, and limited intracellular entry. Cationic nonviral nanocarriers have, therefore, been widely used to encapsulate and make these new therapeutics more efficient. Unfortunately, a substantial number of these materials can produce severe problems associated with toxicity, immune or inflammatory responses, and serum instability, making them unsuitable for clinical applications. Consequently, safe and effective delivery materials have become the bottleneck for the widespread use of gene therapy and RNAi.
Recent and exciting developments are now paving the way for the clinical application of these new therapeutics. For example, high throughput screening platform technologies have been described and proof-of-concept has been demonstrated by identifying cationic polymers and lipid-like-materials suitable for systemic gene and RNAi delivery.11,12 Rational design approaches, on the other hand, are also showing great potential in creating successful delivery materials (e.g. DLin-KC2-DMA13 and cyclodextrin-containing polymer14). Nevertheless, it would be favorable to use previously FDA-approved materials for new therapeutics delivery, as they have specific benefits such as safety and sustained release. One such material being studied is poly(lactic-co-glycolic acid) (PLGA), a specific polymer that can be applied to form nanoparticles densely loaded with siRNA for sustained gene silencing.15 Beyond assuring the safety and efficiency of delivery materials, more progress is needed to screen other factors, including the physicochemical properties of nanocarriers, targeting ligands, and formulation methods—all of which can affect the delivery of new therapeutics in vivo.
Targeted delivery
It is widely believed that active targeting, through the modification of nanoparticles with ligands, has the potential to enhance the therapeutic efficacy and reduce the side effects relative to conventional therapeutics.16 The ability to actively target specific cells rather than tissues also allows ligand-conjugated nanocarriers to outperform first generation, non-targeted nanosystems. While the necessity of targeted delivery depends on various factors (e.g. delivery vehicles, drugs, and diseases), a myriad of important benefits have been demonstrated.3,16-19
In cancer therapy, the presence of targeting ligands can greatly enhance the retention and cellular uptake of nanoparticles via receptor-mediated endocytosis—even although tumor accumulation is largely determined by the physicochemical properties of nanoparticles.16,17 This can then lead to higher intracellular drug concentration and increase therapeutic activity, which is particularly important for bioactive macromolecules (e.g. DNA and siRNA) that require intracellular delivery for bioactivity.16 In the case of endothelial targeting for cardiovascular diseases or immunological tissue targeting, nanoparticle localization is guided by ligand-receptor interactions rather than EPR.18 Similarly, ligand-mediated targeting is of importance for the transcytosis of nanodrugs across tight epithelial and endothelial barriers (e.g. blood-brain barrier).19 Additionally, targeted delivery has been applied, in some instances, to combat multidrug resistance (MDR).3 It is also envisioned that long-circulating targeted nanoparitcles may be able to locate and fight migrating cancer cells.
Despite three targeted nanoparticle systems now in phase I/II clinical trials,3 the clinical translation of targeted delivery is not as smooth as we expect. One possible barrier stems from the complexity behind manufacturing viable targeted nanoparticles. Targeted nanoparticle fabrication usually requires multiple steps—biomaterial assembly, ligand coupling/insertion, and purification—and could cause batch-to-batch variation and quality concern. The recent development of single-step synthesis of targeted nanoparticles by self-assembling pre-functionalized biomaterials provides a simple and scalable manufacturing strategy.14,20 Another important consideration is targeting ligands. Among others, some variables that must be considered include ligand biocompatibility, cell specificity, binding affinity, mass production, and purity.21 For example, to achieve maximal specificity, the ideal ligand would be able to recognize the most over-expressed receptors on target cells relative to healthy ones. Other factors that could also affect cell targeting include ligand surface density and arrangement, as well as spacer type and length dividing ligand molecules and nanoparticles.22 Nevertheless, with advances in ligand engineering and screening, and nanoparticle optimization, targeted delivery will become a mainstay in the next generation of drug therapy.
Co-delivery
Combination therapy has shown several potential advantages (e.g. synergistic effects and reversal of drug resistance) and may prove more effective than single drug therapy.23 However, due to the distinct pharmacokinetic profiles of individual drugs, the synergistic drug ratio optimized in vitro will undoubtedly change after the conventional administration of drug ‘cocktails’—an outcome that could in turn lead to insufficient therapeutic results in vivo. To this end, lipid- and/or polymer-based nanoscale systems, previously developed for single drug delivery, have been applied to facilitate co-delivery. For some drug combinations, we can successfully tune the relative dosage of various drugs in single particle levels, and simultaneously deliver them to target sites with a maintained drug ratio.24 For other combinations, we need to develop novel delivery vehicles with desired functionalities that enable co-encapsulation of hydrophobic and hydrophilic drugs, active targeting, and/or temporally controlled release.25,26 Such features are especially essential for the co-delivery of drugs and nucleic acids, which requires intracellular delivery in order to elicit bioactivity.27,28 For example, in the case of co-delivering chemotherapy and RNAi therapy to treat multidrug resistant cancers, the ideal nanoparticle would be expected to first release siRNA to reduce the expression of MDR transporters, followed by the release of anticancer drugs.
Another exciting advancement in co-delivery is the ability to combine targeted imaging and therapeutic agents within the same particle—allowing us to visualize sites of targeted drug delivery and deliver therapeutics simultaneously (“theranostics”).29 This technology is innovative in concept and holds significant promise for making large medical impacts within the next few decades. It could provide us with critical information on intracellular targets, ensure that therapeutic agents are efficiently reaching their target sites, and enable the effective early detection and treatment of diseases. Current research is primarily focused on the design of such multifunctional nanosystems and proof-of-concept tests,30-33 but more systematic in vivo studies are needed.
Future research in this arena will also likely help us trace the absorption, distribution, metabolism and excretion of nanoparticles in vivo. It is important that we understand the pharmacokinetics of a given drug delivery system in order to improve upon formulations, estimate clinical doses, and guarantee safety.34 Currently, radionuclide labeling is the only technique that can be used to provide in situ quantitative information; but radio emitters may be too unstable to conjugate with nanomaterials. With the help of recently developed in vivo imaging probes like magnetic nanoparticles,7 quantum dots,35 gold nanoparticles,36 and carbon nanotubes,37 more imaging modalities may become available to track the distribution of nanotherapeutics in the body.
MEM/NEM devices for drug delivery
Parallel to the development of particulate delivery systems, the field of micro-/nanoelectromechanical (MEM/NEM) device-based drug delivery has also made significant headway over the past decade. In particular, implantable microchips containing nanosized reservoirs have been developed to deliver drugs for long time periods in a precisely controlled manner; microneedles are being tested in painless transdermal drug delivery; and the incorporation of nanofeatures (e.g. nanopores, nanochannels, and nanoparticles) in microfabricated systems are perfecting drug delivery and immunoisolation techniques.38-41 Intriguingly, these devices can be further modified to deliver new therapeutics, achieve targeted delivery, and co-deliver multiple agents.41,42 Substantial efforts are also being put into creating intelligent devices that could potentially sense when and how much dose is needed and then automatically release it from reservoirs.42 To do this, one feat that must be met is the continuous and stable monitoring of physical and biochemical conditions, in situ. The recent development of nanotechnology-based sensors (e.g. nanowire and nanotube) may offer new ways to address this concern, and could even facilitate device miniaturization.43
In addition to drug delivery, micro- and nanofabricated devices have shown potential in developing nanocarriers with controlled physicochemical properties (e.g. size, shape, and surface chemistries). By using perfluoropolyether molds, fabricated by traditional top-down approaches, polymeric particles at the submicron scale can be replicated with variable shapes and controllable surface chemistries.44 Compared to bulk synthesis, microfluidic devices have also recently been used for nanoprecipitation synthesis of smaller and more homogeneous PLGA-PEG nanoparticles.45 Nonetheless, micro- and nanotechnologies, which can be universally used to control the biophysicochemical properties of various nanosystems, are still in great demand.
Beyond the aforementioned opportunities and challenges, the following are also essential for the development of next generation drug delivery systems: (1) detailed physicochemical characterization of nanosystems (which will require sophisticated and state-of-art techniques); (2) restriction of undesired uptake by non-target organs (e.g. liver and spleen); (3) improvement of stimuli-triggered or programmable drug release systems (e.g. pH, temperature, light, enzyme, ultrasound, magnetic field, and electric current); (4) furthering the means by which we can understand the biological principles of disease and its microenvironment, the biological barriers that hinder drug delivery, and endosomal trafficking pathways; and (5) identification of biological markers attributable to diseased cells. With continual advancement in these areas, we can expect that the field of drug delivery will benefit greatly from the emergence of finely engineered nanomaterials and devices.
Tissue Engineering
Tissue engineering is an evolving interdisciplinary field integrating biology, engineering, materials science, and medicine, that focuses on the development of biological substitutes to restore, replace, maintain or enhance tissue and organ function.46 Over the past few decades, continued progress in this specific field has lead to the creation of implantable tissues, some of which are already used in humans (e.g. skin and cartilage) or have entered clinical trials (e.g. bladder and blood vessels).47 Nevertheless, most tissue engineering strategies rely on the principle that under appropriate bioreactor conditions, cells seeded or recruited into three-dimensional (3D) biocompatible scaffolds are able to reassemble into functional structures resembling native tissues. Early artificial scaffolds were designed to provide cells structural integrity on a macroscopic level, but only achieved moderate success. It is now widely accepted that to recapitulate proper tissue functionality, scaffolds should also establish a tissue specific microenvironment to maintain and regulate cell behavior and function.48
Within tissues, cells are surrounded by extracellular matrix (ECM) which is characterized by a natural web of hierarchically organized nanofibers.49 This integral nanoarchitecture is important because it provides cell support and directs cell behavior via cell-ECM interactions. Furthermore, ECM plays a vital role in storing, releasing and activating a wide range of biological factors, along with aiding cell-cell and cell-soluble factor interactions.50 Thus, the ability to engineer biomaterials that closely emulate the complexity and functionality of ECM is pivotal for successful regeneration of tissues. Recent advances in nanotechnology, however, have enabled the design and fabrication of biomimetic microenvironment at the nanoscale, providing an analog to native ECM.48 Notably, these technologies have been applied to create nanotopographic surfaces and nanofeatured scaffolds, and to encapsulate and control the spatiotemporal release of drugs (e.g. growth factors). In turn, these nanodevices offer a means to direct cellular behaviors that range from cell adhesion to gene expression.
Cell-nanotopography interactions
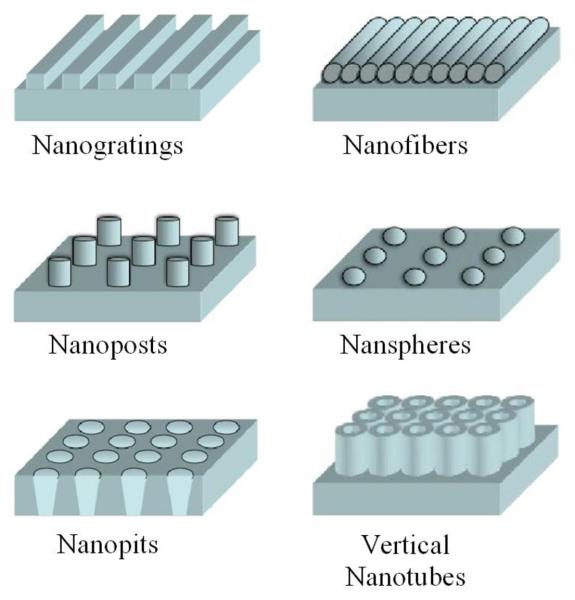
Living cells are highly sensitive to local nanoscale topographic patterns within ECM.49 In the pursuit to control cell function by underlying nanotopographic cues, engineered substrates with different nanofeatures have become rapidly adopted (Figure 2). Top-down lithographic techniques are now utilized to create various nanopatterns, such as gratings, pillars, and pits, in a precisely controlled manner.51 Techniques like micelle lithography, anodization, and electrospinning can also be used to create an array of nanospheres, vertical nanotubes, and nanofibers.52-54 Additionally, less-ordered nanotopographies are now being fabricated by polymer demixing, chemical etching, electrospinning, and phase separation processes.55
Figure 2.
Schematic of fabricated nanotopographic features used to guide cell behaviors via cell-nanotopography interactions.
These advances in the design of nanoscale substrates have enabled investigators to explore cell-nanotopography interactions, and have allowed for the manipulation of cell morphology, signaling, orientation, adhesion, migration, proliferation, and differentiation. In particular, it has been determined that nanogratings and aligned nanofibers can govern the alignment and elongation of many different cell types;54,56-59 the differentiation of mesenchymal stem cells can be regulated by polymer nanogratings,58 disordered nanopits,60 or vertically aligned TiO2 nanotubes;53,61 endothelial cells cultured onanogratings can be organized into multicellular band structures, forming aligned capillary-like tubes;56 and cell adhesion strength and apopotosis can be controlled by the combination of cell signaling epitopes and nanotopography.52,62 In the near future, we can expect the emergence of more striking results from different combinations of cell type, topography geometry and scale, and substrate material. Nonetheless, the design and fabrication of next-generation nanotopographic substrates will be guided by a greater understanding of the mechanisms by which cells respond to and sense nanofeatures.
Nanofabricated scaffolds
While critical insights into 2D cell-nanotopography interactions are now enabling us to direct cell behavior, considerable efforts have been made to develop 3D artificial scaffolds at the nanoscale for tissue engineering applications. Nanofibrous scaffolds are now under wide investigation as they exhibit a very similar physical structure to protein nanofibers in ECM.48 Among the three dominant nanofabrication methods, electrospinning is a very simple and practical technique, suitable for the creation of aligned and complex 3D structures; self-assembly technology emulates the process of ECM assembly and can thus produce very thin nanofibers; and phase separation allows for continuous fiber network fabrication with tunable pore structure, and the formation of sponge-like scaffolding.63 Nanocomposites based scaffolds (e.g. nano-hydroxyapatite/collagen) are, on the other hand, very popular in hard-tissue engineering, particularly for the reconstruction of bone tissue.64 Beyond nanofibers and nanocomposites, carbon nanotubes have also attracted attention due to their mechanical strength and electrical conductivity, and because they can be readily incorporated into 3D architectures.65
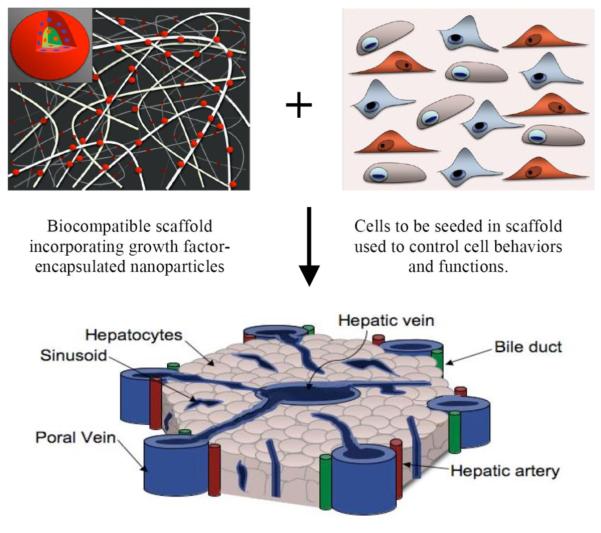
The potential of nanofibrous and nanocomposite scaffolds for the regeneration of various tissues (e.g. nerve, bone, cardiac/skeletal muscle, and blood vessels) is now under extensive investigation (Figure 3). Notwithstanding the great achievements behind the fabrication of nanoscale scaffolds, there is still plenty of room for improvement. For example, the integration of fine nanofeatures into microfabricated 3D scaffolds could provide better control of cell function via cell-nanotopography interactions. Microscale scaffolds with controlled features (e.g. pore size, geometry, network interconnectivity, and mechanical strength) have been created by a set of microfabrication technologies.66 However, in conjunction with nanofabrication technologies, scaffolds could be further decorated with nanotopographic patterns, such as grooves and ridges, to better match the nano-architecture of ECM. A particular case is the E-beam and photolithography modifications recently applied to fabricate tubular scaffold with multiple nanofeatures for vascular tissue engineering.67 Another example that defines the benefits of nanotopographic patterning is the addition of chemically etched, nanoscale roughness to the surface of porous PLGA scaffolds—a method that has been shown to enhance cell adhesion and growth, as well as the expression of matrix components.68 Moreover, several advanced techniques like multi-photon polymerization69 and layer-by-layer assembly70 have shown that nanotopographic cues hold the potential to exponentially improve scaffold design.
Figure 3.
Nanotechnology applications in engineering complex tissues. Cells seeded into biocompatible and nanostructured scaffolds are able to reassemble into functional structures that resemble native tissues, under the stimulation of growth factors spatiotemporally delivered by nanoparticles. Complex tissues, like this lobule of the liver, could be engineered with the help of devices that are equipped with nanotechnology.
Controlled release
Achieving localized and controlled delivery of biological factors in 3D scaffolds represents another key for tissue regeneration and growth. For example, the controlled release of angiogenic factors, such as vascular endothelial growth factors and basic fibroblast growth factors, can specifically enhance vascularization essential for maintaining continuous blood supply to developing tissues.71 The strategy of biomacromolecule encapsulation by direct entrapment or chemical conjugation to scaffolds can provide sustained release characteristics; however, the ability to control release kinetics with these methods is limited. Therefore, polymeric micro-/nanoparticles, pre-loaded with growth factors, have been incorporated into porous scaffolds and hydrogels.72 Using PLGA microspheres or nanospheres, single or multiple biological factors can be released in a spatiotemporally controlled manner.73,74 Furthermore, individual biological factors can be provided with distinct release properties by tuning particle formulation and composition, a modification that could help drive tissue growth to completion.
In addition to polymeric carriers, biological factors can be incorporated with nanofibrous scaffolds by either modified electrospinning or self-assembly techniques.75,76 Guided by this principle, nanofibers with core-shell structures can now be prepared by co-axial electrospinning and internally loaded with growth factors.75 To further enhance the release kinetics of growth factors from these particular scaffolds, adjustments can also be made to control the thickness and porosity of the polymer shell. Despite these efforts, the application of nanotechnology to control the release of biological factors from scaffolds is still in its infancy if compared to the great achievements of nanoparticle-based drug delivery.72
Indeed, many nanoscale systems including lipid- and dendrimer-based nanocarriers and inorganic nanomaterials, could also help to control the delivery of growth factors from artificial scaffolds. Some of these nanocarriers even have the unique ability to co-encapsulate multiple drugs and control the release of each agent in a temporal fashion (e.g. lipid-polymer hybrid nanoparticle25), or to trigger drug release by responding to environmental changes, such as pH, temperature, light, and mechanical stress. With modifications like cell specific ligands or signaling molecules, targeting nanoparticles immobilized on a scaffold's surface may be able to enhance cell adhesion and guide cell migration. Additionally, these controlled-release nanotechnologies can be applied to deliver other biological molecules (e.g. DNA and siRNA) to cells to regulate cell behavior; an idea that has been confirmed following the very recent development of poly(β-amino esters)-DNA nanoparticles used to genetically engineering stem cells for enhanced angiogenesis.77
Aside from the benefits discussed above, nanotechnology is also expected to play an important role in creating novel tissue regeneration strategies (e.g. cell sheet engineering78), and in surmounting other important obstacles in tissue engineering—such as the development and characterization of new biomaterials and stem cell engineering. For example, high throughput assays based on micro-/nanotechnologies are emerging for the cell-based screening of biomaterials.79 Despite the ongoing challenges, we can image that the achievement of functional, artificial tissues/organs will have extremely strong impact on regenerative medicine, as well as other medical fields. One exciting opportunity that lies ahead is the idea of “organ-on-a-chip”80 which, in the foreseeable future, will replace the expensive and life-costing animal testing used for drug development and for evaluation/optimization of nanoparticulate systems for drug delivery.
Summary
Nanotechnology is becoming the driving force behind a variety of evolutionary and revolutionary changes in the medical field. The impact of nanotechnology on drug delivery has helped to improve the efficacy of available therapeutics and will likely enable the creation of entirely new therapeutic entities. For tissue engineering, nanotechnology has also opened the door to new approaches that could stimulate the reconstruction of complex tissue architectures. We are optimistic about the future of nanomedicine, given the wide array of innovative nanoscale materials and technologies that stand on the horizon. With the clarification of nanotechnology-specific medical regulations and a continued influx of investments and time, we believe that nanomedicine will not only improve conventional therapies, but also bridge the shortcomings of conventional medicine to help people on both global and individual levels.
Acknowledgment
This work was supported by National Institutes of Health Grants U54-CA119349 and EB006365; and the Koch-Prostate Cancer Foundation Award in Nanotherapeutics.
Footnotes
Conflict of Interest. O.C.F. and R.L. have financial interest in BIND Biosciences and Selecta Biosciences.
REFERENCES AND NOTES
- 1.Wagner V, Dullaart A, Bock AK, Zweck A. Nat. Biotechnol. 2006;24:1211–1217. doi: 10.1038/nbt1006-1211. [DOI] [PubMed] [Google Scholar]
- 2.Zhang L, Gu FX, Chan JM, Wang AZ, Langer RS, Farokhzad OC. Clin. Pharmacol. Ther. 2008;83:761–769. doi: 10.1038/sj.clpt.6100400. [DOI] [PubMed] [Google Scholar]
- 3.Davis ME, Chen Z, Shin DM. Nat. Rev. Drug Discov. 2008;7:771–782. doi: 10.1038/nrd2614. [DOI] [PubMed] [Google Scholar]
- 4.Jain KK. The Handbook of Nanomedicine. Humana Press; Totowa: 2008. p. p353. [Google Scholar]
- 5.Occupational Health & Safety Report: Nanomedicine Market to Surpass $160 Billion by 2015. http://ohsonline.com/articles/2009/06/29/report-on-nanomedicine-market.aspx (6/1/2010)
- 6.Bangham AD, Horne RW. J. Mol. Biol. 1964;8:660–668. doi: 10.1016/s0022-2836(64)80115-7. [DOI] [PubMed] [Google Scholar]
- 7.Riehemann K, Schneider SW, Luger TA, Godin B, Ferrari M, Fuchs H. Angew. Chem. Int. Ed. 2009;48:872–897. doi: 10.1002/anie.200802585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsumura Y, Maeda H. Cancer Res. 1986;46:6387–6392. [PubMed] [Google Scholar]
- 9.Putnam D. Nat. Mater. 2006;5:439–451. doi: 10.1038/nmat1645. [DOI] [PubMed] [Google Scholar]
- 10.Whitehead KA, Langer R, Anderson DG. Nat. Rev. Drug Discov. 2009;8:129–138. doi: 10.1038/nrd2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson DG, Peng W, Akinc A, Hossain N, Kohn A, Padera R, Langer R, Sawicki JA. Proc. Natl. Acad. Sci. U.S.A. 2004;101:16028–16033. doi: 10.1073/pnas.0407218101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akinc A, Zumbuehl A, et al. Nat. Biotechnol. 2008;26:561–569. doi: 10.1038/nbt1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Semple SC, Akinc A, et al. Nat. Biotechnol. 2010;28:172–176. doi: 10.1038/nbt.1602. [DOI] [PubMed] [Google Scholar]
- 14.Davis ME. Mol. Pharm. 2009;6:659–668. doi: 10.1021/mp900015y. [DOI] [PubMed] [Google Scholar]
- 15.Woodrow KA, Cu Y, Booth CJ, Saucier-Sawyer JK, Wood MJ, Saltzman WM. Nat. Mater. 2009;8:526–533. doi: 10.1038/nmat2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bartlett DW, Su H, Hildebrandt IJ, Weber WA, Davis ME. Proc. Natl. Acad. Sci. U.S.A. 2007;104:15549–15554. doi: 10.1073/pnas.0707461104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pirollo KF, Chang EH. Trends Biotechnol. 2008;26:552–558. doi: 10.1016/j.tibtech.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 18.Farokhzad OC, Langer R. ACS Nano. 2009;3:16–20. doi: 10.1021/nn900002m. [DOI] [PubMed] [Google Scholar]
- 19.Gabathuler R. Neurobiol. Dis. 2010;37:48–57. doi: 10.1016/j.nbd.2009.07.028. [DOI] [PubMed] [Google Scholar]
- 20.Gu F, Zhang L, Teply BA, Mann N, Wang A, Radovic-Moreno AF, Langer R, Farokhzad OC. Proc. Natl. Acad. Sci. U.S.A. 2008;105:2586–2591. doi: 10.1073/pnas.0711714105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Allen TM. Nat. Rev. Cancer. 2002;2:750–763. doi: 10.1038/nrc903. [DOI] [PubMed] [Google Scholar]
- 22.Jung H, Yang T, Lasagna MD, Shi J, Reinhart GD, Cremer PS. Biophys J. 2008;94:3094–3103. doi: 10.1529/biophysj.107.115519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greco F, Vicent MJ. Adv. Drug Deliv. Rev. 2009;61:1203–1213. doi: 10.1016/j.addr.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 24.Mayer LD, Janoff AS. Mol. Interv. 2007;7:216–223. doi: 10.1124/mi.7.4.8. [DOI] [PubMed] [Google Scholar]
- 25.Sengupta S, Eavarone D, Capila I, Zhao GL, Watson N, Kiziltepe T, Sasisekharan R. Nature. 2005;436:568–572. doi: 10.1038/nature03794. [DOI] [PubMed] [Google Scholar]
- 26.Zhang L, Radovic-Moreno AF, Alexis F, Gu FX, Basto PA, Bagalkot V, Jon S, Langer RS, Farokhzad OC. ChemMedChem. 2007;2:1268–1271. doi: 10.1002/cmdc.200700121. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y, Gao S, Ye WH, Yoon HS, Yang YY. Nat. Mater. 2006;5:791–796. doi: 10.1038/nmat1737. [DOI] [PubMed] [Google Scholar]
- 28.Saad M, Garbuzenko OB, Minko T. Nanomedicine. 2008;3:761–776. doi: 10.2217/17435889.3.6.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Debbage P, Jaschke W. Histochem. Cell Biol. 2008;130:845–875. doi: 10.1007/s00418-008-0511-y. [DOI] [PubMed] [Google Scholar]
- 30.Bagalkot V, Zhang L, Levy-Nissenbaum E, Jon S, Kantoff PW, Langer R, Farokhzad OC. Nano Lett. 2007;7:3065–3070. doi: 10.1021/nl071546n. [DOI] [PubMed] [Google Scholar]
- 31.Park JH, von Maltzahn G, Ruoslahti E, Bhatia SN, Sailor MJ. Angew. Chem. Int. Ed. 2008;47:7284–7288. doi: 10.1002/anie.200801810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nasongkla N, Bey E, Ren JM, Ai H, Khemtong C, Guthi JS, Chin SF, Sherry AD, Boothman DA, Gao JM. Nano Lett. 2006;6:2427–2430. doi: 10.1021/nl061412u. [DOI] [PubMed] [Google Scholar]
- 33.McCarthy JR, Weissleder R. Adv. Drug Deliv. Rev. 2008;60:1241–1251. doi: 10.1016/j.addr.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sanhai WR, Sakamoto JH, Canady R, Ferrari M. Nat. Nanotechnol. 2008;3:242–244. doi: 10.1038/nnano.2008.114. [DOI] [PubMed] [Google Scholar]
- 35.Dubertret B, Skourides P, Norris DJ, Noireaux V, Brivanlou AH, Libchaber A. Science. 2002;298:1759–1762. doi: 10.1126/science.1077194. [DOI] [PubMed] [Google Scholar]
- 36.Chanda N, Kattumuri V, Shukla R, Zambre A, Katti K, Upendran A, Kulkarni RR, Kan P, Fent GM, Casteel SW, Smith CJ, Boote E, Robertson JD, Cutler C, Lever JR, Katti KV, Kannan R. Proc. Natl. Acad. Sci. U.S.A. 2010;107:8760–8765. doi: 10.1073/pnas.1002143107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De La Zerda A, Zavaleta C, Keren S, Vaithilingam S, Bodapati S, Liu Z, Levi J, Smith BR, Ma TJ, Oralkan O, Cheng Z, Chen XY, Dai HJ, Khuri-Yakub BT, Gambhir SS. Nat. Nanotechnol. 2008;3:557–562. doi: 10.1038/nnano.2008.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Santini JT, Jr., Cima MJ, Langer R. Nature. 1999;397:335–338. doi: 10.1038/16898. [DOI] [PubMed] [Google Scholar]
- 39.Ainslie KM, Desai TA. Lab Chip. 2008;8:1864–1878. doi: 10.1039/b806446f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prausnitz MR. Adv. Drug Deliv. Rev. 2004;56:581–587. doi: 10.1016/j.addr.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 41.Tasciotti E, Liu X, Bhavane R, Plant K, Leonard AD, Price BK, Cheng MM, Decuzzi P, Tour JM, Robertson F, Ferrari M. Nat. Nanotechnol. 2008;3:151–157. doi: 10.1038/nnano.2008.34. [DOI] [PubMed] [Google Scholar]
- 42.Staples M, Daniel K, Cima MJ, Langer R. Pharm. Res. 2006;23:847–863. doi: 10.1007/s11095-006-9906-4. [DOI] [PubMed] [Google Scholar]
- 43.Patolsky F, Zheng G, Lieber CM. Nanomedicine. 2006;1:51–65. doi: 10.2217/17435889.1.1.51. [DOI] [PubMed] [Google Scholar]
- 44.Gratton SE, Williams SS, Napier ME, Pohlhaus PD, Zhou Z, Wiles KB, Maynor BW, Shen C, Olafsen T, Samulski ET, Desimone JM. Acc. Chem. Res. 2008;41:1685–1695. doi: 10.1021/ar8000348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Karnik R, Gu F, Basto P, Cannizzaro C, Dean L, Kyei-Manu W, Langer R, Farokhzad OC. Nano Lett. 2008;8:2906–2912. doi: 10.1021/nl801736q. [DOI] [PubMed] [Google Scholar]
- 46.Langer R, Vacanti JP. Science. 1993;260:920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 47.Khademhosseini A, Vacanti JP, Langer R. Sci. Am. 2009;300:64–71. doi: 10.1038/scientificamerican0509-64. [DOI] [PubMed] [Google Scholar]
- 48.Goldberg M, Langer R, Jia X. J. Biomater. Sci. 2007;18:241–268. doi: 10.1163/156856207779996931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stevens MM, George JH. Science. 2005;310:1135–1138. doi: 10.1126/science.1106587. [DOI] [PubMed] [Google Scholar]
- 50.Taipale J, Keski-Oja J. FASEB J. 1997;11:51–59. doi: 10.1096/fasebj.11.1.9034166. [DOI] [PubMed] [Google Scholar]
- 51.Bettinger CJ, Langer R, Borenstein JT. Angew. Chem. Int. Ed. 2009;48:5406–5415. doi: 10.1002/anie.200805179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang J, Grater SV, Corbellini F, Rinck S, Bock E, Kemkemer R, Kessler H, Ding J, Spatz JP. Nano Lett. 2009;9:1111–1116. doi: 10.1021/nl803548b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Park J, Bauer S, von der Mark K, Schmuki P. Nano Lett. 2007;7:1686–1691. doi: 10.1021/nl070678d. [DOI] [PubMed] [Google Scholar]
- 54.Xu CY, Inai R, Kotaki M, Ramakrishna S. Biomaterials. 2004;25:877–886. doi: 10.1016/s0142-9612(03)00593-3. [DOI] [PubMed] [Google Scholar]
- 55.Norman JJ, Desai TA. Ann. Biomed. Eng. 2006;34:89–101. doi: 10.1007/s10439-005-9005-4. [DOI] [PubMed] [Google Scholar]
- 56.Bettinger CJ, Zhang Z, Gerecht S, Borenstein JT, Langer R. Adv. Mater. 2008;20:99–103. doi: 10.1002/adma.200702487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gerecht S, Bettinger CJ, Zhang Z, Borenstein JT, Vunjak-Novakovic G, Langer R. Biomaterials. 2007;28:4068–4077. doi: 10.1016/j.biomaterials.2007.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yim EK, Pang SW, Leong KW. Exp. Cell Res. 2007;313:1820–1829. doi: 10.1016/j.yexcr.2007.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim DH, Lipke EA, Kim P, Cheong R, Thompson S, Delannoy M, Suh KY, Tung L, Levchenko A. Proc. Natl. Acad. Sci. U.S.A. 2010;107:565–570. doi: 10.1073/pnas.0906504107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dalby MJ, Gadegaard N, Tare R, Andar A, Riehle MO, Herzyk P, Wilkinson CD, Oreffo RO. Nat. Mater. 2007;6:997–1003. doi: 10.1038/nmat2013. [DOI] [PubMed] [Google Scholar]
- 61.Oh S, Brammer KS, Li YS, Teng D, Engler AJ, Chien S, Jin S. Proc. Natl. Acad. Sci. U.S.A. 2009;106:2130–2135. doi: 10.1073/pnas.0813200106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ranzinger J, Krippner-Heidenreich A, Haraszti T, Bock E, Tepperink J, Spatz JP, Scheurich P. Nano Lett. 2009;9:4240–4245. doi: 10.1021/nl902429b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Madurantakam PA, Cost CP, Simpson DG, Bowlin GL. Nanomedicine. 2009;4:193–206. doi: 10.2217/17435889.4.2.193. [DOI] [PubMed] [Google Scholar]
- 64.Murugan R, Ramakrishna S. Compos. Sci. Technol. 2005;65:2385–2406. [Google Scholar]
- 65.Edwards SL, Werkmeister JA, Ramshaw JA. Expert Rev. Med. Devic. 2009;6:499–505. doi: 10.1586/erd.09.29. [DOI] [PubMed] [Google Scholar]
- 66.Borenstein JT, Weinberg EJ, Orrick BK, Sundback C, Kaazempur-Mofrad MR, Vacanti JP. Tissue Eng. 2007;13:1837–1844. doi: 10.1089/ten.2006.0156. [DOI] [PubMed] [Google Scholar]
- 67.Seunarine K, Meredith DO, Riehle MO, Wilkinson CDW, Gadegaard N. Microelectron. Eng. 2008;85:1350–1354. [Google Scholar]
- 68.Pattison MA, Wurster S, Webster TJ, Haberstroh KM. Biomaterials. 2005;26:2491–2500. doi: 10.1016/j.biomaterials.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 69.LaFratta CN, Fourkas JT, Baldacchini T, Farrer RA. Angew. Chem. Int. Ed. 2007;46:6238–6258. doi: 10.1002/anie.200603995. [DOI] [PubMed] [Google Scholar]
- 70.Bettinger CJ, Weinberg EJ, Kulig KM, Vacanti JP, Wang YD, Borenstein JT, Langer R. Adv. Mater. 2006;18:165–169. doi: 10.1002/adma.200500438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Langer R. ACS Nano. 2009;3:756–761. doi: 10.1021/nn900350p. [DOI] [PubMed] [Google Scholar]
- 72.Zhang SF, Uludag H. Pharm. Res. 2009;26:1561–1580. doi: 10.1007/s11095-009-9897-z. [DOI] [PubMed] [Google Scholar]
- 73.Ma PX. Adv. Drug Deliv. Rev. 2008;60:184–198. doi: 10.1016/j.addr.2007.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Richardson TP, Peters MC, Ennett AB, Mooney DJ. Nat. Biotechnol. 2001;19:1029–1034. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- 75.Sun ZC, Zussman E, Yarin AL, Wendorff JH, Greiner A. Adv. Mater. 2003;15:1929–1932. [Google Scholar]
- 76.Hosseinkhani H, Hosseinkhani M, Khademhosseini A, Kobayashi H, Tabata Y. Biomaterials. 2006;27:5836–5844. doi: 10.1016/j.biomaterials.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 77.Yang F, Cho SW, Son SM, Bogatyrev SR, Singh D, Green JJ, Mei Y, Park S, Bhang SH, Kim BS, Langer R, Anderson DG. Proc. Natl. Acad. Sci. U.S.A. 2010;107:3317–3322. doi: 10.1073/pnas.0905432106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Elloumi-Hannachi I, Yamato M, Okano T. J. Intern. Med. 2010;267:54–70. doi: 10.1111/j.1365-2796.2009.02185.x. [DOI] [PubMed] [Google Scholar]
- 79.Anderson DG, Levenberg S, Langer R. Nat. Biotechnol. 2004;22:863–866. doi: 10.1038/nbt981. [DOI] [PubMed] [Google Scholar]
- 80.Huh D, Matthews BD, Mammoto A, Montoya-Zavala M, Hsin HY, Ingber DE. Science. 2010 doi: 10.1126/science.1188302. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]