Abstract
Several HMGB1-specific antagonists have provided beneficial results in multiple models of inflammatory disease–preclinical trials including arthritis. Since no HMGB1-specific targeted therapy has yet reached the clinic, we have performed in vitro studies to investigate whether any of a selection of well-established antirheumatic drugs inhibit HMGB1 release as part of its mode of action. Freshly purified peripheral blood monocytes from healthy donors were stimulated in cultures with LPS and IFNγ to cause HMGB1 and TNF release detected in ELISPOT assays. Effects on the secretion were assessed in cultures supplemented with dexamethasone, cortisone, chloroquine, gold sodium thiomalate, methotrexate, colchicine, etanercept or anakinra. Pharmacologically relevant doses of dexamethasone, gold sodium thiomalate and chloroquine inhibited the extracellular release of HMGB1 in a dose-dependent mode. Immunostaining demonstrated that dexamethasone caused intracellular HMGB1 retention. No effects on HMGB1 secretion were observed in cultures with activated monocytes by any of the other studied agents. TNF production in LPS/IFNγ-activated monocytes was readily downregulated by dexamethasone and, to some extent, by chloroquine and etanercept. We conclude that dexamethasone, gold sodium thiomalate and chloroquine share a capacity to inhibit HMGB1 release from activated monocytes.
INTRODUCTION
HMGB1 is a ubiquitous nonhistone nuclear protein as well as an extracellular molecule regulating innate and adaptive immunity. Preclinical trials based on HMGB1-specific therapeutic targeting in several disease models have demonstrated that extracellular HMGB1 plays an important functional role in both infectious and sterile inflammation (reviewed in [1,2]).
HMGB1 reaches the extracellular milieu via active or passive cellular release. The pathways are differentiated on the basis of molecular mechanisms and release kinetics. Active secretion of HMGB1 occurs when cells including macrophages, monocytes, NK cells, dendritic cells, endothelial cells, platelets, neurons and astrocytes are exposed to exogenous pathogen-derived molecules like lipopolysaccharide (LPS) or endogenously derived inflammatory mediators like IL-1β, nitric oxide, IFNβ and IFNγ (3–6). Active release is initiated through plasma membrane receptor interactions with subsequent intracellular signal transduction leading to a slow release of HMGB1. Active secretion of HMGB1 from macrophages/monocytes begins 8–12 hours after ligation of appropriate cell surface receptors. This represents a significantly delayed onset of release as compared with most other proinflammatory mediators produced by these cells. Signaling via NF-κB (7), phosphatidylinositol 3-kinase (PI3K) (8), p38MAPK and ERK1/2 (9), and Janus kinase 2 (JAK 2) (6), all have been implicated in the regulation of inducible HMGB1 secretion, depending on the mode of induction and the cell lineage. There is a continuous shuttle of HMGB1 between the nucleus and the cytosol in resting cells: a shuttling that is tightly controlled by the phosphorylation and acetylation status of the two nuclear localization signals in HMGB1 (10,11). During cell activation, phosphorylation or acetylation of HMGB1 prevents the reentry of HMGB1 into the nucleus. Once in the cytoplasm of macrophages/monocytes, HMGB1 is loaded into secretory lysosomes (12). This accumulation is regulated by an ATP-binding cassette protein transporter (ABC-transporter), the multidrug resistance-related protein 1 (MRP1). Macrophages from Mrp1−/− mice have a markedly reduced HMGB1 secretion. The transport by MRP1 requires covalent linkage of HMGB1 to glutathione (13). The exocytosis of the HMGB1-containing secretory lysosomes critically depends on lysophosphatidylcholine (LPC) (12) enzymatically generated from phosphatidylcholine by secretory phospholipase A2 (sPLA2) produced 8–12 hours after LPS activation of monocytes/macrophages (14).
Passive HMGB1 release occurs from dying cells regardless of cell lineage. HMGB1 leakage during necrosis is an immediate process, since HMGB1 is only weakly attached to the chromatin of a living cell. The kinetics of the release pattern during necrosis is thus in distinct contrast to that of active HMGB1 export from living macrophages (15). The contribution from apoptotic cells to the extracellular pool of HMGB1 was initially regarded as nonexistent, since HMGB1 is strongly bound to DNA, histones and nucleosomes during programmed cell death due to hypoacetylation. However, further studies have demonstrated that apoptotic cells undergoing secondary necrosis also may release HMGB1 (16).
Both our research work and that of other groups have identified HMGB1 to be involved in the pathogenesis of chronic arthritis (reviewed in [2]). Experimental studies in arthritis based on HMGB1 antagonists have generated encouraging results. No HMGB1 blocking therapy is yet approved for clinical use, and we thus set out to study regulatory effects on HMGB1 release by well-established antirheumatic compounds. In vitro stimulated human primary blood monocytes were used as target cells to evaluate effects on HMGB1 and TNF secretion. The monocyte cultures were activated with LPS and IFNγ, potent exogenous and endogenous stimuli known to induce HMGB1 and TNF release (17,18). The key element for LPS-induced HMGB1 release involves a translocation of the constitutively expressed nuclear HMGB1 to secretory lysosomes exocytosed to the extracellular milieu (12). LPS stimulates the TLR4 complex via the PI3K pathway to activate calcium-dependent classical protein kinase C (cPKC) to phosphorylate nuclear HMGB1, which finally results in HMGB1 secretion from monocytes/macrophages (8). The regulation of IFNγ-induced HMGB1 release is not fully resolved, but relies on TNF- and Janus kinase 2-dependent mechanisms (6). IFNγ stimulation also upregulates the expression of HMGB1 mRNA in cultured monocytes (19). Our results demonstrate that treatments with dexamethasone, chloroquine or gold sodium thiomalate have the ability to inhibit HMGB1 release.
MATERIALS AND METHODS
In Vitro Cell Cultures
Human PBMCs were purified from healthy donors by using Ficoll centrifugation (Ficoll-Paque Plus, GE Healthcare, Uppsala, Sweden). The washed cells were resuspended in 80 μL of buffer consisting of PBS with 0.5% FCS and 2 mmol/L EDTA per 107 total cells and 20 μL of magnetic MACS anti-CD14 MicroBeads (Miltenyi biotec, Bergisch Gladbach, Germany) per 107 total cells and incubated for 15 min at 6–12°C. After washing, the cells were resuspended in 500 μL phosphate buffered saline (PBS) and run through a column placed in a magnetic field. The magnetic anti-CD14 cell separation yielded a cell population consisting of 95% monocytes. Cells were cultured at 37°C and 5% CO2 in RPMI supplemented with 5% human AB serum, 10 mmol/L HEPES, 100 U/mL penicillin, 100 μg/mL streptomycin and 292 μg/mL L-Glutamine (Gibco Invitrogen, Paisley, UK). RAW 264.7 mouse monocytic cell line was cultured in DMEM with 4.5 g/L glucose supplemented with 5% heat-inactivated FCS (Sigma Chemical Co., St. Louis, MO, USA), 10 mmol/L HEPES, 100 U/mL penicillin, 100 μg/mL streptomycin and 50 μmol/L 2-ME (Gibco Invitrogen).
HMGB1 ELISPOT Assay
An in-house HMGB1 ELISPOT assay was performed as previously described (18). Briefly, multiscreen 96-well HTS Plate Clear (MSIPS4510, Millipore, Stockholm, Sweden) was coated with 40 μg/mL mouse mAb IgG2b 2G7 (noncommercial antibody, originally from Critical Therapeutic Inc., Boston, MA, USA, available upon request) and blocked with cell-specific medium containing 5% human AB serum. Purified monocytes were added at concentrations of 1,000, 2,000 or 4,000 cells/well and preincubated for 1 h with indicated concentrations of dexamethasone, cortisone, methotrexate, chloroquine diphosphate salt, colchicine (all from Sigma, Steinheim, Germany), gold sodium thiomalate (Myocrisin, Aventis Pharma, Sanofi-Aventis, France), etanercept (Enbrel, Wyeth, UK) or anakinra (Kineret, Amgen, The Netherlands) and then stimulated with 10 ng/mL LPS (L-6529, Sigma), and 10 ng/mL human rIFNγ (Biosite, San Diego, CA, USA) for 24 h. As dexamethasone and cortisone were diluted in absolute ethanol, control experiments with cells subjected to equivalent amounts of ethanol also were performed. The addition of ethanol to monocyte cultures did not affect the secretion of HMGB1 or TNF (data not shown). Cell viability was assessed at every experimental setup and determined to be 90% to 100% by Trypan blue (Merck, Darmstadt, Germany) exclusion. Polyclonal antigen affinity-purified rabbit anti-HMGB1 antibodies (BD Biosciences PharMingen, San Diego, CA, USA), 0.5 μg/mL, were added and the plates incubated overnight at 4°C. After washing, the plates were incubated with biotinylated donkey antirabbit antibody (Jackson ImmunoResearch Lab, West Grove, PA, USA), 0.8 μg/mL, washed and further incubated with streptavidine-horseradish peroxidase (Mabtech AB, Stockholm, Sweden). After washing, a color reaction was induced by the addition of tetrametylbenzidine (TMB) substrate (Mabtech AB). The number of spots in each well was analyzed with an AID EliSpot Reader System (AID, Strassberg, Germany). The specificity of the ELISPOT assay was tested by coating the plate with an isotype mouse control IgG2b mAb against glucose oxidase Aspergillus niger (DakoCytomation, Glostrup, Denmark), by using an irrelevant polyclonal rabbit IgG fraction (DakoCytomation) at the detection step, or by omitting the detection anti-HMGB1 antibody. Each experiment was repeated at least three times and the assay was found to be specific for HMGB1 secretion.
TNF ELISPOT Assay
TNF ELISPOT was performed according to the manufacturer’s instructions (R&D Systems, Minneapolis, MN, USA). Briefly, multiscreen 96-well HTS Plates were coated overnight at 4°C with the capture antibody. Human primary monocytes at concentrations 1,000, 2,000 or 4,000 cells/well were added and pre-treated with drugs for 1 h and stimulated for 7 h with LPS and IFNγ, as in the HMGB1 ELISPOT assay. Cells were removed, a biotinylated TNF detection antibody added and the plates incubated overnight at 4°C. Spots were developed by using the streptavidine-horseradish peroxidase/TMB system, as described above.
Apoptosis Assay
Human primary monocytes were isolated and cultured as described above. Cells were plated in 6-well plates with 250,000 cells/well to obtain equal cell density as in the ELISPOT plates. Cells were pre-incubated and stimulated similarly as for the HMGB1 ELISPOT. After washing with cold phosphate buffered saline (pH 7.4) cells first were labeled with Annexin V-biotin and PI and then with streptavidine-FITC (R&D Systems, Minneapolis, MN, USA) according to instructions from the supplier. Apoptosis then was analyzed by flow cytometry (FACSort, Becton Dickinson, Franklin Lakes, NJ, USA).
Immunocytochemistry
RAW 264.7 cells were cultured in 8-well chamber slides (BD Biosciences Falcon, Bedford, MA, USA) at a concentration of 125,000 cells/mL. Cells were preincubated for 1 h with 1,000 nmol/L dexamethasone and then stimulated with 0.1 μg/mL LPS and 1 ng/mL IFNγ for 24 h. Slides were fixed with 4% formaldehyde (Apoteket, Gothenburg, Sweden) for 10 min and stored in PBS at 4°C until staining. Cells were permeabilized with 20 mmol/L Hepes, pH 7.4, 300 mmol/L sucrose, 50 mmol/L NaCl, 3 mmol/L MgCl2, and 0.5% (vol/vol) Triton X-100 (Sigma, St. Louis, MO, USA) for 3 min followed by washing in PBS containing 0.1% saponin (Riedel-de Haën, Seelze, Germany). Throughout the staining procedure, the slides were washed with PBS/saponin, and all incubations were performed at room temperature. Slides were blocked with 2% FCS for 5 min and cells stained with the anti-HMGB1 mouse mAb 2G7 (2 μg/mL) for 1 h. Subsequently, cells were incubated with Alexa 488 conjugated goat antimouse IgG2b (Invitrogen, Molecular probes, Eugene, OR, USA) diluted 1:1000 for 30 min. Cell nuclei were counterstained with Hoechst (Pierce, Rockford, IL, USA), 20 μg/mL for 5 min, and finally slides were mounted with PBS/glycerol. Analysis was conducted by using a Polyvar 2 UV microscope (Reichert-Jung, Vienna, Austria).
Statistical Analysis
Kruskal–Wallis nonparametric analysis of variance (ANOVA) was used to test statistical significance. All pairwise comparisons were adjusted for by using the Dunn multiple comparisons test. The computer software program GraphPad Prism version 5 for Windows (GraphPad Software, San Diego, CA, USA) was used for all tests.
RESULTS
Dexamethasone but Not Cortisone Downregulated HMGB1 and TNF Release from Activated Monocytes
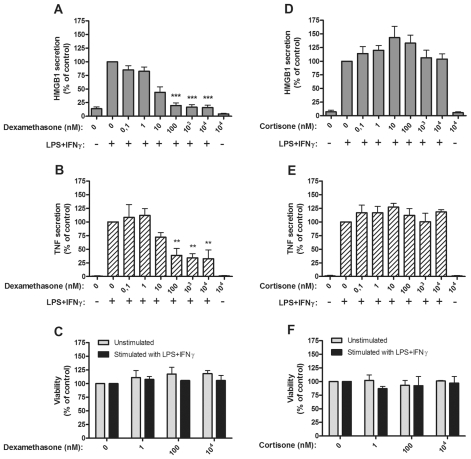
CD14 purified monocytes from peripheral blood of healthy donors were activated by LPS/IFNγ and HMGB1, and TNF secretion was assessed with ELISPOT assays. Dexamethasone, a potent synthetic corticosteroid compound, mediated strong inhibitory effects (P < 0.001) on both HMGB1 and TNF release in doses ranging from 102 to 104 nmol/L in cultures of activated monocytes (Figure 1A, B). The inhibition was not a consequence of cell toxicity, as cell viability was unaffected at all studied dexamethasone concentrations (Figure 1C).
Figure 1.
Dexamethasone, but not cortisone, inhibited HMGB1 and TNF release from human monocytes. Effects of dexamethasone on HMGB1 and TNF release and on cell viability (A–C), and effects of cortisone on HMGB1 or TNF release and cell viability (D–F), are demonstrated. Human primary monocytes were pretreated with dexamethasone (A, B) or cortisone (D, E) for 1 h and subsequently stimulated with LPS/IFNγ for 24 or 7 h. Secretion of HMGB1 and TNF was detected by ELISPOT. Data from at least three experiments were normalized by denoting the number of spots from LPS/IFNγ stimulated cells as 100% and subsequently calculating the effect achieved by addition of the drug; P values were calculated by Kruskal–Wallis nonparametric ANOVA test. *P < 0.05; **P < 0.01; ***P < 0.001. Cell viability was assessed by Annexin-V staining (C, F).
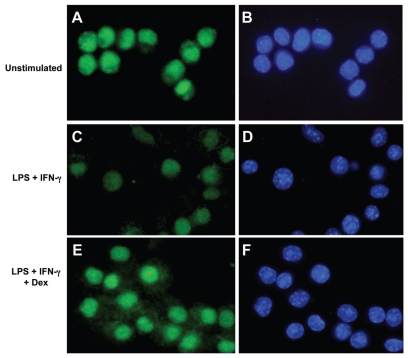
Intracellular HMGB1 immunostaining was performed to examine whether the dexamethasone-induced inhibition of HMGB1 release was caused by intracellular entrapment. Since primary monocytes express very limited cytoplasmic area, these cells are technically difficult to evaluate regarding nuclear and cytoplasmic staining by UV-microscopy. These problems were circumvented by performing these studies in the murine monocytic cell line RAW 264.7 instead. The HMGB1 staining was considerably weaker in cells activated with LPS/IFNγ for 24 h than it was in activated cells co-cultured with dexamethasone or in un-stimulated cells (Figure 2). Since LPS/IFNγ-stimulated cells secreted more HMGB1 in the ELISPOT assay than the other studied cultures, we interpret the weak intracellular HMGB1 staining to reflect a depletion of the preexisting HMGB1 pool after its extracellular translocation. The contrasting observation in LPS/IFNγ-activated cells co-cultured with dexamethasone indicated increased cytoplasmic HMGB1 staining and strong nuclear HMGB1 signals compared with cells stimulated with LPS/IFNγ alone, or with unstimulated cells (see Figure 2). The results in dexamethasone exposed cultures could possibly be explained by inhibited secretory capacity rather than by impaired HMGB1 synthesis.
Figure 2.
Dexamethasone generated intracellular HMGB1 accumulation in RAW 264.7 cells activated by LPS/IFNγ. RAW 264.7 cells monocytic cell line cells were cultured for 24 h without exogenous stimulus (A, B), or with LPS/IFNγ (C, D) or with LPS/IFNγ plus 103 nmol/L dexamethasone (E, F). The cells were then fixed and stained by immunofluorescence to identify HMGB1 (green) or cell nuclei (hoechst blue). HMGB1 was dominantly expressed intranuclearly in unstimulated cells with some cells expressing additional cytoplasmic HMGB1 (A). Cells activated by LPS/IFNγ demonstrated much weaker HMGB1 signals both in the nucleus and cytoplasm (C), compared with cells in panels A and E. Cells activated by LPS/IFNγ in the presence of dexamethasone expressed the strongest cytoplasmic staining (E) of the three studied cell cultures and the intensity of the nuclear signal was at least equal to that in unstimulated cells and considerably stronger than in activated cells cultured without dexamethasone (C).
Cortisone is a precursor of cortisol, the well-established antiinflammatory mediator. Cortisone did not influence HMGB1 or TNF release or cell viability at any studied dose ranging from 10−1 to 104 nmol/L (Figure 1D–F). Single intravenous injection of 30 mg cortisone to patients generates serum levels of approximately 103 nmol/L (20).
Gold Sodium Thiomalate (GST) and Chloroquine Both Inhibited HMGB1 Release
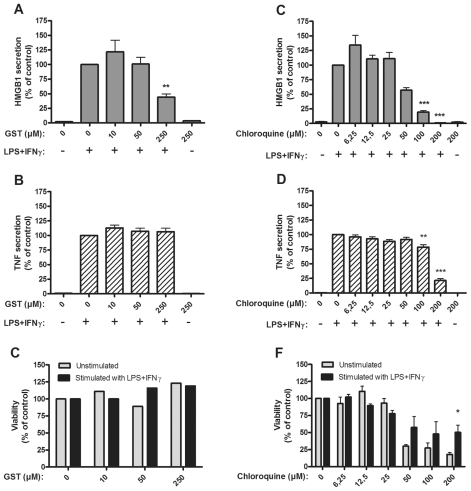
Gold salts represent the first widely used disease-modifying antirheumatic drug (DMARD) to treat rheumatoid arthritis. The most commonly used gold-containing compound, gold sodium thiomalate (GST), mediated divergent effects on HMGB1 versus TNF release after LPS/IFNγ activation of monocytes (P < 0.01) (Figure 3A–C). Addition of 250 μmol/L GST, which is pharmacologically relevant, significantly inhibited HMGB1 release, while no studied dose of GST had any effect on TNF secretion.
Figure 3.
Gold sodium thiomalate and chloroquine inhibited HMGB1 release, but only chloroquine inhibited TNF release. Effects of gold sodium thiomalate (GST) on HMGB1 and TNF release and on cell viability (A–C), and effects of chloroquine on HMGB1 or TNF release and on cell viability (D–F). Human primary monocytes were pretreated with GST or chloroquine for 1 h and subsequently stimulated with LPS/IFNγ for 24 or 7 h. Secretion of HMGB1 and TNF was detected by ELISPOT. Data from at least three experiments were normalized by denoting the number of spots from LPS/IFNγ stimulated cells as 100% and subsequently calculating the effect achieved by addition of the drug; P values were calculated by Kruskal–Wallis nonparametric ANOVA test. **P < 0.01; ***P < 0.001. Cell viability was assessed by Annexin-V staining.
The antimalarial compounds chloroquine and hydroxychloroquine also are used as DMARDs with beneficial effects in lupus and in several forms of chronic arthritis. Activated monocytes cultured with 100 to 200 μmol/L chloroquine exhibited reduced release of both HMGB1 and TNF (Figure 3D, E). Cell viability in cultures supplemented with 50–200 μmol/L chloroquine was reduced, but not in a dose-dependent mode (Figure 3F). Annexin V staining demonstrated that programmed cell death was the mode of death (data not shown). It is thus conceivable that apoptosis contributed to the inhibited HMGB1 and TNF secretion in the cultures.
Methotrexate or Colchicine Did Not Modulate HMGB1 or TNF Release
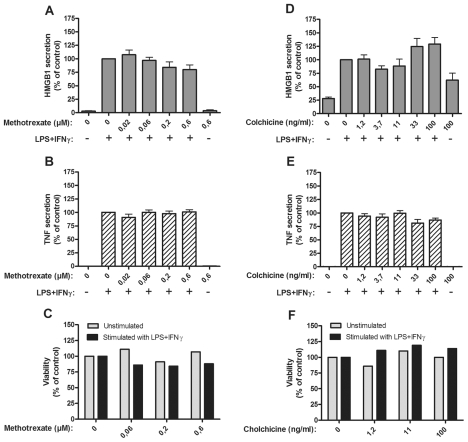
A single dose of 10 to 20 mg methotrexate given once weekly is a well-established DMARD therapy in rheumatoid arthritis. Administration of a single dose of 6 mg methotrexate generates a peak plasma level of approximately 0.2 μmol/L (21). No effects on HMGB1 or TNF secretion were detected in LPS/IFNγ stimulated monocytes that were cultured with 0.02–0.6 μmol/L methotrexate (Figure 4A–C).
Figure 4.
Methotrexate or colchicine had no effect on HMGB1 and TNF release. Effects of methotrexate on HMGB1 and TNF release and on cell viability (A–C), and effects of colchicine on HMGB1 and TNF release and on cell viability (D–F), are demonstrated. Human primary monocytes were pretreated with methotrexate or colchicine for 1 h and subsequently stimulated with LPS/IFNγ for 24 or 7 h. Secretion of HMGB1 and TNF was detected by ELISPOT. Data from at least three experiments were normalized by denoting the number of spots from LPS/IFNγ stimulated cells as 100% and subsequently calculating the effect achieved by addition of the drug; P values were calculated by Kruskal–Wallis non-parametric ANOVA test and found to be nonsignificant. Cell viability was assessed by Annexin-V staining.
Colchicine is a natural product with a long history of being used as an antiinflammatory agent in flares of gout and in other IL-1β-dependent diseases. Addition of colchicine in doses ranging from 1 to 100 ng/mL to the activated monocyte cultures did not interfere with the secretion of HMGB1 or TNF (Figure 4D–F).
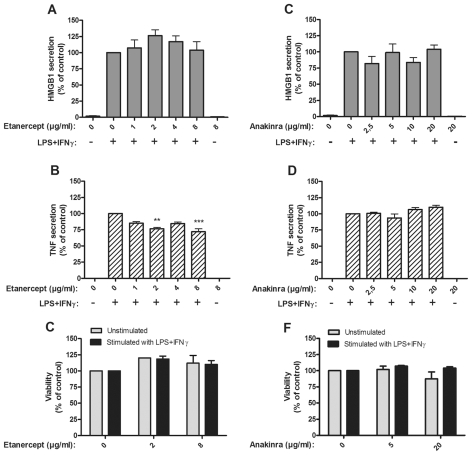
No Effects on HMGB1 Release Occurred in Cultures Treated with TNF-or IL1-Blocking Agents
Etanercept is a modified, soluble TNF type II receptor molecule successfully used for treatment of several different chronic inflammatory disorders. Subcutaneous injection of 25 mg etanercept generated a peak serum level of approximately 3 μg/mL (22). Addition of 1–8 ug/mL etanercept to LPS/IFNγ stimulated monocytes did not reduce HMGB1 release (Figure 5A). Somewhat diminished TNF secretion was observed in cultures with 2 or 8 ug/mL (Figure 5B).
Figure 5.
Blockade of TNF or IL-1β did not suppress HMGB1 release. Effects of soluble TNF receptor (etanercept) on HMGB1 and TNF release and on cell viability (A–C), and effects of IL-1RA (anakinra) on HMGB1 and TNF release and on cell viability (D–F), are demonstrated. Human primary monocytes were pretreated with etanercept or anakinra for 1 h and stimulated with LPS/IFNγ for 24 or 7 h. Secretion of HMGB1 and TNF was detected by ELISPOT. Data from at least three experiments were normalized by denoting the number of spots from LPS/IFNγ stimulated cells as 100% and subsequently calculating the effect achieved by addition of the drug; P values were calculated by Kruskal–Wallis nonparametric ANOVA test and found to be nonsignificant. **P < 0.01; ***P < 0.001. Cell viability was assessed by Annexin-V staining.
Anakinra, IL-1 receptor antagonist (IL-1RA), like TNF-blocking agents, belongs to the expanding group of biological drugs that has revolutionized the treatment of chronic inflammation. Monocytes cultured with anakinra (2.5 to 20 μg/mL) did not display any altered HMGB1 or TNF secretion in response to LPS/IFNγ (Figure 5D–F). Therapy given to patients with anakinra doses of 1 mg/kg or 10 mg/kg resulted in plasma concentrations of approximately 3 ug/mL and 30 ug/mL, respectively (23).
DISCUSSION
The most distinct result of the present study is that dexamethasone, chloroquine and gold salts, in clinically relevant doses, have been recognized as pharmaceutical inhibitors of HMGB1 release in experimental settings used by us. It should however be pointed out that it is not clear how LPS/IFNγ activation of monocytes relates to the pathophysiology of HMGB1 release in vivo during the course of clinical disorders. Despite this important objection, we believe these observations to be of potential interest. It is well documented that HMGB1 is a valid therapeutic target molecule in many different models of disease combined with the notion that no HMGB1-specific antagonists are yet approved for clinical use. Preclinical studies have demonstrated that HMGB1-neutralizing agents including anti-HMGB1 antibodies (4,24–27), thrombomodulin (28) or soluble HMGB1 receptors like sRAGE (29) ameliorate experimental sepsis, arthritis, ischemia-reperfusion injury, stroke, acute pancreatitis and additional experimental conditions. HMGB1 receptor antagonists like truncated HMGB1 (the A box domain) (24,30) or RAGE-specific antibodies (31) also have performed well in multiple preclinical models including sepsis and arthritis. The principle of sequestering HMGB1 in the nucleus and thereby preventing its extracellular release has provided protection in experimental sepsis and arthritis following activation of the cholinergic antiinflammatory pathway (32) and use of compounds like cisplatin or oxaliplatin (33, 34).
We previously have reported that treatment with glucocorticosteroids via intraarticular injections or systemic administration downregulates HMGB1 and TNF expression in tissues from patients with chronic arthritis or myositis (35). Our present results demonstrating dexamethasone-induced inhibition of TNF secretion from cultured monocytes stimulated by LPS is most likely explained by the well-known corticosteroid regulation of NF-κB activation. The studied doses of dexamethasone that downregulated TNF and HMGB1 secretion in activated monocytes ranged from 102 to 104 nmol/L, which is pharmacologically relevant, since systemic treatment with 3 mg dexamethasone generates a plasma concentration of 60 nmol/L (20). However, the molecular background for the diminished HMGB1 secretion in our study remains to be clarified. LPS-stimulated HMGB1 secretion in monocytes is mediated by calcium-dependent classical PKC, not by NF-κB or MAPKs, two factors known to be inhibited by corticosteroids (8). However, our immuno-fluorescence stainings reflecting the cellular HMGB1 localization demonstrated that cells activated by LPS/IFNγ in the presence of dexamethasone accumulated HMGB1 intracellularly. The HMGB1 retention was much stronger than in LPS/IFNγ stimulated cells cultured without dexamethasone (see Figure 2). These observations indicate that dexamethasone blocked the secretion of HMGB1. Active release of HMGB1 needs lysophosphatidylcholine, which is generated from phosphatidylcholine by sPLA2 (12). It has been demonstrated in several studies that corticosteroids are potent inhibitors of sPLA2 synthesis in many cell types after different modes of stimulation including LPS (14). It is thus plausible that an important mechanism to explain the inhibitory effect by dexamethasone on HMGB1 release is due to suppressed production of sPLA2, an enzyme required for HMGB1 exocytosis.
The absence of cortisone-mediated effects on TNF or HMGB1 release is not surprising, since primary monocytes lack the 11β-hydroxysteroid dehydrogenase type 1 enzyme required for conversion of cortisone to cortisol, the functional end-product of this potent endogenous anti-inflammatory system (36).
Gold compounds such as GST reduce the symptoms of rheumatoid arthritis, although their mechanism of action is not well defined. Previously, we have published that pharmacologically relevant doses of GST may inhibit HMGB1 release from activated, cultured monocytic cell lines (37). Using identical GST concentrations, we now confirm these observations in fresh, primary monocytes. GST did not regulate TNF secretion from either primary monocytes or monocytic cell lines. The divergent results on HMGB1 versus TNF release might be caused by the fact that GST also inhibits release of IFN-β and nitric oxide. They are both key endogenous mediators of intracellular HMGB1 transport mechanisms, but are not needed for TNF release (37).
Activated monocytes cultured with 100 to 200 μmol/L chloroquine counteracted both HMGB1 and TNF release. Equivalent intracellular chloroquine levels occur in leukocytes from patients treated with daily oral doses of 400 mg hydroxychloroquine (38). The mechanism of action of antimalarials such as hydroxylchloroquine and chloroquine in autoimmune diseases is not fully understood, but is suggested to be related to the fact that they accumulate in the cellular acid-vesicle system, including lysosomes (39). It is thus a possibility that HMGB1 (that also accumulates in lysosomes during its passage to secretion) may interact there with chloroquine in a process that will prevent further release. The increased apoptotic cell death in monocytes exposed to higher doses of chloroquine may be an additional mechanism for the diminished HMGB1 release (see Figure 3F).
Low-dose methotrexate therapy is the most commonly used DMARD administration in rheumatoid arthritis. The mode of action is believed to involve modulation of T lymphocyte, rather than macrophage/monocyte functions, since T cell-dependent but not independent experimental models respond to low-dose methotrexate therapy (40). Our results concur with this view, since no effects on HMGB1 or TNF release were observed by methotrexate addition to the monocyte cultures.
Colchicine is known to inhibit the intracellular microtubuli system preventing the release of IL-1β as the mechanism for mediating beneficial effects in IL-1β-dependent diseases such as gout and familial Mediterranean fever. The intracellular transport system in monocytes for IL-1β secretion shares features with that for active export of HMGB1 (12). It was thus somewhat surprising that the addition of colchicine to the LPS/IFNγ-stimulated monocytes had no effect on HMGB1 secretion (see Figure 4). The background could be that lysosomal pools of HMGB1 and IL-1β in activated monocytes are readily differentiated (12). Exocytosis of IL-1β containing vesicles is activated as an early event following monocyte activation, and occurs in association with a rise in ATP that also is sufficient to trigger assembly of the inflammasome. This fails, however, to stimulate the early release of HMGB1 containing vesicles, which are released much later following production and accumulation of lysophosphatidylcholine (12).
The effects on HMGB1 expression of systemic TNF-blocking therapy have been studied in a small cohort of patients with chronic arthritis (41). Systemic administration of anti-TNF monoclonal antibodies did not modulate the synovial HMGB1 expression. In line with this result, we did not observe a downregulation of HMGB1 release when monocytes were activated by LPS/IFNγ in the presence of etanercept. Taken together, these results suggest that HMGB1 may represent a TNF-independent molecule to consider for future therapeutic targeting in patients that are refractory to TNF- blocking treatment. Higher doses of etanercept inhibited TNF secretion in the monocyte cultures. The interpretation of this result is complicated by the fact that etanercept competes with the detecting anti-TNF mAb in the ELISPOT assay and may reflect a technical rather than a true biological issue.
IL-1RA (anakinra) did not influence HMGB1 or TNF secretion in monocytes stimulated by LPS/IFNγ implying that IL-1 is not required for the activation. However, there is another possibility that anakinra may play a functional role in HMGB1-induced clinical diseases. HMGB1 and IL-1β have been demonstrated to form complexes that work in strong proinflammatory synergy compared with the individual components on their own (42). Blocking the IL-1 type I receptor with IL-1RA abrogates the function of these complexes.
Dexamethasone, chloroquine, GST and oxaliplatin (33) thus all inhibit HMGB1 release in our experimental systems. The clinical relevance of this contribution is presently hard to interpret. Additional studies will be needed to study the effects after other modes of HMGB1 release and to further explore the detailed mechanisms in action.
ACKNOWLEDGMENTS
Financial support was provided through the regional agreement on medical training and clinical research (ALF) between Stockholm County Council and Karolinska Institutet, the Swedish Association against Rheumatism, the Swedish Medical Research Council, the Freemason Lodge Barnhuset in Stockholm, and King Gustaf V’s Foundation.
Footnotes
DISCLOSURE
The authors declare that they have no competing interests as defined by Molecular Medicine, or other interests that might be perceived to influence the results and discussion reported in this paper.
Online address: http://www.molmed.org
REFERENCES
- 1.Yang H, Tracey KJ. Targeting HMGB1 in inflammation. Biochim. Biophys. Acta. 1799:149–56. doi: 10.1016/j.bbagrm.2009.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersson U, Harris HE. The role of HMGB1 in the pathogenesis of rheumatic disease. Biochim. Biophys. Acta. 1799:141–8. doi: 10.1016/j.bbagrm.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Semino C, Angelini G, Poggi A, Rubartelli A. NK/iDC interaction results in IL-18 secretion by DCs at the synaptic cleft followed by NK cell activation and release of the DC maturation factor HMGB1. Blood. 2005;106:609–16. doi: 10.1182/blood-2004-10-3906. [DOI] [PubMed] [Google Scholar]
- 4.Wang H, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–51. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- 5.Wang H, et al. Proinflammatory cytokines (tumor necrosis factor and interleukin 1) stimulate release of high mobility group protein-1 by pituicytes. Surgery. 1999;126:389–92. [PubMed] [Google Scholar]
- 6.Rendon-Mitchell B, et al. IFN-gamma induces high mobility group box 1 protein release partly through a TNF-dependent mechanism. J. Immunol. 2003;170:3890–7. doi: 10.4049/jimmunol.170.7.3890. [DOI] [PubMed] [Google Scholar]
- 7.Tang D, et al. The anti-inflammatory effects of heat shock protein 72 involve inhibition of high-mobility-group box 1 release and proinflammatory function in macrophages. J. Immunol. 2007;179:1236–44. doi: 10.4049/jimmunol.179.2.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oh YJ, et al. HMGB1 is phosphorylated by classical protein kinase C and is secreted by a calcium-dependent mechanism. J. Immunol. 2009;182:5800–9. doi: 10.4049/jimmunol.0801873. [DOI] [PubMed] [Google Scholar]
- 9.Kawahara K, et al. C-reactive protein induces high-mobility group box-1 protein release through activation of p38MAPK in macrophage RAW264.7 cells. Cardiovasc. Pathol. 2008;17:129–38. doi: 10.1016/j.carpath.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 10.Youn JH, Shin JS. Nucleocytoplasmic shuttling of HMGB1 is regulated by phosphorylation that redirects it toward secretion. J. Immunol. 2006;177:7889–97. doi: 10.4049/jimmunol.177.11.7889. [DOI] [PubMed] [Google Scholar]
- 11.Bonaldi T, et al. Monocytic cells hyperacetylate chromatin protein HMGB1 to redirect it towards secretion. Embo. J. 2003;22:5551–60. doi: 10.1093/emboj/cdg516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gardella S, et al. The nuclear protein HMGB1 is secreted by monocytes via a non-classical, vesicle-mediated secretory pathway. EMBO Rep. 2002;3:995–1001. doi: 10.1093/embo-reports/kvf198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harris HE, Raucci A. Alarmin(g) news about danger: workshop on innate danger signals and HMGB1. EMBO Rep. 2006;7:774–8. doi: 10.1038/sj.embor.7400759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Touqui L, Alaoui-El-Azher M. Mammalian secreted phospholipases A2 and their pathophysiological significance in inflammatory diseases. Curr. Mol. Med. 2001;1:739–54. doi: 10.2174/1566524013363258. [DOI] [PubMed] [Google Scholar]
- 15.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–5. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 16.Bell CW, Jiang W, Reich CF, 3rd, Pisetsky DS. The extracellular release of HMGB1 during apoptotic cell death. Am. J. Physiol. Cell Physiol. 2006;291:C1318–25. doi: 10.1152/ajpcell.00616.2005. [DOI] [PubMed] [Google Scholar]
- 17.Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-gamma: an overview of signals, mechanisms and functions. J. Leukoc. Biol. 2004;75:163–89. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- 18.Wahamaa H, et al. HMGB1-secreting capacity of multiple cell lineages revealed by a novel HMGB1 ELISPOT assay. J. Leukoc. Biol. 2007;81:129–36. doi: 10.1189/jlb.0506349. [DOI] [PubMed] [Google Scholar]
- 19.Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat. Rev. Immunol. 2005;5:331–42. doi: 10.1038/nri1594. [DOI] [PubMed] [Google Scholar]
- 20.Czock D, Keller F, Rasche FM, Haussler U. Pharmacokinetics and pharmacodynamics of systemically administered glucocorticoids. Clin. Pharmacokinet. 2005;44:61–98. doi: 10.2165/00003088-200544010-00003. [DOI] [PubMed] [Google Scholar]
- 21.Shiozawa K, et al. Serum levels and pharmacodynamics of methotrexate and its metabolite 7-hydroxy methotrexate in Japanese patients with rheumatoid arthritis treated with 2-mg capsule of methotrexate three times per week. Mod. Rheumatol. 2005;15:405–9. doi: 10.1007/s10165-005-0434-6. [DOI] [PubMed] [Google Scholar]
- 22.Korth-Bradley JM, Rubin AS, Hanna RK, Simcoe DK, Lebsack ME. The pharmacokinetics of etanercept in healthy volunteers. Ann. Pharmacother. 2000;34:161–4. doi: 10.1345/aph.19126. [DOI] [PubMed] [Google Scholar]
- 23.Granowitz EV, et al. Pharmacokinetics, safety and immunomodulatory effects of human recombinant interleukin-1 receptor antagonist in healthy humans. Cytokine. 1992;4:353–60. doi: 10.1016/1043-4666(92)90078-6. [DOI] [PubMed] [Google Scholar]
- 24.Yang H, et al. Reversing established sepsis with antagonists of endogenous high-mobility group box 1. Proc. Natl. Acad. Sci. U. S. A. 2004;101:296–301. doi: 10.1073/pnas.2434651100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abraham E, Arcaroli J, Carmody A, Wang H, Tracey KJ. HMG-1 as a mediator of acute lung inflammation. J. Immunol. 2000;165:2950–4. doi: 10.4049/jimmunol.165.6.2950. [DOI] [PubMed] [Google Scholar]
- 26.Kim JY, et al. HMGB1 contributes to the development of acute lung injury after hemorrhage. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005;288:L958–65. doi: 10.1152/ajplung.00359.2004. [DOI] [PubMed] [Google Scholar]
- 27.Liu K, et al. Anti-high mobility group box 1 monoclonal antibody ameliorates brain infarction induced by transient ischemia in rats. Faseb. J. 2007;21:3904–16. doi: 10.1096/fj.07-8770com. [DOI] [PubMed] [Google Scholar]
- 28.Van de Wouwer M, et al. The lectin-like domain of thrombomodulin interferes with complement activation and protects against arthritis. J. Thromb. Haemost. 2006;4:1813–24. doi: 10.1111/j.1538-7836.2006.02033.x. [DOI] [PubMed] [Google Scholar]
- 29.Hofmann MA, et al. RAGE and arthritis: the G82S polymorphism amplifies the inflammatory response. Genes Immun. 2002;3:123–35. doi: 10.1038/sj.gene.6363861. [DOI] [PubMed] [Google Scholar]
- 30.Kokkola R, et al. Successful treatment of collagen-induced arthritis in mice and rats by targeting extracellular high mobility group box chromosomal protein 1 activity. Arthritis Rheum. 2003;48:2052–8. doi: 10.1002/art.11161. [DOI] [PubMed] [Google Scholar]
- 31.Lutterloh EC, et al. Inhibition of the RAGE products increases survival in experimental models of severe sepsis and systemic infection. Crit. Care. 2007;11:R122. doi: 10.1186/cc6184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huston JM, et al. Transcutaneous vagus nerve stimulation reduces serum high mobility group box 1 levels and improves survival in murine sepsis. Crit. Care Med. 2007;35:2762–8. doi: 10.1097/01.CCM.0000288102.15975.BA. [DOI] [PubMed] [Google Scholar]
- 33.Pan P, et al. Low-dose cisplatin administration in murine cecal ligation and puncture prevents the systemic release of HMGB1 and attenuates lethality. J. Leukoc. Biol. 2009;86:625–32. doi: 10.1189/JLB.1108713. [DOI] [PubMed] [Google Scholar]
- 34.Ostberg T, et al. Oxaliplatin retains HMGB1 intranuclearly and ameliorates collagen type II-induced arthritis. Arthritis Res. Ther. 2008;10:R1. doi: 10.1186/ar2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.af Klint E, et al. Intraarticular glucocorticoid treatment reduces inflammation in synovial cell infiltrations more efficiently than in synovial blood vessels. Arthritis Rheum. 2005;52:3880–9. doi: 10.1002/art.21488. [DOI] [PubMed] [Google Scholar]
- 36.Thieringer R, et al. 11 Beta-hydroxysteroid dehydrogenase type 1 is induced in human monocytes upon differentiation to macrophages. J. Immunol. 2001;167:30–5. doi: 10.4049/jimmunol.167.1.30. [DOI] [PubMed] [Google Scholar]
- 37.Zetterstrom CK, et al. Pivotal advance: inhibition of HMGB1 nuclear translocation as a mechanism for the anti-rheumatic effects of gold sodium thiomalate. J. Leukoc. Biol. 2008;83:31–8. doi: 10.1189/jlb.0507323. [DOI] [PubMed] [Google Scholar]
- 38.French JK, Hurst NP, O’Donnell ML, Betts WH. Uptake of chloroquine and hydroxychloroquine by human blood leucocytes in vitro: relation to cellular concentrations during antirheumatic therapy. Ann. Rheum. Dis. 1987;46:42–5. doi: 10.1136/ard.46.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tett S, Cutler D, Day R. Antimalarials in rheumatic diseases. Baillieres Clin. Rheumatol. 1990;4:467–89. doi: 10.1016/s0950-3579(05)80004-4. [DOI] [PubMed] [Google Scholar]
- 40.Lange F, et al. Methotrexate ameliorates T cell dependent autoimmune arthritis and encephalomyelitis but not antibody induced or fibroblast induced arthritis. Ann. Rheum. Dis. 2005;64:599–605. doi: 10.1136/ard.2004.026120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sundberg E, et al. Systemic TNF blockade does not modulate synovial expression of the pro-inflammatory mediator HMGB1 in rheumatoid arthritis patients—a prospective clinical study. Arthritis Res. Ther. 2008;10:R33. doi: 10.1186/ar2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sha Y, Zmijewski J, Xu Z, Abraham E. HMGB1 develops enhanced proinflammatory activity by binding to cytokines. J. Immunol. 2008;180:2531–7. doi: 10.4049/jimmunol.180.4.2531. [DOI] [PubMed] [Google Scholar]