Abstract
Background
Circadian variations in the absorption, distribution, protein binding, elimination and metabolism of drugs account for many of the administration-time-dependent differences in their pharmacokinetics. The aim of this study is to determine whether the time of intrathecal injection influences spinal anesthesia.
Methods
Ninety patients scheduled for orthopedic surgery were randomly assigned to three groups. Each group received spinal anesthesia with 0.5% bupivacaine 10 mg at different times; group AM (8 am to 12:00), group Noon (12:00 to 4:00 pm) and group PM (4:00 pm to 8:00 pm). Sensory and motor blockade were assessed by pinprick and a four-point modified Bromage scale. Time to first postoperative analgesic requirement and side effects such as hypotension, bradycardia, nausea, and shivering were recorded.
Results
No significant differences were found among the three groups in peak sensory blockade, duration of motor block to Bromage 1 or side effects, but time to first postoperative analgesic requirement (P = 0.008), and recovery time of S1 sensation to pinprick were significantly prolonged in group Noon compared with the other groups (P = 0.03).
Conclusions
The tine of administration of spinal local anesthetics influences the duration of local anesthesia.
Keywords: circadian effect, hour of administration, local anesthetics
INTRODUCTION
Many studies have been conducted in animals and humans demonstrating circadian time-dependent differences in acute and chronic toxicity, pharmacokinetics and pharmacodynamics of local anesthetic agents. Most studies were done with amide-type agents demonstrating a dosing-time dependency in toxicity [1-3].
Debon et al. [4] reported a significantly longer effect of epidural ropivacaine during the first stage of labor in the diurnal period (morning and afternoon) when compared with the nocturnal period (evening and night). The analgesia duration was 20-28% longer in the diurnal period and the longest anesthesia duration occurred at 3:00 pm.
Because important differences in the duration of action of local anesthetic with respect to time of administration have been demonstrated in several fields (transcutaneous, dental and epidural, etc), we designed this study to determine whether the time of administration influences spinal hyperbaric bupivacaine. Our study may be the first to investigate the influence of the time of administration of spinal local anesthetics on the duration of anesthesia, because no studies have been published concerning about that.
MATERIALS AND METHODS
This study protocol was approved by our hospital ethics committee, and written informed consent was obtained from all study participants. Ninety patients with Class 1 and 2 of the American Society of Anesthesiologists Physical Status classification scheduled for orthopedic surgery were randomly assigned to three groups. Each group received spinal anesthesia with 0.5% bupivacaine 10 mg at different times: group AM (8 am to 12:00), group Noon (12:00 to 4:00 pm) and group PM (4:00 pm to 8:00 pm). The baricity of bupivacaine was 1.026 at 20℃ and 1.021 at 37℃. Patients were excluded from the study if they had abnormal coagulation profiles, skin infections or they refused regional anesthesia.
No premedication was given. An intravenous infusion of 500-700 ml lactated Ringer's solution was started on arrival in the operating room. All patients were monitored using noninvasive blood pressure monitoring, pulse oximetry and electrocardiography. Spinal anesthesia was performed at the L3-L4 or L4-L5 level in the sitting position using a 27-gauge Quincke needle. After free flow of cerebrospinal fluid was observed, a total volume of 2 ml spinal solution was administered to each patient over 10-15 s. Patients were put into the prone position immediately after intrathecal injection. Episodes of bradycardia (heart rate < 50 beats/min) or perioperative hypotension (systolic blood pressure < 20% of baseline) were recorded and were treated with a loading of fluid, intravenous ephedrine or atropine. Sensory block level was measured using pinprick testing and the degree of motor block was assessed using a four-point modified Bromage scale [5] by asking the patient to flex the hip, knee, and ankle joints (0, full flexion of the knees and feet; 1, just able to flex knees, full flexion of feet; 2, unable to flex knees, flexion of feet; 3, unable to move legs or feet, full motor block). Sensory and motor evaluations were performed at 1 min intervals for the first 5 min and then every 5 min until the end of surgery. Further evaluation was then done at 10 min intervals in the postanesthesia care unit until recovery time of S1 sensation and duration of motor block to Bromage 1. Peak sensory blockade and side effects such as hypotension, bradycardia, nausea, shivering were recorded. Patients were given analgesics when VNRS (verbal numerical rating scale) scores were 5 or more.
Results are presented as mean ± standard deviation, median (range) or number of patients. Comparison of age, body weight, height, administered fluid, time to first postoperative analgesic requirement, duration of motor block to Bromage 1, and recovery time of S1 sensation to pinprick among groups were conducted using one-way ANOVA, followed by a Tukey's HSD post-hoc test to determine intergroup differences. Because peak sensory blockade values were not normally distributed, the Kruskal-Wallis test was used. Chi-square test was used to analyze nonparametric data such as gender, and frequency of hypotension, bradycardia, nausea, and shivering. P values of < 0.05 were considered statistically significant.
RESULTS
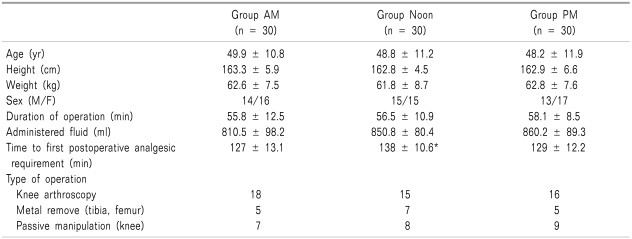
The three groups were comparable with respect to age, weight, height, gender distribution, operation duration, administered fluid and type of operation (Table 1).
Table 1.
Demographic and Clinical Variance and Operation Type
Values are mean ± SD. Group AM: were performed spinal anesthesia at 8 am to 12:00. Group Noon: were performed spinal anesthesia at 12:00 to 4:00 pm. Group PM: were performed spinal anesthesia at 4:00 to 8:00 pm. *P < 0.05 compared with other two groups.
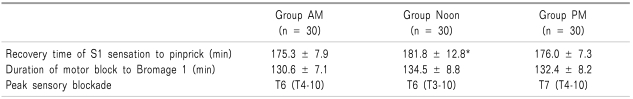
There were no significant differences in peak sensory blockade or duration of motor block to Bromage 1 among the three groups. Time to first postoperative analgesic requirement (P = 0.008) and recovery time of S1 sensation (P = 0.03) were significantly longer in group Noon than in the other two groups (Table 2).
Table 2.
Sensory and Motor Blockade
Values are mean ± SD or median and range. Group AM: were performed spinal anesthesia at 8 am to 12:00. Group Noon: were performed spinal anesthesia at 12:00 to 4:00 pm. Group PM: were performed spinal anesthesia at 4:00 to 8:00 pm. *P < 0.05 compared with othertwo groups.
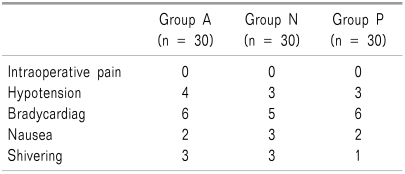
Side effects such as hypotension, bradycardia, nausea, and shivering did not significantly differ among the three groups (Table 3).
Table 3.
Side Effects During Operation and Recovery Period
Values are mean ± SD. Group AM: were performed spinal anesthesia at 8 am to 12:00. Group Noon: were performed spinal anesthesia at 12:00 to 4:00 pm. Group PM: were performed spinal anesthesia at 4:00 to 8:00 pm. No significant differences are found the three groups.
DISCUSSION
Anesthesiologists choose a particular local anesthetic in part because of the differences in onset and duration of effect [5]. As for many other drugs, efficacy and toxicity of local anesthetics depend on time of administration. We found that recovery time of S1 sensation recorded after administration of the same dose of intrathecal bupivacaine was significantly longer in group Noon (12:00 to 4:00 pm) than in the other two groups according to time of drug administration. Our results are comparable to previous studies, which exhibited a circadian rhythm of the duration of local anesthesia induced by lidocaine, bupivacaine and mepivacaine, with the longest anesthesia duration being in the afternoon, at 3:00 pm [1,2,4,6]. Several mechanisms are believed to be involved. Membrane nerve cell permeability to ions, namely, the cell potassium concentration and the K+ efflux from cells and circadian rhythm of catecholamine release are lowest around 3:00 pm [4]. Chassard et al. [2] found the duration of intrathecal plain bupivacaine analgesia (2 mg) exhibited a temporal pattern with 25% variation during the daytime, and peaked at around 12:00. However, duration of motor block to Bromage 1, and side effects such as hypotension, bradycardia, nausea, and shivering did not significantly differ among the three groups.
Administration-time differences in the pharmacokinetics of local anesthetics were explored in rodents using lidocaine, bupivacaine, etidocaine and mepivacaine. Changes in plasma levels and tissue concentrations, especially in the heart and brain, are particularly important when evaluating the toxicity of these medications to the central nervous and cardiovascular systems. Circadian variations in the absorption, distribution, protein binding, elimination, and metabolism of drugs account for many of the administration-time dependent differences in the pharmacokinetics of local anesthetics [6-10].
A limitation of our study was that we couldn't record postoperative pain level and the need for analgesia was influenced by the time of day the operation was performed. In our pilot study, some group PM (4:00 to 8:00 pm) patients in wards rejected their report after surgery owing to sleep disturbance. Therefore, we assessed sensory and motor blockade during operation and in the recovery room. In addition, differences in time to first postoperative analgesic requirement and recovery time of S1 sensation among groups in our study were clinically less important, although they were statistically significant. Circadian variation in the occurrence and intensity of pain has been described in many clinical situations [4,10-12]. Knowledge of these temporal patterns should be the first step in designing treatments for pain relief. Pain evaluation questionnaires that are used in clinical wards rarely address the hour of onset or the hours of lowest pain of the patients. Lack of information regarding the biological rhythms of pain could account in part for the fact that pain often persists despite continuous administration of opioids.
In conclusion, we found that the time of administration of spinal local anesthetics influences the duration of anesthesia. However, further studies in the field of spinal anesthesia are needed to confirm our findings. To maximize the effectiveness of intrathecal local anesthetics, awareness of the biologic rhythms in local anesthetics and pain intensity is a necessity.
ACKNOWLEDGEMENTS
This study was supported by Wonkwang University in 2009.
References
- 1.Chassard D, Bruguerolle B. Chronobiology and anesthesia. Anesthesiology. 2004;100:413–427. doi: 10.1097/00000542-200402000-00034. [DOI] [PubMed] [Google Scholar]
- 2.Chassard D, Duflo F, de Queiroz Siqueira M, Allaouchiche B, Boselli E. Chronobiology and anaesthesia. Curr Opin Anaesthesiol. 2007;20:186–190. doi: 10.1097/ACO.0b013e328136c55e. [DOI] [PubMed] [Google Scholar]
- 3.Debon R, Boselli E, Guyot R, Allaouchiche B, Lemmer B, Chassard D. Chronopharmacology of intrathecal sufentanil for labor analgesia: daily variations in duration of action. Anesthesiology. 2004;101:978–982. doi: 10.1097/00000542-200410000-00024. [DOI] [PubMed] [Google Scholar]
- 4.Debon R, Chassard D, Duflo F, Boselli E, Bryssine B, Allaouchiche B. Chronobiology of epidural ropivacaine: variations in the duration of action related to the hour of administration. Anesthesiology. 2002;96:542–545. doi: 10.1097/00000542-200203000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Chin YJ. Mechanism of action of local anesthetics. Korean J Pain. 1994;7:155–161. [Google Scholar]
- 6.Bruguerolle B, Labrecque G. Rhythmic pattern in pain and their chronotherapy. Adv Drug Deliv Rev. 2007;59:883–895. doi: 10.1016/j.addr.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Bruguerolle B, Boulamery A, Simon N. Biological rhythms: a neglected factor of variability in pharmacokinetic studies. J Pharm Sci. 2008;97:1099–1108. doi: 10.1002/jps.21044. [DOI] [PubMed] [Google Scholar]
- 8.Labrecque G, Vanier MC. Biological rhythms in pain and in the effects of opioid analgesics. Pharmacol Ther. 1995;68:129–147. doi: 10.1016/0163-7258(95)02003-9. [DOI] [PubMed] [Google Scholar]
- 9.Smolensky MH, Peppas NA. Chronobiology, drug delivery, and chronotherapeutics. Adv Drug Deliv Rev. 2007;59:828–851. doi: 10.1016/j.addr.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 10.Boscariol R, Gilron I, Orr E. Chronobiological characteristics of postoperative pain: diurnal variation of both static and dynamic pain and effects of analgesic therapy. Can J Anaesth. 2007;54:696–704. doi: 10.1007/BF03026866. [DOI] [PubMed] [Google Scholar]
- 11.Anastasopoulou-Sampani D, Sampanis E, Karargiris G. The need for analgesia in elective cholecystectomies influenced by the time of day the operation is performed. Acta Anaesthesiol Scand. 1996;40:955. doi: 10.1111/j.1399-6576.1996.tb04567.x. [DOI] [PubMed] [Google Scholar]
- 12.Aya AG, Vialles N, Mangin R, Robert C, Ferrer JM, Ripart J, et al. Chronobiology of labour pain perception: an observational study. Br J Anaesth. 2004;93:451–453. doi: 10.1093/bja/aeh223. [DOI] [PubMed] [Google Scholar]