Abstract
Elastin-like polypeptides (ELPs) have found utility in tissue engineering applications, not only because they are biocompatible, biodegradable, and non-immunogenic, but also because their amino acid sequence and molecular weight can be precisely controlled at the genetic or synthetic level, affording exquisite control over final protein functionality. This review presents a basic overview of ELP properties and modifications that are relevant to tissue engineering, as well as a discussion of the application of ELPs to cartilage, intervertebral disc, vascular graft, liver, ocular, and cell sheet engineering.
Keywords: hydrogel, tissue engineering, cartilage, liver, elastin, crosslinking, vascular graft
1. Introduction
Biomaterials used in tissue engineering applications must be able to fill and assume the shape of a replacement tissue, void or defect, support the necessary cellular processes needed to regenerate tissue that will function like native tissue, enable efficient nutrient and waste exchange to support those cells, supply the necessary physical properties required for a given tissue, and remain fixed within the target area for a chosen period of time [1–3]. These goals have historically been targeted by a variety of synthetic and naturally occurring polymers that can be processed into various forms, such as felts, meshes, foams, and gels. A common obstacle in designing optimal biomaterials for many applications is the difficulty in meeting multiple and often competing design goals with any one strategy. For example, synthetic polymers are often biocompatible, but they lack cues for cell attachment and tissue growth, which has led to the need for modification of many of these materials with bioactive sequences and factors. Conversely, naturally occurring polymers derived from extracellular matrix proteins, offer the advantage of biological signaling, but can be antigenic and their physical properties are more difficult to modulate than synthetic polymers.
Human elastin provides unique physical characteristics to the connective tissue in which it is found (skin, ligament, arteries, specialized cartilages), lending both strength and extensibility. Elastin is a 68 kDa protein comprised of approximately 800 amino acid residues [4], containing two main domains: highly hydrophobic domains and crosslinking domains, which give the tissue its mechanical properties, and at the same time, allow it to form a crosslinked network with neighboring molecules [5]. A large number of polymers have been newly synthesized for tissue engineering using human elastin sequences for their building blocks, with the goal of precise control of the physical and functional properties of the polypeptide at the genetic or chemical synthesis level. One class of artificial repetitive polypeptides derived from elastin are elastin-like polypeptides (ELPs), which are polymers of the pentapeptide motif VPGVG that is observed to recur in the tropoelastin gene across species [4]. ELPs are thermally responsive and can undergo a reversible inverse temperature phase transition, whereby ELP molecules will hydrophobically self-associate with an increase in temperature above a characteristic transition temperature (Tt), forming a highly viscous fluid termed a coacervate. The fourth amino acid, V in each pentapeptide repeat of poly(VPGVG) can be substituted with any amino acid, and provides a simple and rational way to tune the Tt of an ELP [6]. In addition to the type and fraction of the 4th –guest- residue X– in the VPGXG repeat, the Tt is also dependent on ELP MW and concentration. In fact, the Tt of ELPs follows the Hofmeister almost perfectly, albeit at much lower concentrations than are used to salt proteins out, and provide a rational and predictable method to isothermally trigger the phase transition of an ELP by the addition of salt.
ELPs are attractive for tissue engineering for at least five important reasons: first, because ELPs can be genetically encoded, their synthesis from a synthetic gene in a heterologous host (e.g., bacteria or eukaryotic cell) provides complete control over the amino acid sequence and MW, two variables that are not easy to precisely control in synthetic polymers. Second, ELPs are readily expressed form a plasmid-borne gene in E. coli to relatively high yields (~500 mg/L growth), which also makes them attractive for tissue engineering applications where large quantities of polymer are often required. Third, they can be readily purified from E. coli –and other– cell lysates in batch process by exploiting their inverse temperature phase transition without the need for chromatography, which greatly simplifies large scale purification of ELPs [7]. Fourth, ELPs can be engineered to approach the viscoelastic properties of native elastin upon crosslinking. Finally, they are biocompatible, biodegradable, and nonimmunogenic [8].
The temperature sensitivity of ELPs may be exploited in tissue engineering applications for those applications that may benefit from biomaterial formulations that are injectable and may be triggered in some way to form a solid matrix after defect filling. The inherent thermal transition properties of ELP provide a natural trigger for coacervation, and reversibility of the phase transition enables recovery of ELP from applications that desire a scaffold-free outcome. ELPs may also be designed to be mixed with a biocompatible crosslinker that is also triggered by temperature or other external stimulus in order to form a mechanically robust scaffold after crosslinking. These features allow ELP solutions to be biocompatibly mixed with cells or other bioactive factors prior to gelation (thermal or crosslinking) and to be injected into a defect site to form a mechanically functional scaffold upon implantation. This eliminates the lag time associated with some other implantable scaffolds that only become mechanically robust after the initiation of new tissue formation.
In an effort to further tune the properties of ELPs for a myriad of tissue engineering applications, they have been processed in a variety of ways to form gels [8–13], films [14–16], foams [13], and fibers [17, 18]. Many of these methods will be presented in greater detail in the sections to follow, but generalized processing methods are provided here. Because the fourth residue in the pentapeptide sequence of ELP (VPGXG) may be modified to essentially any amino acid, the possibilities for modification and crosslinking of ELP sequences is vast. For example, the substitution of a primary amine (e.g., lysine or alanine) in the guest residue position has been used to incorporate a reactive site along the ELP backbone for chemical modification, such as crosslinking or peptide functionalization [10, 11, 16, 19, 20]. Direct crosslinking of ELPs has been carried out in this manner to form gels and films. Likewise, lysines have also been used as the site for further chemical modification for the addition of acrylate groups in order to form photocrosslinkable ELP substrates [12].
Cell recognition sequences such as RGD or REDV have also been incorporated into proteins containing the ELP sequence in order to modify their cell adhesive properties [15, 21, 22]. This has been done both recombinantly and synthetically, and has been shown to transform an otherwise non-cell adhesive ELP sequence into a substrate that mimics other native protein substrates [21]. In addition, block copolymers designed at the genetic level enable synthesis of ELPs with alternating blocks containing hydrophobic, hydrophilic, crosslinking, and cell recognition sequences in order to introduce virtually limitless variations in the mechanical, swelling, degradation, and crosslinking properties of the final processed form [13, 23, 24]. This method has also been used to produce silk-elastin like polymers [25] that have application in tissue augmentation, tissue engineering [26], and drug/gene delivery [27–29]. ELPs thus provide a high level of control over polymer design for specific applications.
2. Tissue Engineering Applications
2.1 Cartilage and Intervertebral Disc Tissue Engineering
Different ELP variants have been used for various applications associated with cartilage tissue engineering and regeneration. Early studies indicated the value of uncrosslinked ELPs for promoting rapid accumulation and retention of chondrocyte-associated matrix in a method designed to allow longer-term monolayer culture of chondrocytes without dedifferentiation [30]. In this work, Betre and co-workers entrapped primary chondrocytes in an ELP solution designed to undergo transition and coacervation at 35°C, thereby enabling the entrapment of cells. Because the transition of ELPs is completely reversible, cells and their accumulated matrix could be recovered after 10 days of culture by gentle agitation at room temperature and plated in monolayer culture on Transwell membranes. Two-week and four-week monolayer culture of these cells with their matrix revealed maintenance of the chondrocyte phenotype, as seen by accumulation of type II collagen and sGAG (Figure 1A) [30]. This work demonstrated the utility of ELPs for promoting an environment that would encourage chondrogenesis and led to the use of coacervated ELP as a scaffold for later cartilage tissue engineering investigations. Betre and co-workers later showed that ELPs promoted the synthesis and accumulation of articular cartilage ECM for both primary chondrocytes [31] and adult stem cells (hADAS cells) [32]. Additionally, it was found that ELP promoted the differentiation of stem cells down a chondrocytic pathway without the addition of chondrocyte-specific growth factors [32], suggesting ELPs as valid candidate materials for cartilage tissue engineering in vitro.
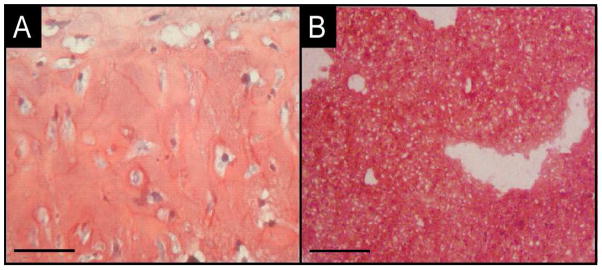
Figure 1.
Safranin-O stained histology of (A) tissue resulting from a two-step recovery method for dedifferentiated chondrocytes using uncrosslinked ELP and (B) cartilage-like tissue synthesized by articular chondrocytes encapsulated in THPP-crosslinked ELP.
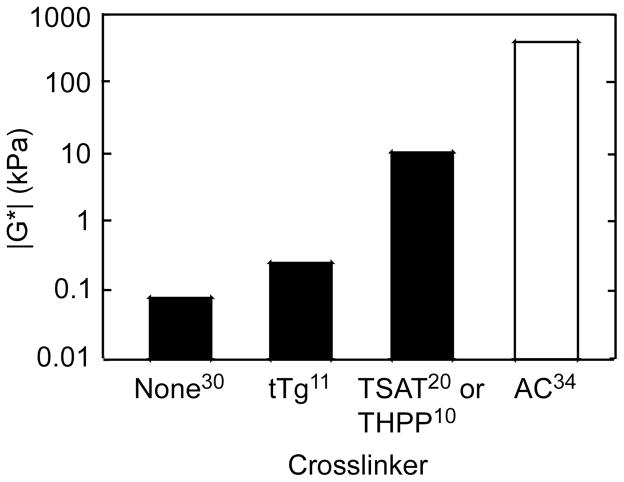
As cartilage is a load bearing tissue [33], conventional thinking suggests that scaffolds used for filling defects in situ should also be capable of bearing load. Uncrosslinked ELPs possess shear moduli that are four orders of magnitude below that of articular cartilage [31, 34] (Figure 2), suggesting that modification of the ELP would be necessary in order to use this method for defect filling. To that end, McHale and co-workers developed glutamine- and lysine-containing ELPs that could be enzymatically crosslinked via tissue transglutaminase in order to increase load-bearing capabilities [11]. ELPs crosslinked in this manner formed turgid hydrogels with shear moduli that were two orders of magnitude greater that of uncrosslinked ELP, and retained the ability to promote the synthesis and retention of cartilage matrix by entrapped primary cells [11]. However, this method of crosslinking did not go to completion in a clinically relevant time, leading to the development of a chemical crosslinking scheme that would allow the evolution of increased mechanical properties in a shorter time frame (< 5 minutes). In this work, Lim and co-workers pioneered the crosslinking of lysine-containing ELPs [10, 20, 23] with (β-[Tris(hydroxymethyl) phosphino] propionic acid (betaine) (THPP). These ELPs possessed mechanical properties three orders of magnitude greater than uncrosslinked ELP after only a five minute reaction time with a biocompatible crosslinker that enabled entrapment of cells [10] (Figure 2). ELPs crosslinked in this method were later used for in vitro tissue engineering as well as injectable scaffolds for cartilage regeneration in a goat model of an osteochondral defect (Figure 3). Both in vitro and in vivo studies resulted in synthesis of new cartilage-like matrix (Figures 1B and Figure 4A, respectively), and showed the ability of ELPs to be used as injectable biomaterials for cartilage regeneration [35]. This work also made use of an ELP containing a histidine (-his) tag, which introduced an epitope by which ELP remaining in the defect site after in vivo incubation could be tracked, demonstrating another useful modification of ELP that can be made at the genetic level [36]. These histidine tagged ELPs were also used to show the degradation profile of his-tagged ELPs in various enzyme solutions (trypsin, collagenase, elastase, or PBS) by tracking hydrogel wet weight over time (Figure 5), suggesting that ELPs may be tuned to degrade in vivo at a chosen rate [36].
Figure 2.
Effect of various crosslinkers on the magnitude of the complex shear modulus (|G*|) compared to uncrosslinked ELP and native articular cartilage. TSAT = tris-succinimidyl aminotriacetate. Superscript numbers refer to bibliography numbers.
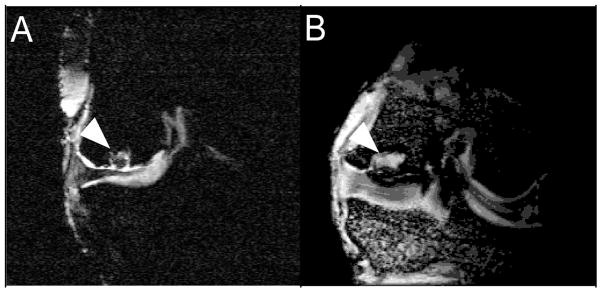
Figure 3.
MRI images of (A) SELP and (B) ELP filling osteochondral defects in goat femoral condyles. White arrows indicate biomaterial.
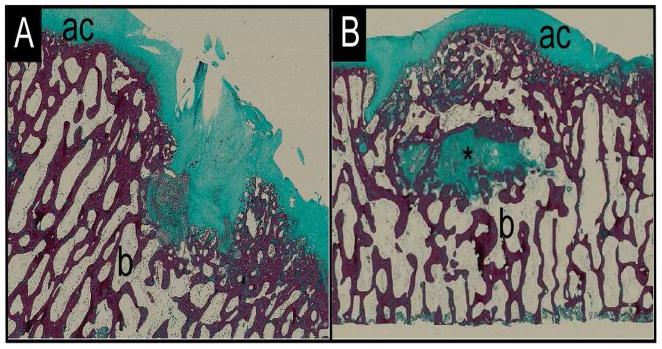
Figure 4.
Histology images of (A) unfilled control and (B) ELP-filled osteochondral defects in goat femoral condyles stained with Masson’s Trichrome. “ac” denotes articular cartilage; “b” denotes bone; and “*” denotes biomaterial.
Figure 5.
Degradation profiles for ELP [KV6]-112-His in various enzyme solutions. ◆ 0.05 % trypsin; ■ 0.01% trypsin; ▲ 0.01% elastase; ● 0.05% collagenase; and × PBS.
More recently, studies were undertaken to understand the effects of physico-chemical properties of ELPs on cartilage matrix synthesis in vitro, towards the goal of obtaining candidate ELP formulations with optimized properties for in vivo use. In this work, sixteen lysine-containing ELP formulations were evaluated for their mechanical properties and their ability to promote cartilage matrix synthesis by encapsulated primary chondrocytes. These ELP formulations varied by frequency of lysine along the ELP backbone, their solution concentration, and molecular weight. This led to such a large number of combinations of variables that an artificial neural network analysis was needed to discern relationships between the properties of ELP-associated biological and mechanical outcomes [37]. This study revealed a significant dependence of mechanical properties, chondrocyte viability, metabolism, and matrix synthesis on crosslink density (lysine frequency), followed by ELP solution concentration, with little dependence of biological or physical outcomes on molecular weight [37]. These studies serve to illustrate the utility of ELPs as uncrosslinked or crosslinked scaffolds for promoting cartilage matrix synthesis and regeneration in vitro and in situ.
Block co-polymers of silk and elastin peptide sequences, termed SELPs, were synthesized by Cappello and co-workers over 30 years ago and have a long history of investigation and development for multiple applications. These polymers are produced recombinantly using similar methods as those used to produce ELPs, and contain an amino acid motif from Bombyx mori (silkworm) silk (GAGAGS) as well as ELP repeats [25]. These SELPs have been investigated as an acellular therapy for cartilage repair in a rabbit model of an osteochondral defect [38]. In this work, SELPs were injected into osteochondral defects on the femoral condyles of rabbit knees and remained in the defect for 12 weeks, demonstrating an ability to deliver SELPs in solution phase and trigger a crosslinking reaction and subsequent gel formation in vivo. This work was later continued in the goat (Figure 3A). SELPs have also been investigated for their ability to promote cartilage matrix synthesis by human mesenchymal stem cells (hMSCs) [26]. The ELP repeats used in this work were largely VPGVG but also included a substituted sequence using K. The final polymer is termed SELP-47 K. hMSCs were encapsulated in SELP-47 K, which is fluid at room temperature and undergoes gelation at 37C, and cultured for 28 days in the presence or absence of transforming growth factor (TGF-β3). Upon evaluation for cell viability, biochemical components of cartilaginous matrix (sGAG and collagen), and gene expression, SELP-47K was found to maintain cell viability in the presence or absence of TGF-β3, and promote increasing accumulation of sGAG and collagen over time in the presence of TGF-β3. These polymers have also been extensively investigated for drug and gene delivery, suggesting the possibility of combining these technologies for drug or gene-directed tissue engineering.
ELPs created from exons of the human tropoelastin gene have been used for cartilage tissue engineering applications in the knee and intervertebral disc (IVD) [39–42]. An ELP created from exons 20 – (21–24)4 (termed EP20-24-24-24-24) was crosslinked with genepin to form a turgid hydrogel, which was subsequently press-fit into osteochondral defects in rabbits. This initial feasibility and biocompatibility study showed retention of the ELP implant in the defect with no significant inflammation and limited local degradation, while allowing for an environment that was more conducive to new hyaline cartilage deposition compared to unfilled controls [39]. It is also noteworthy that compressive mechanical tests showed that the aggregate modulus of these implants was in the range reported for native articular cartilage [39], a potential advantage of this technology for a press-fit type application to cartilage repair. In other studies, a composite scaffold was created, which combined EP4 (four repeats of exons 20–24) with a thiol-modified hyaluronan and a polyethylene glycol diacrylate (PEGDA) crosslinker for nucleus pulposus repair and/or treatment of early degenerative disc degeneration (DDD) [40, 41]. This composite scaffold was first evaluated for its ability to support viability and gene expression of NP-associated genes for pathologic human disc cells. These gels were shown to possess an aggregate modulus of 27.6 kPa and support cell viability and phenotype [40]. However, when the same polymer was injected acellularly into a disc puncture model of DDD in rabbits, there was no evidence of an inflammatory response, but there were no differences between treated and untreated discs in disc volume or histology [41], suggesting limited benefit of injection of the ELP. However, modifications to the current scaffold, such as the addition of cells or bioactive factors, could improve efficacy in this model.
2.2 Vascular Graft Tissue Engineering
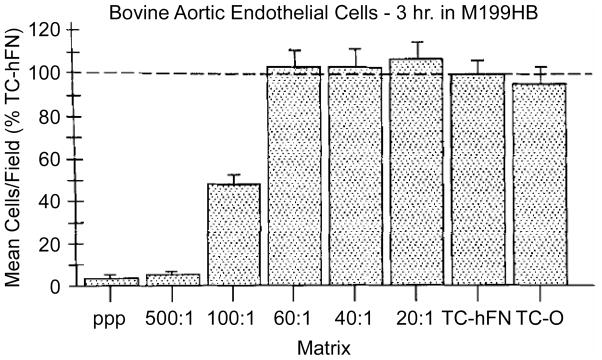
ELPs and peptide-ELP hybrids have also been studied for their potential application in small diameter vascular graft tissue engineering [15, 16, 21, 43–45]. In early work, Nicol and Urry explored the possibility of incorporating cell recognition peptide sequences into ELPs in order to promote endothelial cell adhesion [46]. To this end, Nicol and Urry co-polymerized a peptide containing the RGDS sequence with that of an ELP sequence to create copolymers consisting of various ratios of ELP repeats and RGDS repeats [21]. These biopolymers were induced to undergo a temperature-induced phase transition, and then gamma-irradiated to promote crosslinking of a gel-like, cell adhesive matrix. In this work, cell attachment and spreading on copolymers consisting of at least a 60:1 (x : y basis) ratio of ELP to RGDS were found to be similar to that of cells plated on adsorbed fibronectin, a positive control substrate known to promote endothelial cell attachment and spreading (Figure 6). However, when the ratio of ELP to RGDS was below 60:1, cell attachment and degree of spreading fell sharply. This work suggests not only that cell recognition sequences can be incorporated into ELPs that result in similar levels of cell attachment and growth as a control substratum, but also that the “naked” ELP sequences (e.g. poly(VPGVG)) are poor substrates for cell adhesion [21] and that creating bioactive ELP matrices is probably essential for their application to vascular graft tissue engineering.
Figure 6.
Short-term adhesion (3 h) of BAE cells to elasomeric matrix discs containing a range of concentrations of covalently incorporated GRGDSP sequences. The data are plotted as the mean numbers of attached cells per square eyepiece reticle field expressed as a percentage of the mean number of cells per field attaching to the fibronectin-coated tissue culture plastic. The vertical bar represents one standard error. These results are each the combined data from two experiments. Reproduced from Nicol et al [21] with permission from John Wiley and Sons, Inc.
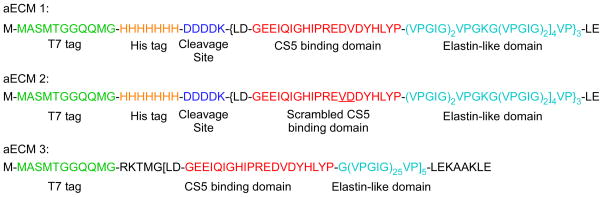
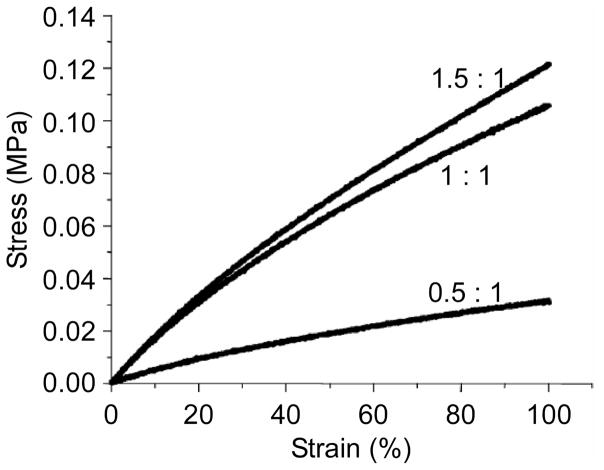
Incorporating peptide motifs that present biological cues to cells has been a focus of recent work that has the dual goals of developing materials that support the development of a confluent endothelial layer while matching the compliance of the surrounding tissue in order to prevent intimal hyperplasia and thrombosis. One approach to achieve these goals has been to design recombinant hybrid materials, termed artificial ECMs (aECMs), that combine a cell-binding domain with the elastic properties of a recombinant elastin sequence [22]. In this work, several aECMs were developed that include the CS5 cell binding domain from fibronectin, which contains the REDV domain that has been shown to be important for endothelial cell binding (Figure 7). The CS5 domain was incorporated into a recombinant polymer that included ELP sequences designed to undergo temperature-driven transition at room temperature and separate ELP domains containing lysine residues either within the ELP sequence or on the N- and C- terminus of the protein to provide a site for chemical crosslinking of the aECM [16, 22, 43–45]. These studies showed that the mechanical properties of these crosslinked aECMs approached that of native elastin, and were tunable depending on crosslinker ratio (Figure 8) [16, 43]. Furthermore, these studies showed that endothelial cells attached to aECMs when the entire CS5 domain was incorporated [44] into the biopolymer, in contrast to low cell attachment when only the REDV sequence from CS5 was incorporated into the biopolymer [47]. Furthermore, the placement of lysine as the crosslinking residue outside of the ELP domain, further increased endothelial cell attachment compared to polymers with the crosslinking domain included in the ELP domain [45].
Figure 7.
Amino acid sequences of the aECM proteins incorporating an ELP sequence that were used for endothelial cell adhesion. Each sequence contains a T7 tag (green) to aid in protein expression and detection. aECM 1 and 2 contain a hexahistidine tag (orange), an enzymatic cleavage site (dark blue), and three cassettes of the CS5 binding domain (red) interspersed with elastin-like repeats (light blue) that contain lysine sites for crosslinking. In aECM 2, the minimal recognition sequence within the CS5 domain has been scrambled to provide a negative control. aECM 3 contains five cassettes of the CS5 binding domain interspersed with twenty-five elastin-like repeats. These cassettes are flanked by lysine residues for site-specific crosslinking. Modified from Heilshorn et al, 2003.
Figure 8.
Stress-strain behavior of films cross-linked in PBS at 25°C with NHS/lysine ratios of 0.05:1, 1:1, and 1.5:1. Reproduced from DiZio et al [43] with permission from the American Chemical Society.
PEGylated aECMs that include the RGD motif have been used to control the biological information that is presented to cells while controlling for non-specific adhesion, without varying the chemical and physical properties of the aECM [15]. This was accomplished by determining the density of RDG-containing PEGylated aECM necessary to mimic adhesion to fibronectin, and then mixing in a PEGylated aECM containing the scrambled RDG sequence in a ratio that preserved cell adhesion as well as crosslinked gel properties [15]. Triblock elastin polymers have also been used to impregnate expanded polytetrafluoroethylene vascular grafts as antithrombogenic coatings and were shown to reduce both platelet aggregation and fibrin deposition in small diameter vascular grafts compared to uncoated controls [48].
2.3 Ocular Tissue Engineering
ELPs have also been investigated for application in ocular tissue engineering. Namely, ELP sequences containing two distinct elastin blocks have been engineered to also contain the CS5 fibronectin domain for cell attachment as well as an enzyme recognition sequence for elastase to aid in biodegradation [49]. In these studies, the ELPs have been adsorbed to glass surfaces and shown to promote epithelial cell attachment, proliferation, and retention of the differentiated phenotype [49]. The goal of this work is to develop naturally-derived polymer scaffolds to be used in ocular surface tissue engineering [50].
2.4 Liver Tissue Engineering/Polyelectrolyte ELPs
In studies geared toward understanding and controlling hepatocyte morphology, differentiation state, and function, ELPs were chemically conjugated to polyelectrolytes and used as a substratum for primary hepatocyte culture [51]. When cultured on ELP alone or ELP conjugated to a negatively charged polyelectrolyte, hepatocytes rapidly spread, while forming spheroids on ELPs conjugated to positively charged polyelectrolytes. These morphological differences translated to important functional differences, as assessed by the ability of the cells to produce albumin and urea, two important markers of hepatocyte function. Cells cultured on ELP surfaces conjugated to a positively charged polyelectrolyte displayed an increased rate of production of both urea and albumin, compared to cells cultured on ELP alone, or ELP conjugated to a negatively charged polyelectrolyte. Furthermore, it was found that ≥ 80% of the culture surface needed to be coated with ELP conjugated to a positively-charged polyelectrolyte in order for spheroid formation to occur, which translated into increased hepatocyte function [51]. These studies point to the ability to use ELPs in liver tissue engineering applications as well as in studies designed to ascertain the function of regenerating hepatic tissue [52]. In an extension of this work, cells were cultured on multilayer substrates formed by depositing alternating layers of ELP and polyelectrolyte. These studies indicate that the thickness as well as the mechanical integrity of the multilayer substratum are important for regulating cell proliferation as well as the formation of focal adhesions [52]. This layer-by-layer fabrication approach using ELPs could be useful in tissue engineering applications requiring controlled layering of cells.
2.5 Cell Sheet Engineering
There is much interest in engineering scaffold-free tissue replacements. To this end, ELPs have been used to form cell-sheet growth substrates from which they can be recovered as simply cells and their ECM [53]. As discussed previously, ELPs by themselves are not particularly cell adhesive. Thus, in this work, ELPs were mixed with RGD-functionalized ELPs and adsorbed onto tissue culture plates. Cells were then mixed with the RGD-containing ELP and plated on the ELP – ELP-RGD coated surface. Cultures were maintained above the transition temperature of the ELP sequences, and after several days, the temperature was lowered below the transition temperature of the ELPs, thereby releasing the cells with their ECM as a sheet, which contained no ELP polymer [53]. This work demonstrates a novel scaffold-free system for engineering tissue replacements in the form of sheets, simply by exploiting the genetic and inverse transition characteristics of ELPs.
3. Conclusions
ELPs are attractive for tissue engineering applications where mechanical properties are of importance along with the ability to precisely control the physicochemical properties of the scaffold. ELPs are very easily synthesized at scales necessary for tissue engineering, and can be easily crosslinked to form foams, gels, and fibers. ELPs are biocompatible, showing no in vivo signs of adverse immune responses. ELPs composed of the poly(VPGXG) motif are slowly degraded in vivo, and their rate of degradation can be further tuned by the explicit incorporation of specific protease recognition sites within the ELP sequence. ELPs are not cell-adhesive, but support cellular viability and phenotype of cells that do not need to adhere to the substrate, while it is trivial to incorporate cell adhesive peptide sequences into ELPs if necessary. ELPs are particularly attractive for recovery of cells and cell sheets for scaffold-free tissue engineering applications by exploiting their inverse temperature phase transition. ELPs are also attractive as co-polymers, as their thermal and non-adhesive properties have the ability to contribute unique features. Future work using ELPs for tissue engineering could focus on more specific characterization of biocompatibility, biotransport and degradability, as well as further manipulation of form (fibers, foams, etc.) in order to perturb nutrient transport and degradation characteristics.
Acknowledgments
This study was supported by grants from the NIH (R01EB02263, P01AR50245, R01GM061232), and the North Carolina Biotechnology Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
4. References
- 1.Hollister SJ. Scaffold Design and Manufacturing: From Concept to Clinic. Adv Mater. 2009;21:3330–3342. doi: 10.1002/adma.200802977. [DOI] [PubMed] [Google Scholar]
- 2.Langer R, Cima LG, Tamada JA, Wintermantel E. Future-directions in biomaterials. Biomaterials. 1990;11:738–745. doi: 10.1016/0142-9612(90)90038-r. [DOI] [PubMed] [Google Scholar]
- 3.Langer R, Vacanti JP. Tissue Engineering. Science. 1993;260:920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 4.Sandberg LB, Soskel NT, Leslie JG. Elastin structure, biosynthesis, and relation to disease states. N Engl J Med. 1981;304:566–579. doi: 10.1056/NEJM198103053041004. [DOI] [PubMed] [Google Scholar]
- 5.Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. The Cell. Vol. 4. Garland Science; New York, NY: 2002. Cell junctions, cell adhesions, and the extracellular matrix; pp. 1065–1125. [Google Scholar]
- 6.Urry DW. Physical chemistry of biological free energy transduction as demonstrated by elastic protein-based polymers. J Phys Chem B. 1997;101:11007–11028. [Google Scholar]
- 7.Meyer DE, Chilkoti A. Purification of recombinant proteins by fusion with thermally responsive polypeptides. Nat Biotechnol. 1999;17:1112–1115. doi: 10.1038/15100. [DOI] [PubMed] [Google Scholar]
- 8.Urry DW, Parker TM, Reid MC, Gowda DC. Biocompatibility of the bioelastic materials, poly(GVGVP) and its gamma-irradiation cross-linked matrix - summary of generic biological test-results. J Bioact Compat Polym. 1991;6:263–282. [Google Scholar]
- 9.Lee J, Macosko CW, Urry DW. Swelling behavior of gamma-irradiation cross-linked elastomeric polypentapeptide-based hydrogels. Macromolecules. 2001;34:4114–4123. [Google Scholar]
- 10.Lim DW, Nettles DL, Setton LA, Chilkoti A. Rapid crosslinking of elastin-like polypeptides with hydroxymethylphosphines in aqueous solution. Biomacromolecules. 2007;8:1463–1470. doi: 10.1021/bm061059m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McHale MK, Setton LA, Chilkoti A. Synthesis and in vitro evaluation of enzymatically cross-linked elastin-like polypeptide gels for cartilaginous tissue repair. Tissue Eng. 2005;11:1768–1779. doi: 10.1089/ten.2005.11.1768. [DOI] [PubMed] [Google Scholar]
- 12.Nagapudi K, Brinkman WT, Leisen JE, Huang L, McMillan RA, Apkarian RP, Conticello VP, Chaikof EL. Photomediated solid-state cross-linking of an elastin-mimetic recombinant protein polymer. Macromolecules. 2002;35:1730–1737. [Google Scholar]
- 13.Srokowski EM, Woodhouse KA. Development and characterisation of novel cross-linked bio-elastomeric materials. J Biomater Sci Polym Ed. 2008;19:785–799. doi: 10.1163/156856208784522038. [DOI] [PubMed] [Google Scholar]
- 14.Cappello J. Bioresorption Of Implanted Protein Polymer-Films Controlled By Adjustment Of Their Silk Elastin Block Lengths. Abstracts of Papers of the ACS. 1994;207:82-BTEC. [Google Scholar]
- 15.Liu JC, Tirrell DA. Cell Response to RGD Density in Cross-Linked Artificial Extracellular Matrix Protein Films. Biomacromolecules. 2008;9:2984–2988. doi: 10.1021/bm800469j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nowatzki PJ, Tirrell DA. Physical properties of artificial extracellular matrix protein films prepared by isocyanate crosslinking. Biomaterials. 2004;25:1261–1267. doi: 10.1016/s0142-9612(03)00635-5. [DOI] [PubMed] [Google Scholar]
- 17.Huang L, McMillan RA, Apkarian RP, Pourdeyhimi B, Conticello VP, Chaikof EL. Generation of synthetic elastin-mimetic small diameter fibers and fiber networks. Macromolecules. 2000;33:2989–2997. [Google Scholar]
- 18.Lee J, Macosko CW, Urry DW. Elastomeric polypentapeptides cross-linked into matrixes and fibers. Biomacromolecules. 2001;2:170–179. doi: 10.1021/bm0000900. [DOI] [PubMed] [Google Scholar]
- 19.Lee J, Macosko CW, Urry DW. Mechanical properties of cross-linked synthetic elastomeric polypentapeptides. Macromolecules. 2001;34:5968–5974. [Google Scholar]
- 20.Trabbic-Carlson K, Setton LA, Chilkoti A. Swelling and mechanical behaviors of chemically cross-linked hydrogels of elastin-like polypeptides. Biomacromolecules. 2003;4:572–580. doi: 10.1021/bm025671z. [DOI] [PubMed] [Google Scholar]
- 21.Nicol A, Gowda DC, Urry DW. Cell-adhesion and growth on synthetic elastomeric matrices containing Arg-Gly-Asp-Ser-3. J Biomed Mater Res. 1992;26:393–413. doi: 10.1002/jbm.820260309. [DOI] [PubMed] [Google Scholar]
- 22.Welsh ER, Tirrell DA. Engineering the extracellular matrix: A novel approach to polymeric biomaterials. I. Control of the physical properties of artificial protein matrices designed to support adhesion of vascular endothelial cells. Biomacromolecules. 2000;1:23–30. doi: 10.1021/bm0002914. [DOI] [PubMed] [Google Scholar]
- 23.Lim DW, Nettles DL, Setton LA, Chilkoti A. In-situ crosslinking of elastin-like polypeptide block copolymers for tissue repair. Biomacromolecules. 2008;9:222–230. doi: 10.1021/bm7007982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nagapudi K, Brinkman WT, Thomas BS, Park JO, Srinivasarao M, Wright E, Conticello VP, Chaikof EL. Viscoelastic and mechanical behavior of recombinant protein elastomers. Biomaterials. 2005;26:4695–4706. doi: 10.1016/j.biomaterials.2004.11.027. [DOI] [PubMed] [Google Scholar]
- 25.Cappello J, Crissman J, Dorman M, Mikolajczak M, Textor G, Marquet M, Ferrari F. Genetic-engineering of structural protein polymers. Biotechnol Prog. 1990;6:198–202. doi: 10.1021/bp00003a006. [DOI] [PubMed] [Google Scholar]
- 26.Haider M, Cappello J, Ghandehari H, Leong KW. In vitro chondrogenesis of mesenchymal stem cells in recombinant silk-elastinlike hydrogels. Pharm Res. 2008;25:692–699. doi: 10.1007/s11095-007-9282-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cappello J, Ghandehari H. Engineered protein polymers for drug delivery and biomedical applications. Adv Drug Deliv Rev. 2002;54:1053–1055. doi: 10.1016/s0169-409x(02)00063-7. [DOI] [PubMed] [Google Scholar]
- 28.Dandu R, Megeed Z, Haider M, Cappello J, Ghandehari H. Silk-elastinlike hydrogels: Thermal characterization and gene delivery; 226th National Meeting of the ACS; 2006. pp. 150–168. [Google Scholar]
- 29.Greish K, Araki K, Li DQ, O’Malley BW, Dandu R, Frandsen J, Cappello J, Ghandehari H. Silk-Elastinlike Protein Polymer Hydrogels for Localized Adenoviral Gene Therapy of Head and Neck Tumors. Biomacromolecules. 2009;10:2183–2188. doi: 10.1021/bm900356j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Betre H, Chilkoti A, Setton LA. A two-step recovery system based on thermally sensitive elastin-like polypeptide scaffolds for cartilage tissue engineering. Proc. Sec. Joint EBMS/BMES Conf; 2002. pp. 829–830. [Google Scholar]
- 31.Betre H, Setton LA, Meyer DE, Chilkoti A. Characterization of a genetically engineered elastin-like polypeptide for cartilaginous tissue repair. Biomacromolecules. 2002;3:910–916. doi: 10.1021/bm0255037. [DOI] [PubMed] [Google Scholar]
- 32.Betre H, Ong SR, Guilak F, Chilkoti A, Fermor B, Setton LA. Chondrocytic differentiation of human adipose-derived adult stem cells in elastin-like polypeptide. Biomaterials. 2006;27:91–99. doi: 10.1016/j.biomaterials.2005.05.071. [DOI] [PubMed] [Google Scholar]
- 33.Buckwalter JA. Articular cartilage. Instr Course Lect. 1983;32:349–370. [PubMed] [Google Scholar]
- 34.Setton LA, Mow VC, Howell DS. Mechanical behavior of articular cartilage in shear is altered by transection of the anterior cruciate ligament. J Orthop Res. 1995;12:437–482. doi: 10.1002/jor.1100130402. [DOI] [PubMed] [Google Scholar]
- 35.Nettles DL, Kitaoka K, Hanson NA, Flahiff C, Mata BA, Hsu EW, Chilkoti A, Setton LA. In situ crosslinking elastin-like polypeptide gels for articular cartilage regeneration in a goat osteochondral defect model. Tissue Eng. 2008 doi: 10.1089/ten.tea.2007.0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ong SR, Carlson KT, Nettles DL, Lim DW, Chilkoti A, Setton LA. Epitope tagging for tracking elastin-like polypeptides. Biomaterials. 2006;27:1930–1935. doi: 10.1016/j.biomaterials.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 37.Nettles DL, Haider MA, Chilkoti A, Setton LA. Neural network analysis identifies scaffold properties necessary for in vitro chondrogenesis in elastin-like polypeptide biopolymer scaffolds. Tissue Eng. 2010;16:11–20. doi: 10.1089/ten.tea.2009.0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nettles DL, Vail TP, Flahiff CM, Walkenhorst J, Carter AJ, Setton LA. Injectable silk-elastin for articular cartilage defect repair. Trans of the ORS Washington, DC. 2005;30 paper 1366. [Google Scholar]
- 39.Hrabchak C, Rouleau J, Moss IL, Woodhouse K, Akens M, Bellingham C, Keeley F, Dennis M, Yee A. Assessment of biocompatibility and initial evaluation of genipin cross-linked elastin-like polypeptides in the treatment of an osteochondral knee defect in rabbits. Acta Biomater. 2009 doi: 10.1016/j.actbio.2009.12.034. Epub ahead of Print, PMID: 20026437. [DOI] [PubMed] [Google Scholar]
- 40.Moss IL, Gordon L, Woodhouse KA, Whyne C, Yee AJ. Nucleus puolposus tissue repair in intervertebral disc dgeneration: biochemial and mechanical evaluation of a novel human disc cel - hyaluronan/elastin polypeptide scaffold composite. Trans of the ORS San Francisco, CA. 2008;33 Poster 1495. [Google Scholar]
- 41.Moss IL, Whyne CM, Yee AJ, Woodhouse KA. Initial feasibility of thiol-modified hyaluronan and elastin-like polypeptide hydrogels for the treatment of early degenerative disc disease. Trans of the ORS Las Vegas, NV. 2009;34 Poster 1660. [Google Scholar]
- 42.Moss IL, Hrabchak C, Rouleau J, Keeley F, Woodhouse KA, Yee AJ. Genepin-cross-linked elastin-like polypeptide hydrogels for the repair of osteochondral defects. Trans of the ORS Las Vegas, NV. 2009;34 Poster 556. [Google Scholar]
- 43.Di Zio K, Tirrell DA. Mechanical properties of artificial protein matrices engineered for control of cell and tissue behavior. Macromolecules. 2003;36:1553–1558. [Google Scholar]
- 44.Heilshorn SC, DiZio KA, Welsh ER, Tirrell DA. Endothelial cell adhesion to the fibronectin CS5 domain in artificial extracellular matrix proteins. Biomaterials. 2003;24:4245–4252. doi: 10.1016/s0142-9612(03)00294-1. [DOI] [PubMed] [Google Scholar]
- 45.Heilshorn SC, Liu JC, Tirrell DA. Cell-binding domain context affects cell behavior on engineered proteins. Biomacromolecules. 2005;6:318–323. doi: 10.1021/bm049627q. [DOI] [PubMed] [Google Scholar]
- 46.Lin HB, Garciaecheverria C, Asakura S, Sun W, Mosher DF, Cooper SL. Endothelial-cell adhesion on polyurethanes containing covalently attached RGD-peptides. Biomaterials. 1992;13:905–914. doi: 10.1016/0142-9612(92)90113-3. [DOI] [PubMed] [Google Scholar]
- 47.Nicol A, Gowda DC, Parker TM, Urry DW. In: Biotechnology and Bioactive Polymers. Gebelein CG, Carraher CE, editors. Plenum Press; New York: 1994. [Google Scholar]
- 48.Jordan SW, Haller CA, Sallach RE, Apkarian RP, Hanson SR, Chaikof EL. The effect of a recombinant elastin-mimetic coating of an ePTFE prosthesis on acute thrombogenicity in a baboon arteriovenous shunt. Biomaterials. 2007;28:1191–1197. doi: 10.1016/j.biomaterials.2006.09.048. [DOI] [PubMed] [Google Scholar]
- 49.Martinez-Osorio H, Juarez-Campo M, Diebold Y, Girotti A, Alonso M, Arias FJ, Rodriguez-Cabello JC, Garcia-Vazquez C, Calonge M. Genetically Engineered Elastin-Like Polymer as a Substratum to Culture Cells from the Ocular Surface. Curr Eye Res. 2009;34:48–56. doi: 10.1080/02713680802542053. [DOI] [PubMed] [Google Scholar]
- 50.Girotti A, Reguera J, Rodriguez-Cabello JC, Arias FJ, Alonso M, Testera AM. Design and bioproduction of a recombinant multi(bio)functional elastin-like protein polymer containing cell adhesion sequences for tissue engineering purposes. J Mater Sci Mater Med. 2004;15:479–484. doi: 10.1023/b:jmsm.0000021124.58688.7a. [DOI] [PubMed] [Google Scholar]
- 51.Janorkar AV, Rajagopalan P, Yarmush ML, Megeed Z. The use of elastin-like polypeptide-polyelectrolyte complexes to control hepatocyte morphology and function in vitro. Biomaterials. 2008;29:625–632. doi: 10.1016/j.biomaterials.2007.10.022. [DOI] [PubMed] [Google Scholar]
- 52.Swierczewska M, Hajicharalambous CS, Janorkar AV, Megeed Z, Yarmush ML, Rajagopalan P. Cellular response to nanoscale elastin-like polypeptide polyelectrolyte multilayers. Acta Biomater. 2008;4:827–837. doi: 10.1016/j.actbio.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 53.Mie M, Mizushima Y, Kobatake E. Novel extracellular matrix for cell sheet recovery using genetically engineered elastin-like protein. J Biomed Mater Res B Appl Biomater. 2008;86B:283–290. doi: 10.1002/jbm.b.31019. [DOI] [PubMed] [Google Scholar]