Abstract
Stress, strain and modulus are regularly used to characterize material properties of tissue samples. However, when comparing results from different studies it is evident the reported material properties, particularly failure strains, vary hugely. The aim of our study was to characterize how and why specimen length and cross-sectional area (CSA) appear to influence failure stress, strain and modulus in fascicles from two functionally different tendons. Fascicles were dissected from five rat tails and five bovine foot extensors, their diameters determined by a laser micrometer, and loaded to failure at a range of grip-to-grip lengths. Strain to failure significantly decreased with increasing in specimen length in both rat and bovine fascicles, while modulus increased. Specimen length did not influence failure stress in rat tail fascicles, although in bovine fascicles it was significantly lower in the longer 40 mm specimens compared to 5 and 10 mm specimens. The variations in failure strain and modulus with sample length could be predominantly explained by end-effects. However, it was also evident that strain fields along the sample length were highly variable and notably larger towards the ends of the sample than the mid-section even at distances in excess of 5 mm from the gripping points. Failure strain, stress and modulus correlated significantly with CSA at certain specimen lengths. Our findings have implications for the mechanical testing of tendon tissue: while it is not always possible to control for fascicle length and/or CSA, these parameters have to be taken into account when comparing samples of different dimensions.
Keywords: Rat tail tendon, Bovine extensor tendon, Cross-sectional area, Specimen length, Mechanical properties, End-effect
1. Introduction
Mechanical testing is frequently used to characterize tendons, and to describe the effects of interventions such as exercise or medication on their mechanical properties. Force, deformation and stiffness, derived from an ultimate tensile strength test, provide sample specific information about the mechanical behaviour of a tendon sample. From these values, stress, strain and elastic modulus are regularly derived in order to provide quantitative data concerning material properties, with the intention of characterizing the material irrespective of sample dimensions. However, when comparing the results of different studies, it is evident that the reported material properties, in particular the strain values, vary hugely, e.g. from 6–10% (Rigby et al., 1959; Tamiwa, 2007) to 17–20% (Almeida-Silveira et al., 2000; Legerlotz et al., 2007) in rat tendons.
It is well documented that the mechanical properties of tendons are influenced by a range of intrinsic and extrinsic factors including anatomical site (Haraldsson et al., 2005), age (Nakagawa et al., 1996), loading history (Simonsen et al., 1995) or hormonal status (Inhofe et al., 1995). In addition, results can also be influenced by experimental setup, including environmental conditions and test protocol (Schatzmann et al., 1998; Wang et al., 1991). However, even taking these factors into account, there is a remarkably wide range of reported material properties for tendon, with particularly large strain values frequently reported when analyzing the tendons of small mammals such as rats.
A recent study by Tamiwa et al. (2006) suggested that stress is not able to accurately normalize force data for rat tail tendon fascicles, whilst two brief articles published in the 1980s describe an effect of specimen length on stress–strain characteristics in rat tail tendon (Haut, 1986; Sanjeevi et al., 1982). A number of reasons for these variations have been hypothesized, including inhomogeneity of samples (Atkinson et al., 1999) and end-effects (Lam et al., 1988). The influence of each of these effects is important, as it highlights the difficulties in simply using stress and strain parameters to derive true material properties for non-homogenous soft tissues.
The aims of our study were to highlight the influence of specimen length and CSA on apparent material properties, investigate why these occur, and determine if they differ between two functionally different tendons. We hypothesize that the variations recorded in material properties with specimen dimensions are largely influenced by gripping the specimens. However, we further suggest that variations in micro-structure between different sized samples may lead to different material properties.
2. Methods
Tendon fascicles were dissected according to an established protocol (Screen, 2003) from five rat tails and five bovine foot extensors, all from healthy animals sacrificed for other, unrelated reasons. For each fascicle, the diameter was determined by a laser micrometer at multiple points along a 1 cm region in the middle of the fascicle. The smallest diameter recorded was used to calculate CSA, assuming a circular shape. Fascicles were secured in a materials testing machine (Bionix100, MTS, 50N Load cell) by pneumatically driven grips with a serrated surface, exerting a gripping pressure of 3 GPa. Fascicles were loaded to failure at room temperature at a strain rate of 1%/s. During dissection and testing, specimens were kept moist by continually spraying with phosphate buffered saline solution (PBS, Sigma).
Grip-to-grip length was varied and at each length 3–4 fascicles per animal were tested: rat tail fascicles were tested at 5, 10, 20, 40, 60, 80 and 100 mm (103 fascicles in total); bovine extensor fascicles were tested at 5, 10, 20 and 40 mm (67 fascicles in total). Force and deformation were both continuously recorded from the Bionix100 at 50 Hz and engineering stress and strain calculated using the CSA and length of the sample at the start. From the resulting data, the point at which a 0.1 N load was detected was located, and defined as the test start point. The original sample length was corrected accordingly. Stress–strain data were smoothed with a 5-point moving average filter, before calculating the modulus over every 8 values. The modulus across the most linear region of the stress–strain curve and the maximum modulus were found (Fig. 1) and referred to as the linear and maximum modulus, respectively.
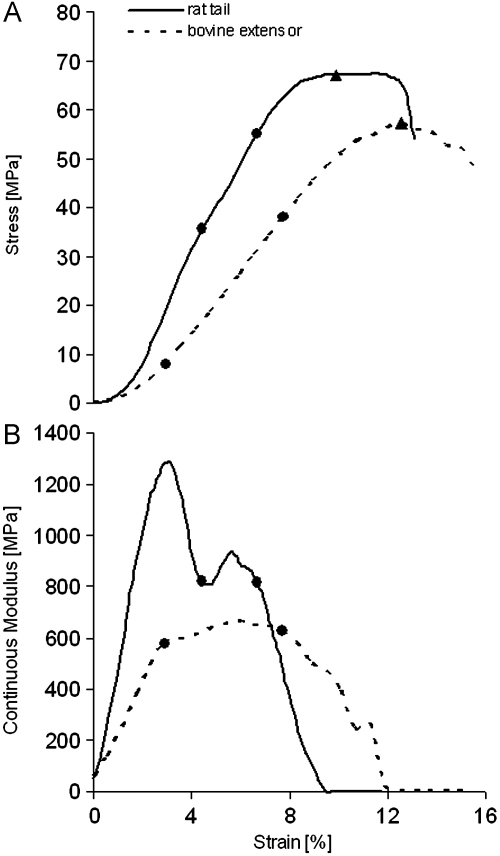
Fig. 1.
Representative samples of bovine extensor and rat tail fascicles tested at 20 mm specimen length. The stress–strain curve shows a different slope for rat tail (broken line) and bovine extensor fascicles (continuous line) (A). The failure stress (maximum stress) is marked by a triangle. Failure strain is defined as the strain at the point of maximum stress. The begin and end of the defined linear region, in which the elastic modulus was calculated, are marked by two dots. The continuous elastic modulus shows a characteristic two-peak course in rat tail fascicle in contrast to a one peak course in bovine extensor fascicles (B).
The end-effect was calculated to establish the effective specimen length, which is longer than the daylight length. Compliance (elongation/linear modulus×CSA) against specimen length was plotted, and the regression line extrapolated to cut the x-axis and indicate the magnitude of the end-effect (Ker, 1981). Material properties were then corrected for end-effects, to determine how they influence the measured data.
To determine the strain distribution along the length of the fascicles, markers (ink) were drawn every 5 mm along bovine extensor fascicles of 20 and 40 mm lengths. The quasi-static tests to failure were filmed (Olympus C-740, 15 Hz) and the strain distribution determined at the beginning of the test, 50% of strain to failure, and in the last few frames prior to failure. Strains were measured in specific sections of the sample for comparison. The grip-section was defined as the distance of the second highest marker to the upper grip and the second lowest marker to the lower grip, resulting in a constant grip-section length of 15 mm for both the 20 and 40 mm long samples. The remaining distance was defined as the mid-section, which was 5 mm long in the 20 mm sample and 25 mm long in the 40 mm sample. Distances between the markers and the grips were measured using an image processing program (ImageJ, National Institutes of Health, USA) and local strains in each section were determined and expressed as a contribution to total sample strain.
2.1. Statistical analyses
A one-way-ANOVA (Post-Hoc test: Tukey) was used to determine the effect of specimen length on mechanical parameters. To describe relationships between mechanical parameters and either CSA or specimen length, Pearsons correlation coefficient was used. Bovine extensor and rat tail fascicles at a specific specimen length were compared by an unpaired t-test, as were mid-section and grip-section strains in short and long samples. Mid-section and grip-section strains within a group of samples of the same length were compared by a paired t-test. The Kolmogorov–Smirnov test did not show any deviation from the normal distribution for any variable. For all statistical tests, significance was established at p≤0.05. Data are presented as mean±SD.
3. Results
Gripping samples during mechanical testing will always lead to stress concentrations at the grips, hence the reported failure properties should be considered a minumum. However, using identical testing methods for all specimens, the values can be compared. Failure stress, strain and modulus, derived directly from the Bionix100 force and extension data, are compared for different sample lengths or diameters in Figs. 2 and 3, respectively.
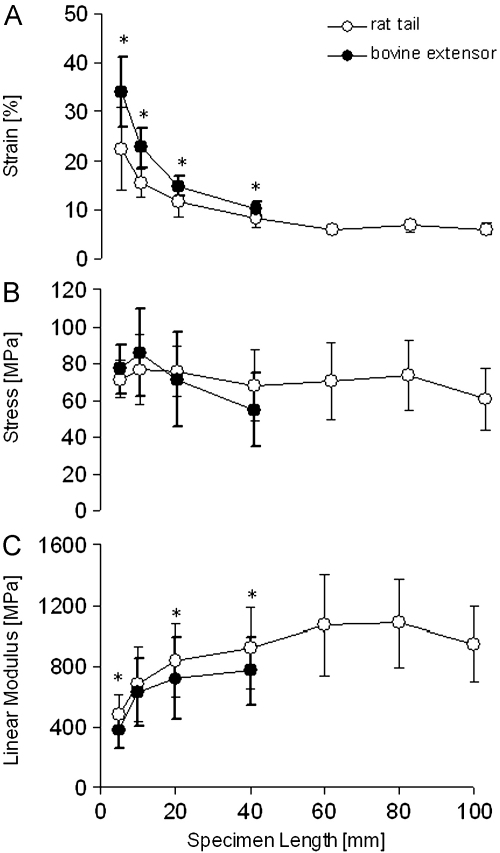
Fig. 2.
Strain at failure (A), stress at failure (B) and elastic modulus (C) at different specimen length in rat tail (open circles) and bovine extensor (filled circles) fascicles. * indicates a significant difference between rat tail and bovine extensor fascicles at a specific specimen length (p≤0.05). Failure strain (A): rat tail=significantly higher strains at 5, 10 and 20 mm compared to 60, 80 and 100 mm lengths, and 5 and 10 mm lenghts compared to 40 mm lengths; bovine extensors=statistically significant reduction in failure strain with every increase in sample length. Stress (B): bovine extensor=lower in the 40 mm compared to 5 and 10 mm long specimens. Linear modulus (C): rat tail=significant differences between 5 mm long fascicles and those 20–100 mm long, as well as 10 mm long samples and those 60 and 80 mm long; bovine extensor fascicles=5 mm long samples experienced a lower modulus than 10–40 mm long samples.
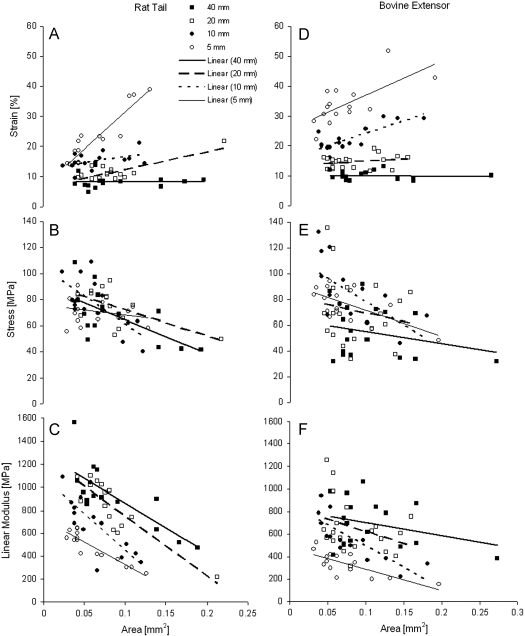
Fig. 3.
Relationship between cross-sectional area and strain, stress and elastic modulus in rat tail (A, C, E) and bovine extensor fascicles (B, D, F) at 5, 10, 20 and 40 mm grip-to-grip length.
3.1. Specimen length
Strain to failure was significantly influenced by specimen length and consistently reduced as the sample length increased. By contrast, specimen length did not influence failure stress in rat tail fascicles, although in bovine fascicles, it was significantly lower in 40 mm than 5 or 10 mm long specimens. The linear modulus increased with increasing specimen length in both sample types. Whilst the effect of specimen length on mechanical parameters was similar in rat tail and bovine extensor fascicles, failure strains were consistently significantly lower in rat tail than bovine extensor fascicles, resulting in a significantly higher linear modulus in rat tail fascicles (Fig. 2). Strain measurements at longer specimen lengths were very consistent as evident from the particularly small standard deviations.
3.2. Cross-sectional area
Failure strain, stress and linear modulus all correlated significantly with CSA at certain specimen lengths. In bovine extensor fascicles, correlations were only apparent in short specimen lengths (5 and 10 mm lengths), where CSA positively correlated with strain and negatively correlated with stress and linear modulus. In rat tail fascicles, failure strain correlated positively with CSA at specimen lengths of 5 and 20 mm, whilst stress and linear modulus correlated negatively with CSA at the majority of sample lengths (10–60 and 100 mm and 5–60 and 100 mm, respectively) (Fig. 3, Table 1).
Table 1.
Correlation (Pearson) of cross-sectional area with strain, stress and linear modulus at different specimen length in bovine extensor and rat tail fascicles.
| Length (mm) | Correlation of CSA with |
||
|---|---|---|---|
| Strain | Stress | Modulus | |
| Bovine extensor | |||
| 5 | 0.681* | −0.647* | −0.744* |
| 10 | 0.795* | −0.632* | −0.803* |
| 20 | 0.260 | −0.176 | −0.325 |
| 40 | −0.079 | −0.260 | −0.259 |
| Rat tail | |||
| 5 | 0.924* | −0.243 | −0.914* |
| 10 | 0.443 | −0.700* | −0.770* |
| 20 | 0.797* | −0.601* | −0.867* |
| 40 | 0.018 | −0.644* | −0.775* |
| 60 | 0.095 | −0.708* | −0.757* |
| 80 | 0.493 | −0.203 | 0.494 |
| 100 | −0.108 | −0.634* | −0.792* |
Indicates a significant correlation (p≤0.05).
3.3. End-effect
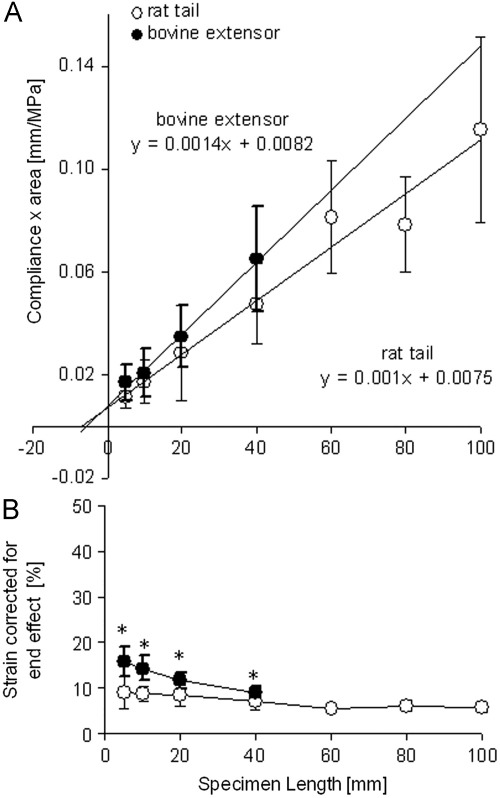
The linear regression lines, indicating that compliance is proportional to length, crossed the x-axis at −5.86 mm (bovine extensor) and −7.50 mm (rat tail) (Fig. 4A). The end-effect at each grip can thus be estimated at 2.93 mm in bovine extensor and 3.75 mm in rat tail samples. These data highlight that, having accounted for end-effects, a single linear modulus value can be used to describe each tendon type, found from the reciprocal of the regression lines to be 1000±165 MPa for rat rail tendons and 714±120 MPa for bovine extensors. Correcting failure strains for end-effects reduced differences between short and long sample lengths; however, significant reductions in failure strain with increase in sample length were still evident (Fig. 4B).
Fig. 4.
Calculation of and correction for the end-effect: (A) compliance against specimen length. The point where the regression line crosses the x-axis gives an indication of the magnitude of the end-effect. (B) Strain corrected for end-effect: bovine extensor=significant reduction in failure strain with every increase in sample length (except 5–10 mm); rat tail=higher strains in 5–20 mm compared to 80–100 mm samples and 5–10 mm compared to 60 mm samples. * indicates a significant difference between rat tail and bovine extensor fascicles at a specific specimen length (p≤0.05).
3.4. Shape of the stress–strain curve
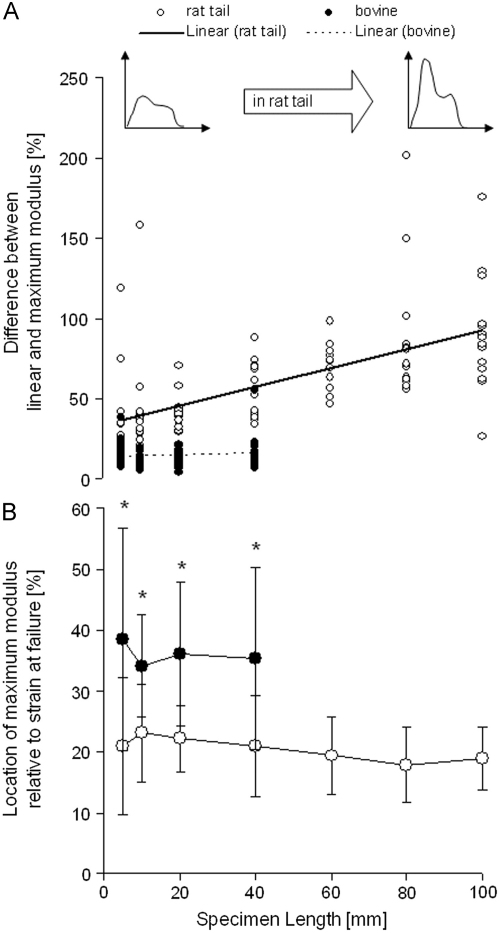
The shape of the stress–strain curves was consistently different for bovine extensor and rat tail fascicles, and in the case of the rat tail fascicles it also changed with specimen length. The continuous modulus curves showed a characteristic two-peak course in rat tail fascicles in contrast to one peak in bovine extensor fascicles (Fig. 1B). In rat tail fascicles, the height of the first peak, rising to the maximum modulus, increased with increasing specimen length (r=0.592; p<0.01), enlarging the difference between the maximum and linear modulus. By contrast, this difference remained constant in bovine extensor fascicles (r=0.107; p=0.387) (Fig. 5A). The location of the maximum modulus on the stress–strain curve, relative to the strain at failure, appeared to be constant and independent of specimen length in both types of tendon fascicles. However, it occurred significantly earlier in rat tail fascicles at 21±7% of the strain at failure compared to 36±13% of failure strain in bovine extensor fascicles (Fig. 5B).
Fig. 5.
Relationship between specimen length and the relative difference between the linear and the maximum elastic modulus (A) and location of the maximum elastic modulus (B). In rat tail (open circles) the peak of the continuous elastic modulus increases with longer specimen length, enlarging the difference between the linear and maximum elastic modulus (r=0.592; p<0.001). In bovine extensor (filled circles) the shape of the curve is not affected by specimen length. The location of the maximum elastic modulus is independent of specimen length, but dependent on tendon structure/function, occurring earlier in rat tail than in bovine extensor fascicles. * indicates a significant difference between rat tail and bovine extensor fascicles at a specific specimen length (p≤0.05).
3.5. Mid-section and grip-section strain
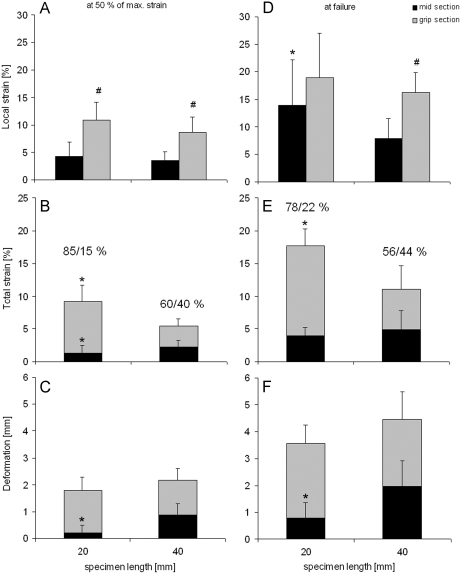
Whilst deformation (Fig. 6F) and subsequently local strains (Fig. 6D) in the grip-section were consistent for both sample lengths, mid-section strains were significantly smaller in larger specimens, highlighting that the variable strain distribution extended well beyond the gripping regions (Fig. 6E). Whilst similar trends were unsurprisingly seen at 50% of failure, local strains in the mid-section of short samples were significantly smaller than in the grip-section at this time point only (Fig. 6A), highlighting that strains did not increase homogenously throughout the test either, but increased more rapidly in the grip-section for both sample lengths (Fig. 6B and E).
Fig. 6.
Mid-section and grip-section strain for bovine extensor fascicles tested at 20 and 40 mm grip-to-grip distance. Data presented as local strain (A, D) proportion of total strain (B, E), and deformation (C, F) at 50% of maximum (A–C) and at failure (D–F). Data were derived from image analysis of filmed tests. * indicates a significant difference between specimens of different length (p≤0.05). # indicates a significant difference between grip-section and mid-section strain within specimens of the same length. The numbers above the columns illustrate the relationship between grip-section (first number) and mid-section strain (second number).
4. Discussion
We have highlighted how the material properties of tendon fascicles appear to be influenced by both specimen length and CSA. Measurement of grip-to-grip failure strains yielded significantly larger values in shorter specimens, with a subsequent reduction in modulus in these samples, while Fig. 3 also highlighted how failure stress and modulus both decreased with Increasing sample diameter. Increased total strains at short specimen lengths have been described previously (Haut, 1986). Further studies have suggested that this might be due to the end-effect, where extra unseen sample length within the grips contributes heavily to overall strain measurements in short specimens, whereas the response of longer samples more closely reflects true material properties (Bennett et al., 1986; Lam et al., 1988). A correction for end-effects in the current data notably reduced the magnitude of differences in strain between short and long samples, and also enabled us to find a single modulus value for each tendon type, indicating that the apparent changes in sample properties with length are likely to be almost entirely artifactual. One method to avoid this is to use an extensometer, which provides a method of measuring sample length without including the grip -region, and thus eliminating end-effect artifacts.
Returning to the uncorrected data, Fig. 3 indicates that material properties are also influenced by CSA. While this is at odds with Fig. 4, in which a single modulus value was appropriately found for each tendon type, it may explain the large standard deviations for each sample length in this data, if modulus is additionally influenced by CSA. A correlation between CSA and failure stress cannot be directly related to end-effects. It is possible that some of the variation is still related to gripping, as collagen fibers in the centre of a larger fascicle experience less gripping pressure and are therefore more likely to shear and add to the end-effect, thereby increasing strain. However, in rat tail specimens of 10, 40, 60 and 100 mm length, no correlation between CSA and strain was observed, but CSA did correlate with stress and modulus.
The smaller stress values of larger diameter samples may also be related to structural differences in the internal organization of samples. This may encompass fiber organization, interactions between subfascicles connected by connective tissue sheaths, or the relative contribution of collagen fascicles and connective tissue sheaths. It has been suggested that the decrease in the amount of areolar connective tissue in smaller samples might lead to an increase in modulus (Danylchuk et al., 1978). Alternatively, the proportion of fiber bundles running parallel to the axis of force might be larger in smaller specimens (Butler et al., 1986). Indeed, on a macro-scale, a study of human patella tendon has shown that small (∼1 mm²) and large (∼20 mm²) sections of tendon do not exhibit the same mechanical properties with larger modulus values in small specimens (Atkinson et al., 1999). This has been attributed to structures other than the subfascicle, such as the epitenon or other connective tissue components. Average fascicle CSA has been shown to be negatively correlated with tendon modulus, but positively correlated to the total tendon CSA in equine superficial digital flexor tendon, also indicating an effect of structural organization on mechanical properties (Gillis et al., 1997).
Indeed, differences in composition and structure of functionally different tendons, such as the rat tail and bovine tendons investigated in the current study, may explain many of the differences seen in their mechanical properties. Although failure strain, stress and modulus displayed similar trends with specimen length in both tendons, the shape of the stress–strain curve at different specimen lengths changed in rat tail fascicles only, and the effect of CSA on mechanical properties was more pronounced in rat tail fascicles.
Whilst end-effects explain the variation in failure strain with sample length, Fig. 6 also highlights how local strains vary along with sample length during testing. It has been reported previously that the strains close to the gripping points are higher than in the mid-section of specimens (Butler et al., 1984; Devkota and Weinhold, 2003; Wu et al., 2004). This has been attributed to sample slippage in the grips, or premature failure, resulting from local stress concentrations at the grips (Butler et al., 1984; Wu et al., 2004). While gripping may reduce the sample diameter at these points and lead to greater stress and more extension in these regions, failure occurred throughout the length of the samples, opposing the suggestion of localized damage near the grips, although we cannot exclude grip-related damage triggering the rupturing process. Furthermore, two studies investigating the failure mechanics of tendons have found no reduction in the ultimate tensile strength of tendons failing at the grips compared with those failing in the mid-section (Ng et al., 2005; Smith et al., 1996).
The act of gripping is likely to alter the stiffness of the ends of the sample close to the grips. However, the small diameter of the samples would suggest that this effect should only extend a minimal distance into the sample. It does not readily explain a strain distribution along the sample length, evidently extending in excess of 5 mm from the gripping points. Considering these findings from a micro-structural perspective, they may indicate discontinuity of fibrils. The applied strain is evidently transferred to those fibrils held within the grips, but our data imply that it may not be fully transferred to the adjacent fibrils near the centre of the sample, resulting in reduced strains in this area. Since the grip-section in our experiment was between 5 and 10 mm long at each end (in sum constantly 15 mm), and the mid-section strains were notably higher in the short samples (20 mm) than in the long samples, we conclude that this subunit is on average more than 5 mm and less than 20 mm long.
While tendon fascicles are thought to span the entire length of a tendon (Basso et al., 2001) and to be mechanically and functionally independent (Haraldsson et al., 2008), controversy exists regarding the length and continuity of collagen fibrils in skeletally mature tendons and ligaments (Provenzano and Vanderby, 2006). Fibrils are clearly discontinuous during tendon development (Birk et al., 1997; Provenzano and Vanderby, 2006) and the variable CSA along the length of some tendons, such as the human Achilles tendon (Kongsgaard et al., 2005), suggests that discontinuous subunits are necessary to allow for this change in CSA. However, in mature tendon only two fibril ends have been spotted using transmission electron microscopy to analyze 5639 fibrils (Trotter and Wolfsky, 1989), and no ends have been found in a study analyzing 7275 fibrils (Provenzano and Vanderby, 2006). The extremely high aspect ratio and interweaving of collagen fibrils make imaging complex (Craig et al., 1989), and with a fibril length in excess of 5 mm, the likelihood of seeing an end is low. Since the large aspect ratio of collagen fibrils complicates the experimental assessment of fibril length, theoretical approaches have also been used, estimating a mean collagen fibril length in mature rat tail tendon of 6.4–12.7 mm (Craig et al., 1989). This corresponds positively with our estimation of >5 and <20 mm length of a discontinuous subunit.
4.1. Conclusion
Our findings have implications for the mechanical testing of tendon tissue: while it is not always possible to control for fascicle length and/or CSA, these parameters have to be taken into account when comparing samples of different dimensions. It seems advisable to use longer specimens whenever possible to reduce the variability within a given subgroup of a defined fascicle length, and to avoid end-effects by using an extensometer in a portion of the specimen well clear of the clamps.
Conflict of interest statement
There has been no conflict of interest. There have been no financial or personal relationships with other people or organizations which could inappropriately have influenced our work.
Acknowledgement
This study was supported by the Arthritis Research UK (Grant number 18424). Dr. Riley is an Arthritis Research UK Senior Research Fellow (Grant number 17826). The authors would like to thank Robert Ker for his thoughtful insights and significant input into the development of this manuscript.
References
- Almeida-Silveira M.I., Lambertz D., Perot C., Goubel F. Changes in stiffness induced by hindlimb suspension in rat Achilles tendon. Eur. J. Appl. Physiol. 2000;81(3):252–257. doi: 10.1007/s004210050039. [DOI] [PubMed] [Google Scholar]
- Atkinson T.S., Ewers B.J., Haut R.C. The tensile and stress relaxation responses of human patellar tendon varies with specimen cross-sectional area. J. Biomech. 1999;32(9):907–914. doi: 10.1016/s0021-9290(99)00089-5. [DOI] [PubMed] [Google Scholar]
- Basso O., Johnson D.P., Amis A.A. The anatomy of the patellar tendon. Knee Surg. Sports Traumatol. Arthroscopy. 2001;9(1):2–5. doi: 10.1007/s001670000133. [DOI] [PubMed] [Google Scholar]
- Bennett M.B., Ker R.F., Dimery N.J., Alexander R.M. Mechanical properties of various mammalian tendons. J. Zool. London. 1986;209:537–548. [Google Scholar]
- Birk D.E., Zycband E.I., Woodruff S., Winkelmann D.A., Trelstad R.L. Collagen fibrillogenesis in situ: fibril segments become long fibrils as the developing tendon matures. Dev. Dyn. 1997;208(3):291–298. doi: 10.1002/(SICI)1097-0177(199703)208:3<291::AID-AJA1>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Butler D.L., Grood E.S., Noyes F.R., Zernicke R.F., Brackett K. Effects of structure and strain measurement technique on the material properties of young human tendons and fascia. J. Biomech. 1984;17(8):579–596. doi: 10.1016/0021-9290(84)90090-3. [DOI] [PubMed] [Google Scholar]
- Butler D.L., Kay M.D., Stouffer D.C. Comparison of material properties in fascicle-bone units from human patellar tendon and knee ligaments. J. Biomech. 1986;19(6):425–432. doi: 10.1016/0021-9290(86)90019-9. [DOI] [PubMed] [Google Scholar]
- Craig A.S., Birtles M.J., Conway J.F., Parry D.A. An estimate of the mean length of collagen fibrils in rat tail-tendon as a function of age. Connect. Tissue Res. 1989;19(1):51–62. doi: 10.3109/03008208909016814. [DOI] [PubMed] [Google Scholar]
- Danylchuk K.D., Finlay J.B., Krcek J.P. Microstructural organization of human and bovine cruciate ligaments. Clin. Orthop. Relat. Res. 1978;131:294–298. [PubMed] [Google Scholar]
- Devkota A.C., Weinhold P.S. Mechanical response of tendon subsequent to ramp loading to varying strain limits. Clin. Biomech. (Bristol, Avon) 2003;18(10):969–974. doi: 10.1016/s0268-0033(03)00168-2. [DOI] [PubMed] [Google Scholar]
- Gillis C., Pool R.R., Meagher D.M., Stover S.M., Reiser K., Willits N. Effect of maturation and aging on the histomorphometric and biochemical characteristics of equine superficial digital flexor tendon. Am. J. Vet. Res. 1997;58(4):425–430. [PubMed] [Google Scholar]
- Haraldsson B.T., Aagaard P., Krogsgaard M., Alkjaer T., Kjaer M., Magnusson S.P. Region-specific mechanical properties of the human patella tendon. J. Appl. Physiol. 2005;98(3):1006–1012. doi: 10.1152/japplphysiol.00482.2004. [DOI] [PubMed] [Google Scholar]
- Haraldsson B.T., Aagaard P., Qvortrup K., Bojsen-Moller J., Krogsgaard M., Koskinen S. Lateral force transmission between human tendon fascicles. Matrix Biol. 2008;27(2):86–95. doi: 10.1016/j.matbio.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Haut R.C. The influence of specimen length on the tensile failure properties of tendon collagen. J. Biomech. 1986;19(11):951–955. doi: 10.1016/0021-9290(86)90190-9. [DOI] [PubMed] [Google Scholar]
- Inhofe P.D., Grana W.A., Egle D., Min K.W., Tomasek J. The effects of anabolic steroids on rat tendon. An ultrastructural, biomechanical, and biochemical analysis. Am. J. Sports Med. 1995;23(2):227–232. doi: 10.1177/036354659502300217. [DOI] [PubMed] [Google Scholar]
- Ker R.F. Dynamic tensile properties of the plantaris tendon of sheep (Ovis aries) J. Exp. Biol. 1981;93:283–302. doi: 10.1242/jeb.93.1.283. [DOI] [PubMed] [Google Scholar]
- Kongsgaard M., Aagaard P., Kjaer M., Magnusson S.P. Structural Achilles tendon properties in athletes subjected to different exercise modes and in Achilles tendon rupture patients. J. Appl. Physiol. 2005;99(5):1965–1971. doi: 10.1152/japplphysiol.00384.2005. [DOI] [PubMed] [Google Scholar]
- Lam T.C., Shriven G, Frank C.B. Letters to the editor: Comment on “The influence of specimen length on the tensile failure properties of tendon collagen”. J. Biomech. 1988;21(1):67. doi: 10.1016/0021-9290(86)90190-9. [DOI] [PubMed] [Google Scholar]
- Legerlotz K., Schjerling P., Langberg H., Bruggemann G.P., Niehoff A. The effect of running, strength, and vibration strength training on the mechanical, morphological, and biochemical properties of the Achilles tendon in rats. J. Appl. Physiol. 2007;102(2):564–572. doi: 10.1152/japplphysiol.00767.2006. [DOI] [PubMed] [Google Scholar]
- Nakagawa Y., Hayashi K., Yamamoto N., Nagashima K. Age-related changes in biomechanical properties of the Achilles tendon in rabbits. Eur. J. Appl. Physiol. Occup. Physiol. 1996;73(1–2):7–10. doi: 10.1007/BF00262803. [DOI] [PubMed] [Google Scholar]
- Ng B.H., Chou S.M., Krishna V. The influence of gripping techniques on the tensile properties of tendons. Proc. Inst. Mech. Eng. H. 2005;219(5):349–354. doi: 10.1243/095441105X34239. [DOI] [PubMed] [Google Scholar]
- Provenzano P.P., Vanderby R., Jr. Collagen fibril morphology and organization: implications for force transmission in ligament and tendon. Matrix Biol. 2006;25(2):71–84. doi: 10.1016/j.matbio.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Rigby B.J., Hirai N., Spikes J.D., Eyring H. The mechanical properties of rat tail tendon. J. Gen. Physiol. 1959;43:265–283. doi: 10.1085/jgp.43.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanjeevi R., Somanathan N., Ramaswamy D. A viscoelastic model for collagen fibres. J. Biomech. 1982;15(3):181–183. doi: 10.1016/0021-9290(82)90250-0. [DOI] [PubMed] [Google Scholar]
- Schatzmann L., Brunner P., Staubli H.U. Effect of cyclic preconditioning on the tensile properties of human quadriceps tendons and patellar ligaments. Knee Surg. Sports Traumatol. Arthroscopy. 1998;6(Suppl 1):S56–S61. doi: 10.1007/s001670050224. [DOI] [PubMed] [Google Scholar]
- Screen, H.R., 2003. The contribution of structural components to tendon mechanics. Unpublished PhD Thesis, Queen Mary University of London, London.
- Simonsen E.B., Klitgaard H., Bojsen-Moller F. The influence of strength training, swim training and ageing on the Achilles tendon and m. soleus of the rat. J. Sports Sci. 1995;13(4):291–295. doi: 10.1080/02640419508732242. [DOI] [PubMed] [Google Scholar]
- Smith C.W., Young I.S., Kearney J.N. Mechanical properties of tendons: changes with sterilization and preservation. J. Biomech. Eng. 1996;118(1):56–61. doi: 10.1115/1.2795946. [DOI] [PubMed] [Google Scholar]
- Tamiwa, M., 2007. Dynamic characterisation of rat-tail tendon fascicles. Unpublished PhD thesis, Queen Mary University of London, London.
- Tamiwa, M., Screen, H.R., Bader, D.L., Shelton, J.C., 2006. Testing Environment influences the viscoelastic behaviour of tendon fascicles. Paper presented at the 52nd Annual Meeting of the Orthopaedic Research Society, Chicago.
- Trotter J., Wolfsky C. The length of collagen fibrils in tendon. Trans. Orthop. Res. Soc. 1989;35:180a. [Google Scholar]
- Wang X.T., De Ruijter M.R., Alexander R.M., Ker R.F. The effect of temperature on the tensile stiffness of mammalian tail tendons. J. Zool. London. 1991;223:491–497. [Google Scholar]
- Wu J.Z., Brumfield A., Miller G.R., Metheny R., Cutlip R.G. Comparison of mechanical properties of rat tibialis anterior tendon evaluated using two different approaches. Biomed. Mater. Eng. 2004;14(1):13–22. [PubMed] [Google Scholar]