Abstract
Atherosclerosis is characterized by a chronic inflammatory condition that involves numerous cellular and molecular inflammatory components. A wide array of inflammatory mediators, such as cytokines and proteins produced by macrophages and other cells, play a critical role in the development and progression of the disease. ATP-binding membrane cassette transporter A1 (ABCA1) is crucial for cellular cholesterol efflux and reverse cholesterol transport (RCT) and is also identified as an important target in antiatherosclerosis treatment. Evidence from several recent studies indicates that inflammation, along with other atherogenic-related mediators, plays distinct regulating roles in ABCA1 expression. Proatherogenic cytokines such as interferon (IFN)-γ and interleukin (IL)-1β have been shown to inhibit the expression of ABCA1, while antiatherogenic cytokines, including IL-10 and transforming growth factor (TGF)-β1, have been shown to promote the expression of ABCA1. Moreover, some cytokines such as tumor necrosis factor (TNF)-α seem to regulate ABCA1 expression in species-specific and dose-dependent manners. Inflammatory proteins such as C-reactive protein (CRP) and cyclooxygenase (COX)-2 are likely to inhibit ABCA1 expression during inflammation, and inflammation induced by lipopolysaccharide (LPS) was also found to block the expression of ABCA1. Interestingly, recent experiments revealed ABCA1 can function as an antiinflammatory receptor to suppress the expression of inflammatory factors, suggesting that ABCA1 may be the molecular basis for the interaction between inflammation and RCT. This review aims to summarize recent findings on the role of inflammatory cytokines, inflammatory proteins, inflammatory lipids, and the endotoxin-mediated inflammatory process in expression of ABCA1. Also covered is the current understanding of the function of ABCA1 in modulating the immune response and inflammation through its direct and indirect antiinflammatory mechanisms including lipid transport, high-density lipoprotein (HDL) formation and apoptosis.
INTRODUCTION
Atherosclerosis has been identified as a chronic inflammatory disease of the artery wall. The demonstration of activated T cells and macrophages within the atherosclerotic lesion provides in situ evidence for the inflammatory components of this disease (1,2). Activated T cells and macrophages together produce a wide array of cytokines that can exert both pro- and antiinflammatory effects (3,4). Inflammatory proteins, such as CRP and COX-2, have both been considered markers and mediators of endovascular inflammation and cardiovascular disorders (5). Reverse cholesterol transport (RCT) is a pathway by which accumulated cholesterol is transported from the vessel wall to the liver for excretion, thus preventing atherosclerosis (6). A critical part of RCT is cholesterol efflux, in which accumulated cholesterol is removed from macrophages in the subintima of the vessel wall to high-density lipoprotein (HDL) or apolipoprotein (apo)A-I by a variety of mechanisms, including a pathway dependent on a cell membrane protein called ATP-binding membrane cassette transporter A1 (ABCA1) (7). Evidence from many recent studies indicates that the inflammatory process impairs RCT (8). The molecular links between inflammation and RCT are not completely known, but evidence reveals the expression and activity of ABCA1 has been altered in the inflammatory process in vivo (9). The in vitro results also show that ABCA1 expression is closely associated with many inflammatory cytokines and proteins (10,11), and bacterial lipopolysaccharide (LPS) seems to decrease the expression of ABCA1 and ABCA1-mediated cholesterol efflux (12,13). Interestingly, recent experimental studies reveal that ABCA1 in turn plays a crucial role in the modulation of the inflammatory response where direct and indirect pathways have been found (14–16), indicating that ABCA1 may be a promising therapeutic target for inflammation-related diseases. In this article, we will review the most compelling evidence regarding the role of immuno-inflammatory response in the expression of ABCA1, and the function of ABCA1 in modulation of the inflammation, which may be useful in understanding the molecular mechanism underlying the interaction between inflammation and RCT.
FUNCTION AND EXPRESSION OF ABCA1
The ATP-binding cassette (ABC) transporters are ubiquitous membrane proteins that couple the transport of diverse substrates across cellular membranes to the hydrolysis of ATP (17). ABCA1 is a member of the ABCA subfamily, and recent studies have supported that ABCA1 plays a central role in the HDL cholesterol metabolism and lipid clearance from the foam cell, where ABCA1 mediates the transport of cholesterol, phospholipids and other lipophilic molecules across cellular membranes to lipid-poor HDL apolipoproteins (18,19). ABCA1 is broadly expressed with high levels in macrophages, liver cells, intestinal cells, adrenal gland, endothelial cells and placental trophoblast (20,21). Its expression is tightly controlled at both transcription and post-transcription levels (22). Sterols and fatty acids serve as known modulators of the liver × receptors (LXR)/retinoid × receptor (RXR) pathways, through which the ABCA1 is transcriptionally regulated (23). Cyclic AMP (cAMP), which exerts its effects mainly by activating cAMP- dependent protein kinase A (PKA), has been shown to play an important role in the upregulation of ABCA1 expression in murine models (24). A growing list of natural and synthetic substances and metabolic regulators, such as unsaturated fatty acids, bacterial lipopolysaccharide and drugs, are particularly effective in modulating ABCA1 expression through transcription or posttranscription regulation (25–27). During atherosclerotic lesion development, numerous inflammatory mediators such as growth factors or cytokines, inflammatory hydrolases and oxidases can influence lipoprotein metabolism and arterial wall biology, resulting in the progression and destabilization of the lesion (28,29). To further elucidate the influence of these mediators for the development of atherosclerosis, ABCA1 expression was investigated in cells and tissues treated with inflammatory cytokines and proteins. The levels of these mediators have been found to correlate with the expression of ABCA1 (Table 1), revealing a novel mechanism that could link inflammation to increased atherosclerosis susceptibility.
Table 1.
Regulation of ABCA1 expression by inflammatory cytokines and proteins.
| Substance | Cell types/tissues | Effects | Factors | References |
|---|---|---|---|---|
| Cytokines | ||||
| TNF-α | THP-1 cells | ↓Transcription | NF-κB | 35,42 |
| HepG2 cells | ↓Transcription | PPAR-α. LXRα | 35 | |
| Kidney HK-2 cells | ↓Transcription | LXRα | 12 | |
| Mice peritoneal macrophages | ↑Transcription | NF-κB | 43 | |
| Rabbit adipocytes | ↓Transcription | PPAR-γ, LXRα | 11 | |
| IFN-γ | Mouse peritoneal macrophages | ↓Posttranscription | STAT1 | 30 |
| THP-1 cells | ↓Transcription | STAT3, LXRα | 31,40,58 | |
| IL-1β | HK-2 cells | ↓Transcription | LXRα | 12 |
| THP-1 cells | ↓Transcription | ROS, NF-κB | 10 | |
| Glomerular mesangial cells | ↓Transcription | ? | 33 | |
| Vascular SMCs | ↓Transcription | ? | 33 | |
| PDGF | Vascular SMCs | ↓Transcription | p110, Akt | 36 |
| IL-10 | THP-1 cells | ↑Transcription | STAT3/LXRα/PKA10,37 | |
| RAW264.7 | ↑Transcription | PPAR-γ | 56 | |
| TGF-β1 | THP-1 and mice macrophages | ↑Transcription | ? | 39,40 |
| GM-CSF | Human PBMCs | ↑Transcription | LXRα | 44 |
| Mice peritoneal macrophages | ↑Transcription | PPAR-γ | 45 | |
| Mice alveolar macrophages | ↓Transcription | LXRα | 46,138 | |
| Proteins | ||||
| CRP | THP-1 cells | ↓Transcription | ERK1/2 | 47 |
| Human PBMCs | ↑Transcription | CD64/PI3K/LXRα49,50 | ||
| APN | THP-1 cells | ↑Transcription | PPAR-γ, LXRα | 52,53 |
| Mice liver | ↑Transcription | ? | 54 | |
| COX-2 | THP-1 cells | ↑Transcription | PGE1, PGE2, PGD2 | 57,58 |
| lysoPC | Mouse macrophage | ↑Transcription | PPAR-γ, LXRα | 69 |
| PON1 | Mouse macrophage | ↑Transcription | Lyso-PC | 70 |
| TG2 | TG2-KO mouse macrophage | ↓Transcription | ? | 139 |
| Calpain | Mice peritoneal macrophages | ↓Posttranscription | PEST sequence | 65 |
| Calmodulin | Mice peritoneal macrophages | ↑Posttranscription | Calpain | 66 |
| Lipids | ||||
| Lyso-PC | Mouse macrophage | ↑Transcription | PPAR-γ, LXRα | 69 |
| Linoleic acid | Human PBMCs | ↓Transcription | ? | 79 |
| Palmitic acid | Human PBMCs | ↑Transcription | ? | 79 |
| EPA | THP-1 cells | ↓Posttranscription | cAMP/PKA | 26 |
EPA, eicosapentaenoic acid; lyso-PC, lysophosphatidylcholine; PBMC, peripheral blood mononuclear cell; PGE/D, prostaglandin E/D; PI3K, phosphatidylinositol 3-kinase; PKA, protein kinase A; PON1, paraoxonase 1; TG2, transglutaminase 2.
ROLE OF INFLAMMATORY CYTOKINES AND PROTEINS IN EXPRESSION OF ABCA1
Inflammatory Cytokines
A specified group of proatherogenic cytokines have been shown to inhibit the expression of ABCA1, namely, interferon (IFN)-γ, interleukin (IL)-1β and platelet-derived growth factor (PDGF). IFN-γ, primarily produced by T lymphocytes, is a dimerized soluble cytokine and has a variety of proatherogenic effects. According to Alfaro et al. (30), both ABCA1 mRNA and protein in mouse peritoneal macrophages are rapidly lost after the addition of IFN-γ, with maximal losses of ABCA1 mRNA. The downregulation of ABCA1 mRNA was associated with the rapid phosphorylation and translocation of signal transducer and activator of transcription (STAT)-1 in these cells induced by IFN-γ. Studies performed at our laboratory have also shown that IFN-γ down-regulates ABCA1 expression by inhibiting LXR-α, a transcription regulator for ABCA1, which was consistent with the phosphorylation and nuclear translocation of STAT1 in THP-1 macrophage–derived foam cells after addition of IFN-γ (31), indicating IFN-γ downregulates ABCA1 expression in a JAK/STAT signaling pathway–dependent manner. IL-1β, a prototypic proinflammatory cytokine, has been shown to downregulate ABCA1 as well as ABCA1-mediated cholesterol efflux in macrophages through attenuating ABCA1 promoter activity via ROS- and nuclear factor (NF)-κB–dependent pathways (32). Similar suppression effects by IL-1β are also present in glomerular mesangial cells (33), vascular smooth muscle cells (SMCs) (34), HK-2 cells (35), and HepG2 cells (12). PDGF is a potent inflammatory mitogen that enables vascular SMCs to participate in atherosclerosis. PDGF suppresses endogenous expression of ABCA1 in vascular SMCs where cultured SMCs exposed to PDGF elicit a rapid phosphorylation of Akt, a kinase downstream from phosphatidylinositol 3-kinase, which inhibits activity of the ABCA1 promoter (36).
Some antiinflammatory cytokines such as IL-10 and TGF-β1 have been shown to increase the expression of ABCA1. In human THP-1 cells and peripheral monocytes, IL-10 stimulates transcription of the ABCA1 and redirects macrophage cholesterol handling toward efflux (37). In addition, IL-10 has been reported to override the downregulation of ABCA1 by tumor necrosis factor (TNF)-α (10). Han et al. (38) also found that IL-10 increases cholesterol efflux from macrophages to protect against toxicity of free cholesterol accumulation in the cell through an ABCA1 dependent pathway. TGF-β, a cytokine apparent within the atheroma, is believed to have antiinflammatory properties. TGF-β1 has been found to enhance cholesterol efflux through upregulation of the ABCA1 expression, indicating a protective role for TGF-β1 in controlling cellular lipid accumulation within the intima (39,40).
As one of the most important inflammatory cytokines, TNF-α is specifically active in both human and rodent atherosclerotic plaques (4). However, the exact role of TNF-α in atherosclerosis is not fully clear at the present time because results in different animal models are controversial (4,41). Correspondingly, controversial results about the role of TNF-α in macrophage ABCA1 expression and cholesterol efflux have been described, e.g., TNF-α downregulated ABCA1 expression in THP-1 macrophage–derived foam cells through a NF-κB–dependent pathway (42). However, TNF-α was reported to induce ABCA1 expression in mouse peritoneal macrophages, revealing an antiatherogenic mechanism of TNF-α signaling in macrophages (43). There are two receptors in mice known to elicit TNF-α responses, termed p55 and p75. According to Schreyer et al. (41), the signaling modulated by p55 TNF-α receptor is referred to exert a potent antiatherogenic effect (41). It remains to be determined whether the positive interaction between TNF-α and ABCA1 in mouse macrophages is due to the p55 signaling. Granulocyte-macrophage colony-stimulating factor (GM-CSF) has been implicated in a variety of inflammatory disorders including atherosclerosis. The role of GM-CSF on the ABCA1- mediated cholesterol metabolism has been investigated in different experimental systems. Kazawa et al. (44) showed that GM-CSF significantly upregulated the expression of ABCA1 in human monocyte–derived macrophage. In apoE and GM-CSF double knockout mice, ABCA1 expression in peritoneal macrophages was reduced by 70% compared with apoE−/− mice. These results indicated a positive regulatory role of GM-CSF in macrophage ABCA1 expression (45). However, in pulmonary alveolar macrophages of GM-CSF knockout (KO) mice, according to a recent report by Thomassen et al. (46), GM-CSF knockout results in highly increased ABCA1 expression accompanied by increased LXR-α level, suggesting ABCA1 expression is differently regulated by GM-CSF in a tissue- and species-specific manner.
For now, it is clear that inflammation plays a pivotal role in the progression of atherosclerosis. However, the mechanisms by which this occurs and the relationship between pro- and antiinflammatory cytokines in modulating the development and progress of atherosclerotic lesions remain unclear. The finding that proinflammatory cytokines decrease ABCA1 expression and ABCA1- mediated cholesterol efflux may provide a new basis for understanding the role of cytokines in the pathogenesis of atherosclerosis, and thus investigating the precise cytokine signaling which decreases ABCA1 expression may provide some specific targets for the treatment of atherosclerosis.
Inflammatory Proteins
CRP, a fundamental marker of inflammation, is generally thought to be associated with an increased risk of atherosclerosis. Human macrophages treated with clinically relevant concentrations of CRP are found to have a decreased ability in intracellular cholesterol efflux as well as the ABCA1 expression (47). However, according to new epidemiological findings, the innocent or even antiatherogenic effects for CRP have been implicated (48). Hanriot et al. (49) found that CRP not only increased the secretion of IL-1α, IL-1β, and IL-6, but also upregulated LXR-α and ABCA1 expression in human monocytes, raising the intriguing possibility that CRP could activate yet unidentified pathways to have more complex effects on atherogenesis than previously thought (50). However, because the biological effects of CRP might depend largely on the source of the protein, we should exclude the possibility that CRP’s paradoxical effects reported in different studies might just be caused by LPS contamination of culture medium.
Adiponectin (APN) is one of several important metabolically active proteins secreted from adipocytes (51). Human THP-1 macrophage transduced with the APN gene has decreased lipid accumulation compared with control macrophages for upregulating ABCA1-mediated cholesterol efflux (52). Conversely, the expression of ABCA1 and cellular cholesterol efflux are decreased in APN-KO mice (53,54). COX-2, an inducible enzyme that is responsible for formation of prostanoids, is more often associated with inflammation states (55). This realization has driven the development of COX-2 inhibitors such as celecoxib and rofecoxib Vioxx for antiinflammatory therapy. Nevertheless, selective COX-2 inhibitors are often associated with a heightened risk of myocardial infarction, and the precise mechanisms involved have yet to be satisfactorily elucidated (56,57). The selective COX-2 inhibitor has been shown to significantly reduce ABCA1 expression and increases macrophage foam cell formation (57,58), suggesting a possible explanation for the proatherogenic mechanisms of COX-2 inhibitor.
Myeloperoxidase (MPO) is a central inflammatory enzyme secreted by activated macrophages (59). MPO catalyzes oxidation of tyrosine and nitrite to form reactive intermediates capable of initiating oxidation of lipids in plasma. For example, MPO promotes HDL oxidation in the human artery wall and increases the levels of hypochlorous acid (60). Shao et al. found that HDL or lipid-free apoA-I exposed to hypochlorous acid was less able to remove cholesterol from cultured cells, which was associated with impaired ABCA1-dependent cholesterol transport (60,61). In addition, MPO can enhance the formation of glycolaldehyde, an intermediate in the formation of advanced glycosylation end products (62), which strongly inhibits ABCA1-dependent transport of cholesterol from macrophages to apoA-I (63), suggesting that MPO may play an important role in the development of vascular complications in diabetes mellitus.
Calpains, calcium-activated neutral cysteine proteases, play an important role in the acute inflammatory process through activation of NF-κB and NF-κB–dependent expression of proinflammatory cytokines and adhesion molecules (64). Wang et al. showed that ABCA1 protein degradation is regulated by calpain protease through regulating a sequence rich in proline, glutamic acid, serine, and threonine (PEST) in the cytoplasmic region of ABCA1, while apoA-I can reverse the PEST-dependent ABCA1 degradation by calpain and thereby increase ABCA1 protein level at the cell surface (65). Because cell surface expression of ABCA1 might be much more relevant for its function, the way to stabilize ABCA1 against calpain-mediated degradation should be a novel and potentially important strategy to prevent atherosclerosis. Recently, Iwamoto et al. found calmodulin interacts with ABCA1 to protect from calpain-mediated degradation and upregulates HDL generation, which is consistent with the previous finding that calmodulin increased apoA-I–mediated cell cholesterol release (66). Spiroquinone and diphenoquinone, oxidized products of probucol, have been reported to reduce degradation of ABCA1 without inhibiting its activity or altering transcription of the ABCA1 gene (67). Such function may be also due to its ability to inhibit calpain-mediated degradation.
Inflammatory Lipids
Lysophosphatidylcholine (lyso-PC), the most abundant cellular phospholipid generated by the action of phospholipase A2 (PLA2) on membrane phosphatidylcholine, is associated with a variety of pathological processes such as inflammation and atherosclerosis (68). Lyso-PC was reported to promote the ABCA1- dependent cellular cholesterol efflux (69). In addition, paraoxonase 1 (PON1), a negative acute-phase protein, was also reported to promote the ABCA1- mediated cholesterol efflux from macrophages in a Lyso-PC–dependent manner (70). 15-Lipoxygenase (15-LO), a member of the lipid peroxidizing enzymes family, has been linked with various inflammatory processes in many tissues (71,72). In vitro, 15-LO catalyzes the modification of HDL3, which is less effective in mediating the ABCA1-dependent cholesterol efflux (73).
A typical abnormality in type II diabetic patients is elevated plasma levels of free fatty acids (74). There is a close link between fatty acids, inflammation and an increased risk of cardiovascular disease (75). Stearoyl-CoA desaturase (SCD) catalyzes the rate-limiting step in the biosynthesis of monounsaturated fatty acids and is a critical regulator of energy metabolism, inflammation and diabetes (76). SCD activity has been found to decrease ABCA1 expression and cellular cholesterol efflux by different mechanisms. Sun et al. have shown co-transfection of ABCA1 with either SCD1- or SCD2-inhibited ABCA1-mediated cholesterol efflux in HEK 293 cells and CHO cells (77). In addition, SCD was found to inhibit apolipoprotein-mediated lipid efflux through generating unsaturated fatty acids, which further destabilize ABCA1 protein (78). Unsaturated fatty acids have been found to have a significant impact on ABCA1 expression in human macrophages where the ABCA1 protein level is synchronously suppressed by the w6-unsaturated fatty acid linoleic acid (79). Moreover, our laboratory recently demonstrated that polyunsaturated fatty acid eicosapentaenoic acid reduced serine phosphorylation of ABCA1 protein and impaired ABCA1-dependent cholesterol efflux in THP-1 macrophages (26). Epidemiological studies have reported that a polyunsaturated fatty acid diet in humans may decrease plasma HDL and apoA-I levels (80). Activity of ABCA1 is dramatically lowered in unsaturated fatty acid–treated THP-1 macrophages, and this reduction may result from, at least in part, a regulation of ABCA1, which is a rate-limiting step for generating plasma HDL.
ROLE OF ENDOTOXIN-INDUCED INFLAMMATION IN EXPRESSION OF ABCA1
McGillicuddy et al. (8) provided the first in vivo evidence that endotoxin-induced inflammation impairs cholesterol efflux and RCT in mice, and marked reduction of cholesterol efflux induced by LPS was consistent with downregulation of ABCA1 expression. Recently, the global gene expression profile of circulating leukocytes in a human model of systemic inflammatory were investigated. The global gene expression in circulating leucocytes isolated from male volunteers shows that the expression of the ABCA1 gene is downregulated after infusion of Escherichia coli endotoxin (9). Dysregulated adipose tissue leads to a proinflammatory state in the cells with reduced secretion of APN and increased secretion of several proinflammatory cytokines (81). The inflammation is also regarded to alternate the lipid metabolism in adipose tissue (82,83). In C57BL/6 mice, LPS-mediated inflammation produces a rapid, marked decrease in mRNA levels of a number of type II nuclear hormone receptors, including peroxisome proliferator–activated receptor (PPAR)-γ, LXR-α and thyroid receptor (TR)-α, along with decreased expression of target genes such as ABCA1 (83). In addition, endotoxin-induced inflammatory stress increased cholesterol accumulation in the livers and inhibited expression of LXR-α and ABCA1, which resulted in the reduction of cholesterol efflux (35). Although the hypothesis that inflammation might link to cholesterol metabolism has been confirmed in these studies, the signaling pathway underlying the potential mechanism needs to be further clarified. According to Castrillo et al. (84), LPS strongly blocks the induction of LXR as well as its target gene ABCA1 in cultured mice peritoneal macrophages, where a specific effector of Toll-like receptor 3/4 (TLR3/4), interferon regulatory factor 3 (IRF3), is shown to mediate the crosstalk between LXR and TLR4 signaling by means of transcriptional activity inhibition of LXR on the ABCA1 promoter. In murine macrophages, LPS does not seem to decrease mRNA expression of ABCA1 and ABCA1-mediated cholesterol efflux through reducing LXR protein or by decreasing the binding of nuclear protein to the LXR response element, suggesting an unknown mechanism that requires further exploration (85). Adipocyte enhancer-binding protein 1 (AEBP1), a transcriptional repressor in macrophage, plays a role in the LPS-induced inhibition of ABCA1 (25). A key innate immunity signaling kinase (IRAK-1) has also been reported to associate with the downregulation of ABCA1 expression in response to innate immune signaling (86). These results revealed some novel mechanism of action of LPS-induced innate immune-inflammation response in ABCA1-mediated cholesterol efflux. Although decreased cholesterol efflux may redirect cholesterol to peripheral cells, such as leukocytes, to acutely promote host defense, prolongation of impaired cholesterol efflux may also increase the risk of atherosclerosis observed in chronic infections and inflammatory states (85).
ROLE OF ABCA1 IN REGULATION OF INFLAMMATION AND IMMUNE RESPONSE
New Role for ABCA1 as an Antiinflammatory Mediator
Studies with ABCA1 knockout mice have been critical in demonstrating the relationship between ABCA1 and inflammation (15,16,19,21,87–90). Knockout of ABCA1 in mice increases inflammatory cell infiltration in a number of tissues, including the vessel wall, peritoneal cavity and the blood circulation (19,21). In addition, ABCA1-deficient animals deposit immune complexes in many tissues, indicating a necessary connection between ABCA1 and the immune-inflammation response (90). It should be mentioned that the infiltration of inflammatory cells and cholesterol accumulation observed in ABCA1 knockout mice appear to be restricted primarily to the macrophage, although the expression of ABCA1 is ubiquitous. Thus, it appears that the ABCA1-deficient macrophage may be a more sophistical tool to study the impact of ABCA1 on atherogenesis. This opinion is reinforced by studies from bone marrow transplantation experiments that transplantation of bone marrow from ABCA1-deficient mice into LDLR or apoE-deficient recipients causes an obvious increase in atherosclerosis (91,92), whereas the incidence of atherosclerosis observed in homozygous Tangier patients and ABCA1-deficient mice crossed with either hypercholesterolemic LDLR or apoE-deficient mice are not obviously increased (92). This moderately increased risk for atherosclerosis observed in Tangier patients and ABCA1-deficient mice may be partially due to their low plasma levels of apoB-containing lipoprotein (40–60% normal) (92,93). In addition, overexpression of macrophage ABCA1 by transplantation of bone marrow from human ABCA1 bacterial artificial chromosome (BAC) transgenic mice into LDLR knockout mice inhibits atherosclerotic lesion progression, further confirming the major role of macrophage ABCA1 in affecting atherogenesis (94). Moreover, macrophages isolated from ABCA1-deficient mice have an increased secretion of chemokines, growth factors and cytokines, resulting in an increased ability to respond to a variety of chemotactic factors (88). These changes, together with the proinflammatory condition present in ABCA1-deficient mice, may play a vital role in the development of atherosclerosis. Recently, macrophage ABCA1 has been definitively confirmed to be involved in the HDL-induced expressional downregulation of CD11b and the monocyte transmigration (95). In ABC transporter-deficient macrophages, inflammatory genes are significantly increased via TLR4- and MyD88/TRIF-mediated signal transduction (15). In addition, Pradel et al. (96) investigated the relationship between immunophenotype and ABCA1 expression in different monocyte subsets in the myeloid lineage. The result shows that ABCA1 expression is deficient in the inflammatory monocytes that are more apt to the proinflammatory effect induced by LPS/IFN-γ.
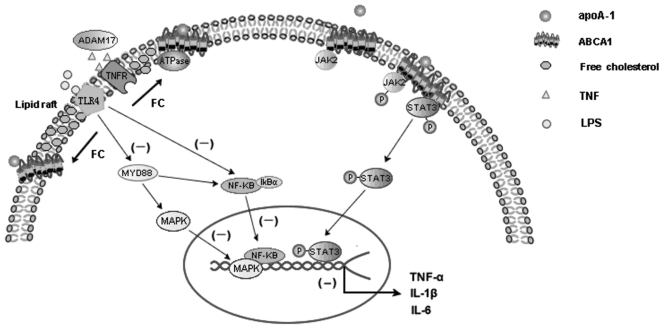
Although the precise mechanism that ABCA1 plays a key role in modulating inflammatory response remains to be elucidated, several studies have shown that the cholesterol export activity of ABCA1 could account for its potent antiinflammatory properties (16,87,95,97) (Figure 1). Zhu et al. showed that the hypersensitivity of ABCA1-deficient macrophages to LPS depended on subtle increases in cell membrane cholesterol and lipid raft content, suggesting an important role of ABCA1 in the regulation of innate immunity through modulation of plasma membrane cholesterol (16,87). Tellier et al. (97) showed purified apoA-I increased the shedding of TNF by ADAM17, a metalloprotease that cleaves TNF and its receptors (TNFR), which is dependent on the reduced lipid raft content in an ABCA1-dependent manner. Along with the fact that ABCA1 expression results in a significant redistribution of cholesterol and sphingomyelin from rafts to nonrafts through its ATPase-related function (98) (see Figure 1), these results unravel potential mechanisms by which ABCA1 decreases inflammation through modulating the lipid composition of plasma membrane lipid rafts. Recently, two candidate STAT3 docking sites in ABCA1 were found to be required for the apoA-I/ABCA1/JAK2 activation of STAT3, suggesting that the macrophage cholesterol exporter ABCA1 functions as a direct antiinflammatory receptor (14) (see Figure 1). These results implicate ABCA1 as a direct molecular link between the cardioprotective effects of cholesterol export from arterial macrophages and suppressed inflammation.
Figure 1.
The primary mechanisms through which ABCA1 has protective effects on macrophage inflammatory response induced by LPS and cytokines. In one way, the cholesterol export activity of ABCA1 could account for its potent antiinflammatory properties. ABCA1-mediated cholesterol efflux disrupts lipid raft membrane microdomains and results in a significant redistribution of cholesterol from rafts to nonrafts via its ATPase-related functions, which in turn activates the ADAM17-dependent cleaving of several transmembrane proteins, including TNF and TNF receptors (TNFR). Furthermore, ABCA1-mediated cholesterol efflux decreased cell membrane LPS-mediated inflammatory signal, such as Toll-like receptor 4 (TLR4), which inhibits the MyD88-mediated activation of MAPK, which then enhanced the expression of proinflammatory cytokines, revealing the suppression role of ABCA1 for LPS-mediated inflammatory signal pathways in macrophages. In another way, ABCA1 can function as a direct antiinflammatory receptor. The interaction of apoA-I with ABCA1-expressing macrophage dramatically increased phosphorylation of JAK2 and thus activates JAK2. The activated JAK2 further activates STAT3, which is independent of the lipid transport function of ABCA1. Two candidate STAT3 docking sites contained in ABCA1 are required for the apoA-I/ABCA1/JAK2 phosphorylation of STAT3. The activated STAT3 further suppressed the ability of LPS to induce the inflammatory cytokines in macrophages.
ATP-binding cassette transporter G1 (ABCG1), another ABC family member, also plays an important role in cholesterol efflux from macrophage foam cells (99). In vitro, ABCG1 induces cholesterol efflux from cholesterol-enriched macrophages to HDL particles, while ABCA1 promotes cholesterol efflux to lipid-poor apoA-1. Thus, ABCA1 and ABCG1 have complementary activity in mediating cholesterol efflux (100). In vivo, increased macrophage ABCG1 expression was significantly promoted, while knockout of macrophage ABCG1 expression significantly reduced macrophage RCT (101). In addition, combined deficiency of ABCA1 and ABCG1 promoted more accumulation of foam cells than individual KO and accelerated atherosclerosis in mice (102), suggesting that additive effects of ABCA1 and ABCG1 in mediating macrophage sterol efflux are central to the antiatherogenic properties of HDL.
In addition to its lipid transport properties, ABCG1 has played a crucial role in the antiinflammatory effects of HDL for its predominant effect in modulating the accumulation of prominent inflammatory macrophage foam cells in various tissues such as lung, liver, spleen or thymus (103–105). Yvan-Charvet et al. found that HDL and apoA-1 exert antiinflammatory effects by promoting cholesterol efflux via ABCG1 and ABCA1 with consequent attenuation of signaling via Toll-like receptors (15). Furthermore, ABCG1 seems to have a more potent antiinflammatory effect than ABCA1, which may be due to the predominant effect of ABCG1 in modulating the lipid composition of membrane lipid rafts (15). Nevertheless, ABCG1 promotes efflux of cholesterol to acceptors without increasing the binding of lipoproteins to cells (106), while ABCA1-mediated lipid efflux requires the direct binding of apolipoprotein to ABCA1, which can further activate the intracellular antiinflammation signaling pathways (14), suggesting some difference in antiinflammation mechanisms between ABCA1 and ABCG1.
Besides mediating macrophage inflammation related to vascular disease, ABCA1 may also play important roles in other chronic inflammatory diseases such as nonalcoholic fatty liver and diabetes mellitus. Specific inactivation of ABCA1 in β-cells of mice showed markedly impaired insulin secretion (107). Similar impairment is also present in patients with Tangier disease; glucose-stimulated insulin secretion from pancreatic β-cells is impaired with the ABCA1 mutation (108). These results indicate ABCA1 in pancreatic β-cells may function as a potent target for prevention and treatment of diabetes.
ABCA1 and Apoptosis
Innate immune responses, as an early warning sign in the immune system, is fine-tuned by specialized counterregulatory mechanisms. Apoptosis, especially in macrophages, is proposed to be involved in this progress (66). Several groups have demonstrated apoptotic cell death in atherosclerotic plaques (109,110). Although the significance of apoptosis in atherosclerosis depends on the stage of the plaque, sustained induction of apoptosis in advanced lesions seems to favor arterial wall inflammation and enhances recruitment of monocytes, leading to increased plaque burden (110). Several investigators have reported that oxidized low-density lipoprotein or free cholesterol, both of which are believed to be major lipid components of macrophages in advanced lesions, rapidly induces apoptosis in macrophages by different mechanisms, such as Fas- and caspase-9–dependent pathways (111–114). Accumulation of free cholesterol in the endoplasmic reticulum is also a likely cause of macrophage apoptosis, which leads to activation of the unfolded protein response and C/EBP homologous protein (CHOP)-induced apoptosis (112). Together with the fact that ABCA1 plays a key role in removing intracellular free cholesterol into vesicles that are translocated to plasma membrane for exocytosis (115), a hypothesis is suggested that there may be a unique role of ABCA1 in protecting free cholesterol loading, endoplasmic reticulum stress and oxidized lipid-mediated apoptosis.
Effective clearance of apoptotic debris by phagocytes is crucial to render the process immunologically silent. Defective clearance of the apoptotic cell in advanced lesions has been found to favor arterial wall inflammation (110). Phosphatidylserine, an anionic phospholipid that resides in the inner leaflet, is exposed on the surface of apoptotic cells, a process considered as an “eat me” signal that is necessary to guarantee recognition by the phagocyte (116). ABCA1 was found to be involved in the efficient exposure of phosphatidylserine on apoptotic somatic cells (116). Syntaxins belong to the family of the N-ethylmaleimide (NEM)- sensitive factor attachment protein receptor and play a role in vesicular transport and membrane fusion. ABCA1 interacts with syntaxin 13 and flotillin-1 in Lubrol WX–insoluble raft microdomains in macrophages and participates in the formation of phagosome (117), revealing that ABCA1 is involved in the endocytic processes. Therefore, ABCA1 may promote the clearance of the apoptotic cell via both the phosphatidylserine signal recognition and the transmembrane receptor–related endocytic function.
ABCG1 was also found to be involved in macrophage apoptosis processes. Baldan et al. found transplantation of ABCG1−/− bone marrow cells into Ldlr−/−or apoE−/− mice resulted in increased numbers of apoptotic cells in atherosclerotic lesions (118). Terasaka et al. found that ABCG1 protects macrophages from oxysterol-induced apoptosis by promoting efflux of 7-ketocholesterol, the major oxysterol present in oxidized LDL and atherosclerotic lesions (119). In advanced lesions, apoptosis of macrophages and other cells may cause increased inflammation and destabilization of atherosclerotic plaques (120). Thus, these results indicate that efflux of oxysterol via macrophage ABCG1 has a protective role in inflammation and advanced atherosclerotic plaques.
ABCA1 in Formation and Function of HDL During Inflammation
The established cardioprotective mechanism of HDL is based on RCT; however, much interest has recently arisen from the antiinflammatory activity of HDL. HDL might protect, at least in part, against the development of atherosclerosis by limiting the effects of a potentially harmful inflammatory response in the vascular wall (121,122). ABCA1 plays a critical role for the biogenesis of HDL. In ABCA1-deficient mice, the absence of ABCA1 results in almost undetectable levels of HDL (21), revealing ABCA1’s essential role in the formation of newly synthesized HDL. The synthesis of HDL requires at least two well-defined steps. In the first step, apoA-I or other apolipoproteins are lipidated through the actions of ABCA1. This process leads to the formation of lipid-poor, nascent HDL particles, also known as pre–β-HDLs. These nascent HDLs are transformed into large-size HDL particles with α-mobility by lecithin:cholesterol acyltransferase (21). However, both the plasma levels and structure of HDL can be significantly altered during the acute and chronic inflammation (123). Acute-phase HDL is characteristically decreased of apoA-I content, whereas serum amyloid A (SAA), an acute-phase response protein, is markedly increased and becomes the major apolipoprotein of HDL during inflammation (124). In vitro, SAA induces cellular lipid release in a similar manner to apoA-I, which requires ABCA1 or ABCA7 in HEK293 cells (125). Although several studies have shown that SAA can remove lipid from cells by ABCA1-independent mechanisms, such as via scavenger receptor B-I (SR-BI) (125–127), it has been suggested that ABCA1-mediated lipidation status of SAA is the major factor governing cholesterol acceptor properties of SAA. Only overexpression of SR-BI in HEK-293 cells devoid of ABCA1 fails to mobilize cholesterol to lipid-free SAA, whereas efficient cholesterol efflux by SAA is observed from fibroblasts and CHO cells both expressing functional ABCA1 (128), indicating ABCA1 is necessary for SAA-mediated cellular cholesterol efflux during inflammation. The direct evidence that ABCA1 is a crucial mediator for SAA-containing HDL biogenesis upon acute inflammation in vivo has been described by Hu et al., who found that HDL is not generated in LPS-treated ABCA1-KO mice, despite SAA being induced by acute inflammation (129). This indicates the same essential role for ABCA1 in mediating the SAA-mediated formation of newly synthesized HDL during inflammation.
ABCA1 Gene Variants and Inflammation
Recent genetic studies have shown that loss-of-function mutations in ABCA1 lead to the allelic disorders Tangier disease and familial hypoalphalipoproteinemia as well as increasing cardiovascular disease risk (130,131). Until now, at least 70 mutations have been identified in the ABCA1 gene. However, most studies focus on how genetic alterations of the ABCA1 gene highlight its role in lipid homeostasis (132). Oram and colleagues found the two intracellular loops of ABCA1 each contain a tetrameric amino acid motif (YXXQ), which might have potential effects on immune function (14). Mutating these sites completely abolished the ability of apoA-I to activate STAT3 without affecting the cholesterol export function of ABCA1, while activated STAT3 in cultured macrophages has been found to abolish LPS-induced inflammatory cytokine production (133), indicating that specific amino acids in the intracellular loops may be responsible for the ABCA1-mediated antiinflammatory function (14). Nevertheless, further clinical genetic studies need to be performed to confirm the role of these alleles in inflammation and immune regulation.
SUMMARY AND CONCLUSIONS
Over the past decade, the relationship between RCT and atherosclerosis has been well studied in animals and humans, and many of the molecular components that mediate the RCT have been elucidated. ABCA1 is a key mediator of cholesterol efflux from cholesterol-loaded macrophages to apoA-I and pre–β-HDL, which is also the most concerned aspect for its antiatherosclerotic effects. Many timely reviews have discussed such effects and their implications for cardiovascular disease protection (134–137). Recent studies indicated inflammation-impaired RCT, which provided a new direction to illuminate the molecular mechanism underlying the role of inflammation in the development of atherosclerosis. Although the molecular connection between inflammation and RCT is not completely known, studies with animal inflammation models and cells stimulated by inflammatory cytokines and proteins revealed that the expression of ABCA1 and cholesterol efflux can be decreased by inflammatory mediators in vitro and inflammatory process in vivo, indicating that ABCA1 may be a potential modulating target in the impaired RCT proceedings during inflammation. Interestingly, ABCA1, in turn, has the antiinflammatory effects that provide new evidence that ABCA1 protects against cardiovascular disease by mechanisms extending well beyond its involvement in cholesterol transport. This effect may also contribute to the novel role of ABCA1 agonists such as PPAR and LXR in the treatment and control of chronic inflammation diseases such as nonalcoholic fatty liver disease, thrombocytopenic purpura and inflammatory diseases of the kidney. However, less attention has been given to this novel function of ABCA1 as well as the factors affecting the mechanical behavior of this transporter. The finding that ABCA1 inhibits inflammatory response, in part because of its function in modulating the fluidity of the plasma membrane as well lipid raft formation, indicates that the antiinflammatory effect of ABCA1 may be secondary to its ability to export lipids from cells, which also indicates complex interactions between the lipid dysfunction and inflammation response and that ABCA1 may be a direct link between the lipid transport and immune-inflammatory response. In the future, a great effort should be directed toward the identification of the major determinants of this interplay’s response, and special attention should be given to the signaling pathways responsible for the antiinflammatory function of ABCA.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the financial support from the National Natural Sciences Foundation of China (30470720), Post-doctor Sciences Foundation of China (2005037157), and The Heng Yang Joint Funds of The Hunan Provincial Natural Sciences Foundation of China (10JJ9019).
Footnotes
DISCLOSURE
The authors declare that they have no competing interests as defined by Molecular Medicine, or other interests that might be perceived to influence the results and discussion reported in this paper.
Online address: http://www.molmed.org
REFERENCES
- 1.Shimada K. Immune system and atherosclerotic disease: heterogeneity of leukocyte subsets participating in the pathogenesis of atherosclerosis. Circ. J. 2009;73:994–1001. doi: 10.1253/circj.cj-09-0277. [DOI] [PubMed] [Google Scholar]
- 2.Tiwari RL, Singh V, Barthwal MK. Macrophages: an elusive yet emerging therapeutic target of atherosclerosis. Med. Res. Rev. 2008;28:483–544. doi: 10.1002/med.20118. [DOI] [PubMed] [Google Scholar]
- 3.Gori AM, et al. The balance between pro-and antiinflammatory cytokines is associated with platelet aggregability in acute coronary syndrome patients. Atherosclerosis. 2009;202:255–62. doi: 10.1016/j.atherosclerosis.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 4.Kleemann R, Zadelaar S, Kooistra T. Cytokines and atherosclerosis: a comprehensive review of studies in mice. Cardiovasc. Res. 2008;79:360–376. doi: 10.1093/cvr/cvn120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alizadeh Dehnavi R, et al. Elevated CRP levels are associated with increased carotid atherosclerosis independent of visceral obesity. Atherosclerosis. 2008;200:417–23. doi: 10.1016/j.atherosclerosis.2007.12.050. [DOI] [PubMed] [Google Scholar]
- 6.Rader DJ, Alexander ET, Weibel GL, Billheimer J, Rothblat GH. The role of reverse cholesterol transport in animals and humans and relationship to atherosclerosis. J. Lipid Res. 2009;50(Suppl):S189–94. doi: 10.1194/jlr.R800088-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang CK, et al. Effect of apolipoprotein A-I on ATP binding cassette transporter A1 degradation and cholesterol efflux in THP-1 macrophage-derived foam cells. Acta. Biochim. Biophys. Sin. (Shanghai) 2004;36:218–26. doi: 10.1093/abbs/36.3.218. [DOI] [PubMed] [Google Scholar]
- 8.McGillicuddy FC, et al. Inflammation impairs reverse cholesterol transport in vivo. Circulation. 2009;119:1135–45. doi: 10.1161/CIRCULATIONAHA.108.810721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmidt WM, Spiel AO, Jilma B, Wolzt M, Muller M. In-vivo effects of simvastatin and rosuvastatin on global gene expression in peripheral blood leucocytes in a human inflammation model. Pharmacogenet. Genomics. 2008;18:109–20. doi: 10.1097/FPC.0b013e3282f44d81. [DOI] [PubMed] [Google Scholar]
- 10.Mei CL, et al. Interleukin-10 inhibits the down-regulation of ATP binding cassette transporter A1 by tumour necrosis factor-alpha in THP-1 macrophage-derived foam cells. Cell. Biol. Int. 2007;31:1456–61. doi: 10.1016/j.cellbi.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 11.Zhao SP, Dong SZ. Effect of tumor necrosis factor alpha on cholesterol efflux in adipocytes. Clin. Chim. Acta. 2008;389:67–71. doi: 10.1016/j.cca.2007.11.025. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y, Moser AH, Shigenaga JK, Grunfeld C, Feingold KR. Downregulation of liver X receptor-alpha in mouse kidney and HK-2 proximal tubular cells by LPS and cytokines. J. Lipid Res. 2005;46:2377–87. doi: 10.1194/jlr.M500134-JLR200. [DOI] [PubMed] [Google Scholar]
- 13.Baranova I, et al. Lipopolysaccharide down regulates both scavenger receptor B1 and ATP binding cassette transporter A1 in RAW cells. Infect. Immun. 2002;70:2995–3003. doi: 10.1128/IAI.70.6.2995-3003.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang C, Liu Y, Kessler PS, Vaughan AM, Oram JF. The macrophage cholesterol exporter ABCA1 functions as an antiinflammatory receptor. J. Biol. Chem. 2009;284:32336–43. doi: 10.1074/jbc.M109.047472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yvan-Charvet L, et al. Increased inflammatory gene expression in ABC transporter-deficient macrophages: free cholesterol accumulation, increased signaling via toll-like receptors, and neutrophil infiltration of atherosclerotic lesions. Circulation. 2008;118:1837–47. doi: 10.1161/CIRCULATIONAHA.108.793869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koseki M, et al. Increased lipid rafts and accelerated lipopolysaccharide-induced tumor necrosis factor-alpha secretion in Abca1-deficient macrophages. J. Lipid Res. 2007;48:299–306. doi: 10.1194/jlr.M600428-JLR200. [DOI] [PubMed] [Google Scholar]
- 17.Hollenstein K, Dawson RJ, Locher KP. Structure and mechanism of ABC transporter proteins. Curr. Opin. Struct. Biol. 2007;17:412–8. doi: 10.1016/j.sbi.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 18.Tang C, Oram JF. The cell cholesterol exporter ABCA1 as a protector from cardiovascular disease and diabetes. Biochim. Biophys. Acta. 2009;1791:563–72. doi: 10.1016/j.bbalip.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 19.Schmitz G, et al. ATP-binding cassette transporter A1 (ABCA1) in macrophages: a dual function in inflammation and lipid metabolism? Pathobiology. 1999;67:236–40. doi: 10.1159/000028100. [DOI] [PubMed] [Google Scholar]
- 20.Langmann T, et al. Molecular cloning of the human ATP-binding cassette transporter 1 (hABC1): evidence for sterol-dependent regulation in macrophages. Biochem. Biophys. Res. Commun. 1999;257:29–33. doi: 10.1006/bbrc.1999.0406. [DOI] [PubMed] [Google Scholar]
- 21.Aiello RJ, Brees D, Francone OL. ABCA1-deficient mice: insights into the role of monocyte lipid efflux in HDL formation and inflammation. Arterioscler. Thromb. Vasc. Biol. 2003;23:972–80. doi: 10.1161/01.ATV.0000054661.21499.FB. [DOI] [PubMed] [Google Scholar]
- 22.Francone OL, Aiello RJ. ABCA1: regulation, function and relationship to atherosclerosis. Curr. Opin. Investig. Drugs. 2002;3:415–9. [PubMed] [Google Scholar]
- 23.Soumian S, Albrecht C, Davies AH, Gibbs RG. ABCA1 and atherosclerosis. Vasc. Med. 2005;10:109–19. doi: 10.1191/1358863x05vm593ra. [DOI] [PubMed] [Google Scholar]
- 24.Lawn RM, et al. The Tangier disease gene product ABC1 controls the cellular apolipoprotein-mediated lipid removal pathway. J. Clin. Invest. 1999;104:R25–31. doi: 10.1172/JCI8119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Majdalawieh A, Ro HS. LPS-induced suppression of macrophage cholesterol efflux is mediated by adipocyte enhancer-binding protein 1. Int. J. Biochem. Cell Biol. 2009;41:1518–25. doi: 10.1016/j.biocel.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 26.Hu YW, et al. Eicosapentaenoic acid reduces ABCA1 serine phosphorylation and impairs ABCA1-dependent cholesterol efflux through cyclic AMP/protein kinase A signaling pathway in THP-1 macrophage-derived foam cells. Atherosclerosis. 2009;204:e35–43. doi: 10.1016/j.atherosclerosis.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 27.Zhang C, et al. NO-1886 upregulates ATP binding cassette transporter A1 and inhibits diet-induced atherosclerosis in Chinese Bama minipigs. J. Lipid Res. 2006;47:2055–63. doi: 10.1194/jlr.M600226-JLR200. [DOI] [PubMed] [Google Scholar]
- 28.Judkins CP, et al. Direct evidence of a role for Nox2 in superoxide production, reduced nitric oxide bioavailability, and early atherosclerotic plaque formation in ApoE−/− mice. Am. J. Physiol. Heart Circ. Physiol. 2010;298:H24–32. doi: 10.1152/ajpheart.00799.2009. [DOI] [PubMed] [Google Scholar]
- 29.DePalma RG, et al. Cytokine signatures in atherosclerotic claudicants. J. Surg. Res. 2003;111:215–21. doi: 10.1016/s0022-4804(03)00075-1. [DOI] [PubMed] [Google Scholar]
- 30.Alfaro Leon ML, Evans GF, Farmen MW, Zuckerman SH. Post-transcriptional regulation of macrophage ABCA1, an early response gene to IFN-gamma. Biochem. Biophys. Res. Commun. 2005;333:596–602. doi: 10.1016/j.bbrc.2005.05.112. [DOI] [PubMed] [Google Scholar]
- 31.Hao XR, et al. IFN-gamma down-regulates ABCA1 expression by inhibiting LXRalpha in a JAK/STAT signaling pathway-dependent manner. Atherosclerosis. 2009;203:417–28. doi: 10.1016/j.atherosclerosis.2008.07.029. [DOI] [PubMed] [Google Scholar]
- 32.Chen M, Li W, Wang N, Zhu Y, Wang X. ROS and NF-kappaB but not LXR mediate IL-1beta signaling for the downregulation of ATP-binding cassette transporter A1. Am. J. Physiol. Cell Physiol. 2007;292:C1493–1501. doi: 10.1152/ajpcell.00016.2006. [DOI] [PubMed] [Google Scholar]
- 33.Zhang GJ, Li H, Li XW. Effects of rapamycin on intracellular cholesterol homeostasis of glomerular mesangial cell in the presence of interleukin-1 beta. Chin. Med. Sci. J. 2008;23:205–11. [PubMed] [Google Scholar]
- 34.Ma KL, Ruan XZ, Powis SH, Moorhead JF, Varghese Z. Anti-atherosclerotic effects of sirolimus on human vascular smooth muscle cells. Am. J. Physiol. Heart Circ. Physiol. 2007;292:H2721–8. doi: 10.1152/ajpheart.01174.2006. [DOI] [PubMed] [Google Scholar]
- 35.Ma KL, et al. Inflammatory stress exacerbates lipid accumulation in hepatic cells and fatty livers of apolipoprotein E knockout mice. Hepatology. 2008;48:770–81. doi: 10.1002/hep.22423. [DOI] [PubMed] [Google Scholar]
- 36.Nagao S, et al. Platelet derived growth factor regulates ABCA1 expression in vascular smooth muscle cells. FEBS Lett. 2006;580:4371–6. doi: 10.1016/j.febslet.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 37.Rubic T, Lorenz RL. Downregulated CD36 and oxLDL uptake and stimulated ABCA1/G1 and cholesterol efflux as anti-atherosclerotic mechanisms of interleukin-10. Cardiovasc. Res. 2006;69:527–35. doi: 10.1016/j.cardiores.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 38.Han X, Kitamoto S, Lian Q, Boisvert WA. Interleukin-10 facilitates both cholesterol uptake and efflux in macrophages. J. Biol. Chem. 2009;284:32950–8. doi: 10.1074/jbc.M109.040899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Argmann CA, et al. Transforming growth factor-beta1 inhibits macrophage cholesteryl ester accumulation induced by native and oxidized VLDL remnants. Arterioscler. Thromb. Vasc. Biol. 2001;21:2011–8. doi: 10.1161/hq1201.099426. [DOI] [PubMed] [Google Scholar]
- 40.Panousis CG, Evans G, Zuckerman SH. TGF-beta increases cholesterol efflux and ABC-1 expression in macrophage-derived foam cells: opposing the effects of IFN-gamma. J. Lipid Res. 2001;42:856–63. [PubMed] [Google Scholar]
- 41.Schreyer SA, Peschon JJ, LeBoeuf RC. Accelerated atherosclerosis in mice lacking tumor necrosis factor receptor p55. J Biol. Chem. 1996;271:26174–8. doi: 10.1074/jbc.271.42.26174. [DOI] [PubMed] [Google Scholar]
- 42.Wang YF, et al. Protective effect of Astragalus polysaccharides on ATP binding cassette transporter A1 in THP-1 derived foam cells exposed to tumor necrosis factor-alpha. Phytother. Res. 2010;24:393–8. doi: 10.1002/ptr.2958. [DOI] [PubMed] [Google Scholar]
- 43.Gerbod-Giannone MC, et al. TNFalpha induces ABCA1 through NF-kappaB in macrophages and in phagocytes ingesting apoptotic cells. Proc. Natl. Acad. Sci. U. S. A. 2006;103:3112–7. doi: 10.1073/pnas.0510345103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kazawa T, et al. Expression of liver X receptor alpha and lipid metabolism in granulocyte-macrophage colony-stimulating factor-induced human monocyte-derived macrophage. Pathol. Int. 2009;59:152–60. doi: 10.1111/j.1440-1827.2009.02343.x. [DOI] [PubMed] [Google Scholar]
- 45.Ditiatkovski M, Toh BH, Bobik A. GM-CSF deficiency reduces macrophage PPAR-gamma expression and aggravates atherosclerosis in ApoE-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2006;26:2337–44. doi: 10.1161/01.ATV.0000238357.60338.90. [DOI] [PubMed] [Google Scholar]
- 46.Thomassen MJ, et al. ABCG1 is deficient in alveolar macrophages of GM-CSF knockout mice and patients with pulmonary alveolar proteinosis. J. Lipid Res. 2007;48:2762–8. doi: 10.1194/jlr.P700022-JLR200. [DOI] [PubMed] [Google Scholar]
- 47.Wang X, et al. C-reactive protein inhibits cholesterol efflux from human macrophage-derived foam cells. Arterioscler. Thromb. Vasc. Biol. 2008;28:519–26. doi: 10.1161/ATVBAHA.107.159467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nordestgaard BG, Zacho J. Lipids, atherosclerosis and CVD risk: is CRP an innocent bystander? Nutr. Metab. Cardiovasc. Dis. 2009;19:521–4. doi: 10.1016/j.numecd.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 49.Hanriot D, et al. C-reactive protein induces pro- and anti-inflammatory effects, including activation of the liver X receptor alpha, on human monocytes. Thromb Haemost. 2008;99:558–69. doi: 10.1160/TH07-06-0410. [DOI] [PubMed] [Google Scholar]
- 50.Filep JG. Perplexity of monocyte responses to C-reactive protein (CRP) Thromb. Haemost. 2008;99:461–2. doi: 10.1160/TH08-02-0072. [DOI] [PubMed] [Google Scholar]
- 51.Maury E, Brichard SM. Adipokine dysregulation, adipose tissue inflammation and metabolic syndrome. Mol. Cell. Endocrinol. 2010;314:1–16. doi: 10.1016/j.mce.2009.07.031. [DOI] [PubMed] [Google Scholar]
- 52.Tian L, et al. Adiponectin reduces lipid accumulation in macrophage foam cells. Atherosclerosis. 2009;202:152–61. doi: 10.1016/j.atherosclerosis.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tsubakio-Yamamoto K, et al. Adiponectin prevents atherosclerosis by increasing cholesterol efflux from macrophages. Biochem. Biophys. Res. Commun. 2008;375:390–4. doi: 10.1016/j.bbrc.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 54.Oku H, et al. Adiponectin deficiency suppresses ABCA1 expression and ApoA-I synthesis in the liver. FEBS Lett. 2007;581:5029–33. doi: 10.1016/j.febslet.2007.09.038. [DOI] [PubMed] [Google Scholar]
- 55.Baber SR, Champion HC, Bivalacqua TJ, Hyman AL, Kadowitz PJ. Role of cyclooxygenase-2 in the generation of vasoactive prostanoids in the rat pulmonary and systemic vascular beds. Circulation. 2003;108:896–901. doi: 10.1161/01.CIR.0000084536.87322.BB. [DOI] [PubMed] [Google Scholar]
- 56.Reiss AB, Anwar F, Chan ES, Anwar K. Disruption of cholesterol efflux by coxib medications and inflammatory processes: link to increased cardiovascular risk. J. Investig. Med. 2009;57:695–702. doi: 10.2310/JIM.0b013e31819ec3c7. [DOI] [PubMed] [Google Scholar]
- 57.Chan ES, et al. Effect of cyclooxygenase inhibition on cholesterol efflux proteins and atheromatous foam cell transformation in THP-1 human macrophages: a possible mechanism for increased cardiovascular risk. Arthritis Res. Ther. 2007;9:R4. doi: 10.1186/ar2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reiss AB, et al. Atheroprotective effects of methotrexate on reverse cholesterol transport proteins and foam cell transformation in human THP-1 monocyte/macrophages. Arthritis Rheum. 2008;58:3675–83. doi: 10.1002/art.24040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ronald JA, et al. Enzyme-sensitive magnetic resonance imaging targeting myeloperoxidase identifies active inflammation in experimental rabbit atherosclerotic plaques. Circulation. 2009;120:592–9. doi: 10.1161/CIRCULATIONAHA.108.813998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bergt C, et al. The myeloperoxidase product hypochlorous acid oxidizes HDL in the human artery wall and impairs ABCA1-dependent cholesterol transport. Proc. Natl. Acad. Sci. U. S. A. 2004;101:13032–7. doi: 10.1073/pnas.0405292101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shao B, et al. Myeloperoxidase impairs ABCA1-dependent cholesterol efflux through methionine oxidation and site-specific tyrosine chlorination of apolipoprotein A-I. J. Biol. Chem. 2006;281:9001–4. doi: 10.1074/jbc.C600011200. [DOI] [PubMed] [Google Scholar]
- 62.Anderson MM, Requena JR, Crowley JR, Thorpe SR, Heinecke JW. The myeloperoxidase system of human phagocytes generates Nepsilon-(carboxymethyl)lysine on proteins: a mechanism for producing advanced glycation end products at sites of inflammation. J. Clin. Invest. 1999;104:103–13. doi: 10.1172/JCI3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Passarelli M, et al. Advanced glycation end product precursors impair ABCA1-dependent cholesterol removal from cells. Diabetes. 2005;54:2198–205. doi: 10.2337/diabetes.54.7.2198. [DOI] [PubMed] [Google Scholar]
- 64.Letavernier E, et al. Targeting the calpain/calpastatin system as a new strategy to prevent cardiovascular remodeling in angiotensin II-induced hypertension. Circ. Res. 2008;102:720–28. doi: 10.1161/CIRCRESAHA.107.160077. [DOI] [PubMed] [Google Scholar]
- 65.Wang N, et al. A PEST sequence in ABCA1 regulates degradation by calpain protease and stabilization of ABCA1 by apoA-I. J. Clin. Invest. 2003;111:99–107. doi: 10.1172/JCI16808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Iwamoto N, Lu R, Tanaka N, Abe-Dohmae S, Yokoyama S. Calmodulin interacts with ATP binding cassette transporter A1 to protect from calpain-mediated degradation and upregulates high-density lipoprotein generation. Arterioscler. Thromb. Vasc. Biol. 2010;30:1446–52. doi: 10.1161/ATVBAHA.110.203927. [DOI] [PubMed] [Google Scholar]
- 67.Arakawa R, et al. Pharmacological inhibition of ABCA1 degradation increases HDL biogenesis and exhibits antiatherogenesis. J. Lipid Res. 2009;50:2299–2305. doi: 10.1194/jlr.M900122-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jackson SK, Abate W, Tonks AJ. Lysophospholipid acyltransferases: novel potential regulators of the inflammatory response and target for new drug discovery. Pharmacol. Ther. 2008;119:104–14. doi: 10.1016/j.pharmthera.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 69.Hou M, et al. Lysophosphatidylcholine promotes cholesterol efflux from mouse macrophage foam cells via PPARgamma-LXRalpha-ABCA1-dependent pathway associated with apoE. Cell Biochem. Funct. 2007;25:33–44. doi: 10.1002/cbf.1374. [DOI] [PubMed] [Google Scholar]
- 70.Rosenblat M, Vaya J, Shih D, Aviram M. Paraoxonase 1 (PON1) enhances HDL-mediated macrophage cholesterol efflux via the ABCA1 transporter in association with increased HDL binding to the cells: a possible role for lysophosphatidylcholine. Atherosclerosis. 2005;179:69–77. doi: 10.1016/j.atherosclerosis.2004.10.028. [DOI] [PubMed] [Google Scholar]
- 71.Jeon SG, et al. 15-Lipoxygenase metabolites play an important role in the development of a T-helper type 1 allergic inflammation induced by double-stranded RNA. Clin. Exp. Allergy. 2009;39:908–17. doi: 10.1111/j.1365-2222.2009.03211.x. [DOI] [PubMed] [Google Scholar]
- 72.Montero A, Badr KF. 15-Lipoxygenase in glomerular inflammation. Exp. Nephrol. 2000;8:14–9. doi: 10.1159/000020643. [DOI] [PubMed] [Google Scholar]
- 73.Pirillo A, Uboldi P, Kuhn H, Catapano AL. 15-Lipoxygenase-mediated modification of high-density lipoproteins impairs SR-BI- and ABCA1-dependent cholesterol efflux from macrophages. Biochim. Biophys. Acta. 2006;1761:292–300. doi: 10.1016/j.bbalip.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 74.Fery F, Paquot N. Etiopathogenesis and pathophysiology of type 2 diabetes [in French] Rev Med Liege. 2005;60:361–8. [PubMed] [Google Scholar]
- 75.Sacks FM, Campos H. Polyunsaturated fatty acids, inflammation, and cardiovascular disease: time to widen our view of the mechanisms. J. Clin. Endocrinol. Metab. 2006;91:398–400. doi: 10.1210/jc.2005-2459. [DOI] [PubMed] [Google Scholar]
- 76.Brown JM, et al. Combined therapy of dietary fish oil and stearoyl-CoA desaturase 1 inhibition prevents the metabolic syndrome and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 30:24–30. doi: 10.1161/ATVBAHA.109.198036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sun Y, et al. Stearoyl-CoA desaturase inhibits ATP-binding cassette transporter A1-mediated cholesterol efflux and modulates membrane domain structure. J. Biol. Chem. 2003;278:5813–20. doi: 10.1074/jbc.M208687200. [DOI] [PubMed] [Google Scholar]
- 78.Wang Y, Kurdi-Haidar B, Oram JF. LXR-mediated activation of macrophage stearoyl-CoA desaturase generates unsaturated fatty acids that destabilize ABCA1. J. Lipid Res. 2004;45:972–80. doi: 10.1194/jlr.M400011-JLR200. [DOI] [PubMed] [Google Scholar]
- 79.Mauerer R, Ebert S, Langmann T. High glucose, unsaturated and saturated fatty acids differentially regulate expression of ATP-binding cassette transporters ABCA1 and ABCG1 in human macrophages. Exp. Mol. Med. 2009;41:126–32. doi: 10.3858/emm.2009.41.2.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mattson FH, Grundy SM. Comparison of effects of dietary saturated, monounsaturated, and polyunsaturated fatty acids on plasma lipids and lipoproteins in man. J. Lipid Res. 1985;26:194–202. [PubMed] [Google Scholar]
- 81.Gustafson B, Hammarstedt A, Andersson CX, Smith U. Inflamed adipose tissue: a culprit underlying the metabolic syndrome and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2007;27:2276–83. doi: 10.1161/ATVBAHA.107.147835. [DOI] [PubMed] [Google Scholar]
- 82.Zechner R, Kienesberger PC, Haemmerle G, Zimmermann R, Lass A. Adipose triglyceride lipase and the lipolytic catabolism of cellular fat stores. J. Lipid Res. 2009;50:3–21. doi: 10.1194/jlr.R800031-JLR200. [DOI] [PubMed] [Google Scholar]
- 83.Lu B, Moser AH, Shigenaga JK, Feingold KR, Grunfeld C. Type II nuclear hormone receptors, coactivator, and target gene repression in adipose tissue in the acute-phase response. J. Lipid Res. 2006;47:2179–90. doi: 10.1194/jlr.M500540-JLR200. [DOI] [PubMed] [Google Scholar]
- 84.Castrillo A, et al. Crosstalk between LXR and toll-like receptor signaling mediates bacterial and viral antagonism of cholesterol metabolism. Mol Cell. 2003;12:805–16. doi: 10.1016/s1097-2765(03)00384-8. [DOI] [PubMed] [Google Scholar]
- 85.Khovidhunkit W, Moser AH, Shigenaga JK, Grunfeld C, Feingold KR. Endotoxin down-regulates ABCG5 and ABCG8 in mouse liver and ABCA1 and ABCG1 in J774 murine macrophages: differential role of LXR. J. Lipid Res. 2003;44:1728–36. doi: 10.1194/jlr.M300100-JLR200. [DOI] [PubMed] [Google Scholar]
- 86.Maitra U, Parks JS, Li L. An innate immunity signaling process suppresses macrophage ABCA1 expression through IRAK-1-mediated downregulation of retinoic acid receptor alpha and NFATc2. Mol. Cell. Biol. 2009;29:5989–97. doi: 10.1128/MCB.00541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhu X, et al. Increased cellular free cholesterol in macrophage-specific Abca1 knock-out mice enhances pro-inflammatory response of macrophages. J. Biol. Chem. 2008;283:22930–41. doi: 10.1074/jbc.M801408200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Francone OL, et al. Increased cholesterol deposition, expression of scavenger receptors, and response to chemotactic factors in Abca1-deficient macrophages. Arterioscler. Thromb. Vasc. Biol. 2005;25:1198–1205. doi: 10.1161/01.ATV.0000166522.69552.99. [DOI] [PubMed] [Google Scholar]
- 89.McNeish J, et al. High density lipoprotein deficiency and foam cell accumulation in mice with targeted disruption of ATP-binding cassette transporter-1. Proc. Natl. Acad. Sci. U. S. A. 2000;97:4245–50. doi: 10.1073/pnas.97.8.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Christiansen-Weber TA, et al. Functional loss of ABCA1 in mice causes severe placental malformation, aberrant lipid distribution, and kidney glomerulonephritis as well as high-density lipoprotein cholesterol deficiency. Am. J. Pathol. 2000;157:1017–29. doi: 10.1016/S0002-9440(10)64614-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.van Eck M, et al. Leukocyte ABCA1 controls susceptibility to atherosclerosis and macrophage recruitment into tissues. Proc. Natl. Acad. Sci. U. S. A. 2002;99:6298–6303. doi: 10.1073/pnas.092327399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Aiello RJ, et al. Increased atherosclerosis in hyperlipidemic mice with inactivation of ABCA1 in macrophages. Arterioscler. Thromb. Vasc. Biol. 2002;22:630–7. doi: 10.1161/01.atv.0000014804.35824.da. [DOI] [PubMed] [Google Scholar]
- 93.Serfaty-Lacrosniere C, et al. Homozygous Tangier disease and cardiovascular disease. Atherosclerosis. 1994;107:85–98. doi: 10.1016/0021-9150(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 94.Van Eck M, et al. Macrophage ATP-binding cassette transporter A1 overexpression inhibits atherosclerotic lesion progression in low-density lipoprotein receptor knockout mice. Arterioscler. Thromb. Vasc. Biol. 2006;26:929–34. doi: 10.1161/01.ATV.0000208364.22732.16. [DOI] [PubMed] [Google Scholar]
- 95.Murphy AJ, et al. High-density lipoprotein reduces the human monocyte inflammatory response. Arterioscler. Thromb. Vasc. Biol. 2008;28:2071–77. doi: 10.1161/ATVBAHA.108.168690. [DOI] [PubMed] [Google Scholar]
- 96.Pradel LC, et al. ATP-binding cassette transporter hallmarks tissue macrophages and modulates cytokine- triggered polarization programs. Eur. J. Immunol. 2009;39:2270–80. doi: 10.1002/eji.200838867. [DOI] [PubMed] [Google Scholar]
- 97.Tellier E, et al. HDLs activate ADAM17-dependent shedding. J. Cell Physiol. 2008;214:687–93. doi: 10.1002/jcp.21265. [DOI] [PubMed] [Google Scholar]
- 98.Landry YD, Denis M, Nandi S, Bell S, Vaughan AM, Zha X. ATP-binding cassette transporter A1 expression disrupts raft membrane microdomains through its ATPase-related functions. J. Biol. Chem. 2006;281:36091–101. doi: 10.1074/jbc.M602247200. [DOI] [PubMed] [Google Scholar]
- 99.Jessup W, Gelissen IC, Gaus K, Kritharides L. Roles of ATP binding cassette transporters A1 and G1, scavenger receptor BI and membrane lipid domains in cholesterol export from macrophages. Curr. Opin. Lipidol. 2006;17:247–57. doi: 10.1097/01.mol.0000226116.35555.eb. [DOI] [PubMed] [Google Scholar]
- 100.Adorni MP, et al. The roles of different pathways in the release of cholesterol from macrophages. J. Lipid Res. 2007;48:2453–62. doi: 10.1194/jlr.M700274-JLR200. [DOI] [PubMed] [Google Scholar]
- 101.Wang X, et al. Macrophage ABCA1 and ABCG1, but not SR-BI, promote macrophage reverse cholesterol transport in vivo. J. Clin. Invest. 2007;117:2216–24. doi: 10.1172/JCI32057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yvan-Charvet L, et al. Combined deficiency of ABCA1 and ABCG1 promotes foam cell accumulation and accelerates atherosclerosis in mice. J. Clin. Invest. 2007;117:3900–8. doi: 10.1172/JCI33372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wojcik AJ, Skaflen MD, Srinivasan S, Hedrick CC. A critical role for ABCG1 in macrophage inflammation and lung homeostasis. J. Immunol. 2008;180:4273–82. doi: 10.4049/jimmunol.180.6.4273. [DOI] [PubMed] [Google Scholar]
- 104.Baldan A, Gomes AV, Ping P, Edwards PA. Loss of ABCG1 results in chronic pulmonary inflammation. J. Immunol. 2008;180:3560–68. doi: 10.4049/jimmunol.180.5.3560. [DOI] [PubMed] [Google Scholar]
- 105.Kennedy MA, et al. ABCG1 has a critical role in mediating cholesterol efflux to HDL and preventing cellular lipid accumulation. Cell Metab. 2005;1:121–31. doi: 10.1016/j.cmet.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 106.Wang N, Lan D, Chen W, Matsuura F, Tall AR. ATP-binding cassette transporters G1 and G4 mediate cellular cholesterol efflux to high-density lipoproteins. Proc. Natl. Acad. Sci. U. S. A. 2004;101:9774–9. doi: 10.1073/pnas.0403506101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Brunham LR, et al. Beta-cell ABCA1 influences insulin secretion, glucose homeostasis and response to thiazolidinedione treatment. Nat. Med. 2007;13:340–47. doi: 10.1038/nm1546. [DOI] [PubMed] [Google Scholar]
- 108.Koseki M, et al. Impaired insulin secretion in four Tangier disease patients with ABCA1 mutations. J. Atheroscler. Thromb. 2009;16:292–296. doi: 10.5551/jat.e599. [DOI] [PubMed] [Google Scholar]
- 109.Seimon T, Tabas I. Mechanisms and consequences of macrophage apoptosis in atherosclerosis. J. Lipid. Res. 2009;50(Suppl):S382–7. doi: 10.1194/jlr.R800032-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gautier EL, et al. Macrophage apoptosis exerts divergent effects on atherogenesis as a function of lesion stage. Circulation. 2009;119:1795–1804. doi: 10.1161/CIRCULATIONAHA.108.806158. [DOI] [PubMed] [Google Scholar]
- 111.Li F, et al. Free cholesterol-induced macrophage apoptosis is mediated by inositol-requiring enzyme 1 alpha-regulated activation of Jun N-terminal kinase. Acta. Biochim. Biophys. Sin. (Shanghai) 2008;40:226–34. doi: 10.1111/j.1745-7270.2008.00396.x. [DOI] [PubMed] [Google Scholar]
- 112.Devries-Seimon T, et al. Cholesterol-induced macrophage apoptosis requires ER stress pathways and engagement of the type A scavenger receptor. J. Cell Biol. 2005;171:61–73. doi: 10.1083/jcb.200502078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yao PM, Tabas I. Free cholesterol loading of macrophages is associated with widespread mitochondrial dysfunction and activation of the mitochondrial apoptosis pathway. J. Biol. Chem. 2001;276:42468–76. doi: 10.1074/jbc.M101419200. [DOI] [PubMed] [Google Scholar]
- 114.Yao PM, Tabas I. Free cholesterol loading of macrophages induces apoptosis involving the fas pathway. J. Biol. Chem. 2000;275:23807–13. doi: 10.1074/jbc.M002087200. [DOI] [PubMed] [Google Scholar]
- 115.Zha X, Gauthier A, Genest J, McPherson R. Secretory vesicular transport from the Golgi is altered during ATP-binding cassette protein A1 (ABCA1)-mediated cholesterol efflux. J. Biol. Chem. 2003;278:10002–5. doi: 10.1074/jbc.C300024200. [DOI] [PubMed] [Google Scholar]
- 116.Venegas V, Zhou Z. Two alternative mechanisms that regulate the presentation of apoptotic cell engulfment signal in Caenorhabditis elegans. Mol. Biol. Cell. 2007;18:3180–92. doi: 10.1091/mbc.E07-02-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bared SM, et al. Association of ABCA1 with syntaxin 13 and flotillin-1 and enhanced phagocytosis in tangier cells. Mol. Biol. Cell. 2004;15:5399–407. doi: 10.1091/mbc.E04-03-0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Baldan A, et al. Impaired development of atherosclerosis in hyperlipidemic Ldlr−/− and ApoE−/− mice transplanted with Abcg1−/− bone marrow. Arterioscler. Thromb. Vasc. Biol. 2006;26:2301–7. doi: 10.1161/01.ATV.0000240051.22944.dc. [DOI] [PubMed] [Google Scholar]
- 119.Terasaka N, Wang N, Yvan-Charvet L, Tall AR. High-density lipoprotein protects macrophages from oxidized low-density lipoprotein-induced apoptosis by promoting efflux of 7-ketocholesterol via ABCG1. Proc. Natl. Acad. Sci. U. S. A. 2007;104:15093–8. doi: 10.1073/pnas.0704602104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Arai S, et al. A role for the apoptosis inhibitory factor AIM/Spalpha/Api6 in atherosclerosis development. Cell Metab. 2005;1:201–13. doi: 10.1016/j.cmet.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 121.Getz GS, Reardon CA. SAA, HDL biogenesis, and inflammation. J. Lipid Res. 2008;49:269–70. doi: 10.1194/jlr.E700012-JLR200. [DOI] [PubMed] [Google Scholar]
- 122.Sampietro T, Bigazzi F, Puntoni M, Bionda A. HDL inflammation and atherosclerosis: current and future perspectives. Future Cardiol. 2006;2:37–48. doi: 10.2217/14796678.2.1.37. [DOI] [PubMed] [Google Scholar]
- 123.van der Westhuyzen DR, de Beer FC, Webb NR. HDL cholesterol transport during inflammation. Curr. Opin. Lipidol. 2007;18:147–51. doi: 10.1097/MOL.0b013e328051b4fe. [DOI] [PubMed] [Google Scholar]
- 124.Kontush A, Chapman MJ. Functionally defective high-density lipoprotein: a new therapeutic target at the crossroads of dyslipidemia, inflammation, and atherosclerosis. Pharmacol. Rev. 2006;58:342–74. doi: 10.1124/pr.58.3.1. [DOI] [PubMed] [Google Scholar]
- 125.Abe-Dohmae S, et al. Serum amyloid A generates high density lipoprotein with cellular lipid in an ABCA1- or ABCA7-dependent manner. J. Lipid Res. 2006;47:1542–50. doi: 10.1194/jlr.M600145-JLR200. [DOI] [PubMed] [Google Scholar]
- 126.van der Westhuyzen DR, Cai L, de Beer MC, de Beer FC. Serum amyloid A promotes cholesterol efflux mediated by scavenger receptor B-I. J. Biol. Chem. 2005;280:35890–5. doi: 10.1074/jbc.M505685200. [DOI] [PubMed] [Google Scholar]
- 127.Stonik JA, et al. Serum amyloid A promotes ABCA1-dependent and ABCA1-independent lipid efflux from cells. Biochem. Biophys. Res. Commun. 2004;321:936–41. doi: 10.1016/j.bbrc.2004.07.052. [DOI] [PubMed] [Google Scholar]
- 128.Marsche G, et al. The lipidation status of acute-phase protein serum amyloid A determines cholesterol mobilization via scavenger receptor class B, type I. Biochem. J. 2007;402:117–24. doi: 10.1042/BJ20061406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hu W, et al. Biogenesis of HDL by SAA is dependent on ABCA1 in the liver in vivo. J. Lipid Res. 2008;49:386–93. doi: 10.1194/jlr.M700402-JLR200. [DOI] [PubMed] [Google Scholar]
- 130.Schippling S, et al. Severe Tangier disease with a novel ABCA1 gene mutation. Neurology. 2008;71:1454–5. doi: 10.1212/01.wnl.0000327870.29639.20. [DOI] [PubMed] [Google Scholar]
- 131.Ishii J, et al. Clinical variant of Tangier disease in Japan: mutation of the ABCA1 gene in hypoalphalipoproteinemia with corneal lipidosis. J. Hum. Genet. 2002;47:366–69. doi: 10.1007/s100380200051. [DOI] [PubMed] [Google Scholar]
- 132.Brunham LR, Singaraja RR, Hayden MR. Variations on a gene: rare and common variants in ABCA1 and their impact on HDL cholesterol levels and atherosclerosis. Annu. Rev. Nutr. 2006;26:105–29. doi: 10.1146/annurev.nutr.26.061505.111214. [DOI] [PubMed] [Google Scholar]
- 133.Matsukawa A, Takeda K, Kudo S, Maeda T, Kagayama M, Akira S. Aberrant inflammation and lethality to septic peritonitis in mice lacking STAT3 in macrophages and neutrophils. J. Immunol. 2003;171:6198–205. doi: 10.4049/jimmunol.171.11.6198. [DOI] [PubMed] [Google Scholar]
- 134.Oram JF, Lawn RM. ABCA1. The gatekeeper for eliminating excess tissue cholesterol. J. Lipid Res. 2001;42:1173–9. [PubMed] [Google Scholar]
- 135.Oram JF, Vaughan AM. ATP-binding cassette cholesterol transporters and cardiovascular disease. Circ. Res. 2006;99:1031–43. doi: 10.1161/01.RES.0000250171.54048.5c. [DOI] [PubMed] [Google Scholar]
- 136.Oram JF, Heinecke JW. ATP-binding cassette transporter A1: a cell cholesterol exporter that protects against cardiovascular disease. Physiol. Rev. 2005;85:1343–72. doi: 10.1152/physrev.00005.2005. [DOI] [PubMed] [Google Scholar]
- 137.Attie AD, Kastelein JP, Hayden MR. Pivotal role of ABCA1 in reverse cholesterol transport influencing HDL levels and susceptibility to atherosclerosis. J. Lipid Res. 2001;42:1717–26. [PubMed] [Google Scholar]
- 138.Uchida K, et al. High-affinity autoantibodies specifically eliminate granulocyte- macrophage colony-stimulating factor activity in the lungs of patients with idiopathic pulmonary alveolar proteinosis. Blood. 2004;103:1089–98. doi: 10.1182/blood-2003-05-1565. [DOI] [PubMed] [Google Scholar]
- 139.Boisvert WA, et al. Leukocyte transglutaminase 2 expression limits atherosclerotic lesion size. Arterioscler. Thromb. Vasc. Biol. 2006;26:563–69. doi: 10.1161/01.ATV.0000203503.82693.c1. [DOI] [PubMed] [Google Scholar]