Abstract
Background and purpose:
In addition to predominant localization at detergent-insoluble, glycolipid-enriched plasma membrane microdomains (DIGs), glycosylphosphatidylinositol (GPI)-anchored proteins (GPI-proteins) have been found associated with lipid droplets (LDs) and adiposomes. Adiposomes are vesicles that are released from adipocytes in response to anti-lipolytic and lipogenic signals, such as H2O2, palmitate and the antidiabetic sulfonylurea drug, glimepiride, and harbour (c)AMP-degrading GPI-proteins, among them the 5-nucleotidase CD73. Here the role of adiposomes in GPI-protein-mediated information transfer was studied.
Experimental approach:
Adiposomes were incubated with isolated rat adipocytes under various conditions. Trafficking of CD73 and lipid synthesis were analysed.
Key results:
Upon blockade of GPI-protein trafficking, CD73 specifically associated with DIGs of small, and to a lower degree, large, adipocytes. On reversal of the blockade, CD73 appeared at cytosolic LD in time- adiposome concentration- and signal (H2O2 > glimepiride > palmitate)-dependent fashion. The salt- and carbonate-resistant association of CD73 with structurally intact DIGs and LD was dependent on its intact GPI anchor. Upon incubation with small and to a lower degree, large adipocytes, adiposomes increased lipid synthesis in the absence or presence of H2O2, glimepiride and palmitate and improved the sensitivity toward these signals. Upregulation of lipid synthesis by adiposomes was dependent on the translocation of CD73 with intact GPI anchors from DIGs to LD.
Conclusions:
The signal-induced transfer of GPI-anchored CD73 from adiposomes via DIGs to LD of adipocytes mediates paracrine upregulation of lipid synthesis within the adipose tissue.
Keywords: microvesicles, exosomes, glycosylphosphatidylinositol, lipid droplets, lipid metabolism, sulfonylureas
Introduction
The glycosylphosphatidylinositol (GPI) anchor is a highly conserved glycolipid structure that is added post-translationally to the carboxy-terminus of many eukaryotic, particularly mammalian, proteins (Nosjean et al., 1997; Ikezawa, 2002). Typically, the resulting GPI-anchored protein (GPI-protein) is embedded in the outer leaflet of the plasma membrane, predominantly in detergent-insoluble glycolipid-enriched plasma membrane microdomains (DIGs) (Varma and Mayor, 1998). DIGs are characterized by resistance toward detergent solubilization and low buoyant density during sucrose gradient centrifugation (Brown and London, 1998). They are highly enriched in cholesterol and (glyco)sphingolipids as well as certain acylated and transmembrane proteins (Brown and London, 1998; Varma and Mayor, 1998; Müller and Frick, 1999).
The functions of GPI-proteins studied so far are of considerable variety, but seem to rely on their cell surface localization. Furthermore, GPI-proteins have been implicated in intercellular communication via either direct cell-to-cell contact mediated by GPI-anchored cell adhesion molecules (Harris and Siu, 2002; Karagogeos, 2003) or transfer of GPI-proteins with ‘messenger function’ from donor to acceptor cells (Zhang et al., 1992; Rooney et al., 1993; Medof et al., 1996; Kooyman et al., 1998; Sloand et al., 1998). So far only limited experimental evidence is available for the molecular mechanisms involved in the transfer of GPI-proteins between cells. GPI-proteins have been demonstrated not to transfer spontaneously from erythrocytes to liposomes, whereas CD4 engineered as a GPI-protein was observed to be efficiently transferred between plasma membranes of HeLa cells, but not of other cell types (Anderson et al., 1996). Consequently, it was suggested that in vivo GPI-proteins are not spontaneously transferred between cell membranes, but some facilitating or catalyzing mechanism is needed (Suzuki and Okumura, 2000). This may involve microvesicles and exosomes that represent vesicular structures differing in size (0.2–1 vs. 0.03–0.20 µm) and buoyant density during sucrose gradient centrifugation (1.25–1.30 vs. 1.13–1.21 g mL−1) but sharing certain phospholipids and proteins of their membrane bilayers (Heijnen et al., 1999; Stoorvogel et al., 2002; Keller et al., 2006).
Exosomes were first identified during reticulocyte maturation (Harding et al., 1983; Pan and Johnstone, 1983) and subsequently demonstrated to be released from various donor cell types. Exosomes were initially thought to correspond to internal vesicles of multivesicular bodies being released into the extracellular space by exocytosis upon fusion with the plasma membrane (Thery et al., 2002; Cocucci et al., 2009). However, GPI-proteins already embedded in the outer leaflet of plasma membranes, such as GPI-anchored acetylcholinesterase, can also be efficiently released (de Gassart et al., 2003), during differentiation of reticulocytes into erythrocytes (Johnstone et al., 1987). This finding argued for release of GPI-proteins in microvesicles during shedding, that is, reverse budding, of the plasma membrane of the donor cells (Wolf, 1967; Poste and Nicolson, 1980). Subsequently, the predominant site of release of both microvesicles and GPI-proteins was identified as DIGs (Del Conde et al., 2005). Compatible with this view was the observed loss of the DIGs-associated GPI-proteins, CD55 and CD59, from human erythrocytes upon their storage (Long et al., 1993) and the spontaneous release of the GPI-proteins, acetylcholinesterase and decay accelerating factor, from human erythrocytes into microvesicles (Bütikofer et al., 1989).
Interestingly, the GPI-proteins, Gce1 and CD73, together with subsets of transmembrane and peripheral membrane proteins were found to be released from plasma membrane DIGs of primary and cultured rodent adipocytes into microvesicles and exosomes, collectively called adiposomes, upon challenge with physiological concentrations of H2O2 or palmitate and pharmacological concentrations of the antidiabetic sulfonylurea drug, glimepiride (Aoki et al., 2007; Müller et al., 2009a). Moreover, both exosomes and microvesicles seem to be involved in cell-to-cell transfer of GPI-proteins. This was deduced from the significant transfer of CD55 and CD59 from microvesicles and exosomes derived from normal erythrocytes to the surface of erythrocytes from patients with paroxysmal nocturnal haemoglobinuria that were deficient in GPI-proteins (Sloand et al., 1998). Effective transfer into the outer leaflet of the plasma membrane was found to require the fatty acyl chains of the GPI anchor (Civenni et al., 1998; Sloand et al., 1998).
The localization and function of GPI-proteins so far found to be transferred from donor to acceptor cells seem to be restricted to the plasma membrane. However, the GPI-proteins, Gce1 and CD73, have recently been attributed an intracellular function in the regulation of lipid metabolism in rat adipocytes (Müller et al., 2008a,d;). Both Gce1 and CD73 are translocated from plasma membrane DIGs to intracellular lipid droplets (LDs) in response to H2O2, palmitate or glimepiride (Müller et al., 2008b,e;). LDs are cytoplasmic organelles that store lipids and cholesterylester in their core and are surrounded by a monolayer of phospholipids and free cholesterol with embedded so-called PAT proteins (Müller and Petry, 2005; Ducharme and Bickel, 2008; Fujimoto et al., 2008). The intrinsic cAMP-specific phosphodiesterase and 5′-nucleotidase activities of Gce1 and CD73, respectively, cause the degradation of cAMP and AMP at the LD surface zone, thereby leading to upregulation of the esterification of fatty acids into lipids and inhibition of lipolysis (Müller et al., 1994b; 2008c;).
Together these findings raised the possibility that Gce1- and CD73-harbouring adiposomes released from donor adipocytes control the lipid metabolism of acceptor adipocytes in a signal-dependent fashion. This view was corroborated in the present study by demonstrating of the transfer of CD73 from adiposomes prepared from donor adipocytes to DIGs and LD of acceptor adipocytes that leads to stimulation of fatty acid esterification. The differences between small and large adipocytes in operating as adiposome acceptor and donor cells, respectively, are compatible with a paracrine role of GPI-protein transfer via adiposomes in vivo.
Methods
Preparation and separation of large and small adipocytes
All animal care and experimental procedures complied with the German animal protection law. Adipocytes were isolated by collagenase digestion of epididymal fat pads from three-months old male Sprague Dawley rats (Charles-River Laboratories (Wilmington, MA, USA; 140–160 g, fed ad libitum) under sterile conditions and washed according to published procedures (Müller et al., 1997a) and then filtered through serial nylon mesh screens with pore sizes of 400, 150 and 75 µm to obtain small (diameter < 75 µm) and large (diameter > 400 µm) adipocytes. After determination of the cell number with a Coulter counter following fixation of aliquots of the suspension with osmic acid as described previously (Cushman and Salans, 1978; Müller et al., 2009b), the adipocytes were adjusted with adipocyte buffer [140 mM NaCl, 4.7 mM KCl, 2.5 mM CaCl2, 1.2 mM MgSO4, 1.2 mM KH2PO4, 20 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid-KOH (HEPES-KOH), pH 7.4] supplemented with 0.2% (w/v) bovine serum albumin (BSA), 100 µg mL−1 gentamycin, 50 units mL−1 penicillin and 50 µg mL−1 streptomycin sulfate to 3.5 × 106 cells mL−1.
Preparation of stromal vascular cells (SVCs)
SVCs were isolated according to previous published protocols (Rodbell, 1964) with the following modifications. After collagenase digestion, the adipocyte suspension (derived from 20 epididymal fat pads) was centrifuged (500× g, 10 min, 20°C). The pellet was suspended in 10 mL of adipocyte buffer and then filtered through a 150-µm mesh to remove undigested clumps, debris and large adipocytes. The filtrate was centrifuged (500× g, 10 min, 20°C). Pelleted cells were resuspended in 5 mL of adipocyte buffer and then consecutively filtered through serial nylon mesh screens with pore sizes of 75 and 30 µm. The last filtrate was centrifuged (1500× g, 10 min, 20°C). The pelleted SVC were washed once in 5 mL of phosphate-buffered saline (PBS), recentrifuged and then suspended in adipocyte buffer supplemented with 100 µg mL−1 gentamycin, 50 units mL−1 penicillin and 50 µg mL−1 streptomycin sulfate at 3.5 × 106 cells mL−1.
Induction of cells
For induction with glucose oxidase (GO), glimepiride or palmitate, the adipocytes or SVC were suspended in adipocyte buffer supplemented with 0.7% (w/v) BSA, 3.5 mM sodium pyruvate and 20 mM d-glucose at 1.0 × 106 cells mL−1 and then incubated (30 min, 37°C) in primary culture in a shaking water bath (100 cycles min−1) under constant bubbling with 95% O2/5% CO2 as outlined previously (Müller and Wied, 1993; Müller et al., 2008d).
Preparation of adiposomes
For preparation of adiposomes harbouring metabolically labelled CD73, untreated rat adipocytes (25 mL, 3.5 × 105 cells mL−1) were washed twice with 50 mL of labelling medium (adipocyte buffer supplemented with 6 mM glucose, 1 mM sodium pyruvate, 0.2% BSA, 25 µg mL−1 gentamycin, 20 units mL−1 penicillin and 20 µg mL−1 streptomycin sulfate) each, and then incubated (2 h, 37°C) in 44 mL of labelling medium in 150 mL culture flasks under 95% O2/5% CO2. Labelling was started by the addition of myo-[U-14C]inositol (50 µCi in 1 mL of labelling medium, 0.1 mM final conc.). After incubation (5 h, 37°C), the adipocytes were induced by the addition of GO (0.5 U mL−1) or left non-induced. Following incubation (12 h, 37°C) and subsequent addition of 5 mL of labelling medium supplemented with 100 mM myo-inositol, the adiposomes were prepared according to published protocols (Müller et al., 2009a,b;) with the following modifications. The adipocyte suspension (50 mL) was transferred (continuous shaking) into polypropylene tubes and then centrifuged (500× g, 1 min, 20°C). The lower layer of fluid below the floating adipocyte layer was removed by suction taking care to avoid contamination with the adipocytes and transferred into new tubes. This procedure was repeated twice. The final lower layer was passed through a 5-µm mesh to remove residual cells. The filtrate was supplemented with dithiothreitol (DTT; final conc. 0.5 mM) and protease inhibitor mix and then centrifuged (3000× g, 20 min, 4°C) to remove residual cell debris. The supernatant was centrifuged (150 000× g, 60 min, 4°C, Ti-40 rotor, Beckman, Krefeld, Germany). After careful aspiration of the supernatants, the pellets were suspended in 30 mL of adiposome buffer (10 mM TRIS-HCl, pH 7.4, 250 mM sucrose, 1 mM ethylenediaminetetraacetic acid, 0.5 mM ethylene glycol tetraacetic acid, 140 mM NaCl, 10 mM MgCl2, 2 mM MnCl2, 1 mM isobutylmethylxanthine, 1 mM DTT, 20 mM sodium fluoride, 25 mM glycerol-3-phosphate, 10 mM sodium pyrophosphate and protease and phosphatase inhibitor mixes) and re-centrifuged as above. The pellet was washed (vortexing) two times with 15 mL of adiposome buffer, each. After determination of the phosphatidylcholine (PC) content, the adiposomes were suspended in adiposome buffer at 1 µg PC per µL.
Incubation of cells with adiposomes
Isolated rat adipocytes or SVCs (3.5 × 106 cells) were incubated in 1.5 mL of adipocyte buffer supplemented with 5.5 mM glucose, 1 mM sodium pyruvate, 0.2% (w/v) BSA, 100 µg mL−1 gentamycin, 50 units mL−1 penicillin and 50 µg mL−1 streptomycin sulfate in the presence of adiposomes at the amounts (in terms of PC content) and periods and temperatures as indicated. Thereafter the mixtures were supplemented with 13.5 mL of adipocyte buffer.
Adipocytes were collected by flotation (500× g, 1 min, 20°C), washed once with 15 mL of adipocyte buffer and then suspended in 1.5 mL of adipocyte buffer (final titer 1.4 × 106 cells mL−1). Two hundred microliter portions of the adipocyte suspension were transferred (continuous shaking) into microfuge tubes (Beckman) pre-filled with 100 µL of dinonylphtalate and then centrifuged (500× g, 1 min, 20°C). The tubes were cut through the dinonylphtalate layer separating the incubation medium in the bottom part from the adipocytes at the top of the upper part that were saved. Care was taken to minimize the volume of dinonylphtalate taken along with the adipocytes. After transfer of the adipocytes into 10 mL polystyrene tubes and washing the upper part of the microfuge tubes once with 1 mL of adipocyte buffer containing protease inhibitor mix, the combined adipocyte suspension and washing fluid were centrifuged (500× g, 2 min, 20°C). After careful aspiration of the lower layer, the adipocytes floating at the top were suspended in 1.5 mL of adipocyte buffer (2 × 106 cells mL−1).
SVCs were collected by centrifugation (500× g, 10 min, 20°C), suspended in 10 mL of adipocyte buffer, washed twice by centrifugation and finally suspended in 2.5 mL of adipocyte buffer (∼106 cells mL−1).
Adsorption of CD73-harbouring adiposomes to AMP-Sepharose beads
The incubation mixtures containing adipocytes and adiposomes were supplemented with 200 µL of AMP-Sepharose beads (50 mg AMP-Sepharose beads in 1 mL of 100 mM HEPES-KOH, pH 7.4, 140 mM NaCl, 1 mM MgCl2, 0.5 mM DTT and protease inhibitor mix) and then incubated with continuous head-over rotation (60 cycles per min) of the tubes. Thereafter, the incubation mixtures were centrifuged under conditions (2500× g, 5 min, 20°C), which did not lead to sedimentation of the unadsorbed adiposomes per se, but were sufficient for the quantitative sedimentation of the Sepharose beads (with the CD73-harbouring adiposomes adsorbed) and concomitant flotation of the adipocytes. The adipocytes at the top of the tubes were recovered together with the lower layer containing the unadsorbed adiposomes by suction, transferred into new tubes and then recentrifuged. The adipocytes and lower layer were recovered together, transferred into new tubes, gently mixed and then further incubated as indicated.
In/on-cell Western blotting
After incubation of the adipocytes and subsequent removal of the medium by suction, the cells were washed three times with adipocyte buffer (0.5 mL per well) and then fixed by the addition of p-formaldehyde (3.7% in PBS, 150 µL per well). After incubation (20 min, 25°C), the formaldehyde was removed by suction. For permeabilization, the adipocytes were washed twice with Triton X-100 (0.35% in PBS) for 10 min, each, with shaking. Permeabilized or intact cells were blocked by the addition of blocking buffer (Odyssey, Li-Cor, Bad Homburg, Germany, 150 µL per well). After incubation (2 h, 25°C) with shaking and removal of the blocking buffer, anti-perilipin A (1:400, final dilution) and anti-CD73 (1:200) antibodies in blocking buffer containing 0.1% Tween 20 (100 µL per well) were added and incubated (2 h, 25°C). Thereafter, the wells were washed five times (10 min each) with washing buffer (0.1% Tween-20 in PBS, 200 µL per well) with shaking and then supplemented (50 µL per well) with anti-rat IgG (Molecular Probes, Eugene, OR, USA, AlexaFluoro-680, 1:1000) and anti-rabbit IgG (Rockland, Gilbertsville, PA, USA, IRDye 800CW, 1:2000). After incubation (1 h, 25°C, protected from light), the wells were washed five times (5 min each) with washing buffer (200 µL per well) under shaking and then imaged by scanning simultaneously at 700 and 800 nm with an Odyssey Infrared Imaging System from Li-Cor Biosciences at 169 µm resolution, medium quality, focus off-set of 3.0 mm and intensity setting of 5 for both channels. Data were calculated as arbitrary units using the corresponding software (Li-Cor Odyssey).
Data analysis
Results are shown as mean ± standard deviation. Differences between various treatments as indicated were analysed by unpaired Student's t-tests or one-way analysis of variance, with Bonferroni post-tests, as applicable with P values < 0.05 considered as significant. Concentration-response curves were fitted using the GraphPad Prism 4.03 software (GraphPad Software Inc., La Jolla, CA, USA). Phosphorimages were quantified by computer-assisted video densitometry using the Storm 860 PhosphorImager system (Molecular Dynamics, Gelsenkirchen, Germany) and transformed into figures using the Adobe Photoshop software (Adobe Systems, Mountain View, CA, USA).
Miscellaneous
Published procedures were used for the preparation of LD and DIGs (from 2.5 × 106 cells per incubation; Müller et al., 2001; 2002; 2008a,d;), determination of 5′-nucleotidase activity (Müller et al., 2008d), [1-14C]palmitate-driven esterification into total radiolabelled acylglycerols (with 2.5 × 105 cells in 1 mL of incubation volume; Müller et al., 2008c), protein concentration and PC content of adiposomes (Müller et al., 2009a), affinity purification of CD73 using AMP-Sepharose (Müller et al., 2008a,d;), sodium dodecylsulfate-polyacrylamide gel electrophoresis [SDS-PAGE; 4–12% Bis-TRIS precast gel, pH 6.4, 4-morpholineethanesulfonic acid (MES)-SDS running buffer] under reducing conditions (Müller et al., 2001), extraction and precipitation of proteins from LD, DIGs and incubation medium under native conditions (Müller et al., 2009b) and treatment of ADIP, DIGs, LD or incubation medium with alkaline Na2CO3, NaCl, detergents, free and cholesterol-bound methyl-β-cyclodextrin (m-βCD) (glycosyl)phosphatidylinositol-specific phospholipase C (GPI-PLC) from Bacillus cereus or NaNO2 (0.25 M, adjusted with sodium acetate to pH 4.0, 3 h at 25°C) (Müller et al., 1997b; 2008c; 2009a,b;).
Materials
Myo-[U-14C]inositol was purchased from Amersham-Buchler (Braunschweig, Germany). Recombinant human insulin, glimepiride (Müller et al., 1994b), β-amidotaurocholate (Müller et al., 1994a) and GPI-2350 (Müller et al., 2005) were made available by the biotechnology and medicinal chemistry departments of Sanofi-Aventis Germany GmbH (Frankfurt, Germany). GO, pre-mixed protease (complete EASYpack) and phosphatase (PhosSTOP) inhibitor cocktails (one tablet for 10 mL) were bought from Roche Molecular Biochemicals (Mannheim, Germany). Dinonylphtalate was from Calbiochem-Merck (Darmstadt, Germany). β-D-lactosyl-N-octanoyl-L-threo-sphingosine (l-t-LacCer) was provided by Avanti Polar Lipids (Alabaster, AL, USA). AMP-Sepharose beads were purchased from LKB/Pharmacia (Freiburg, Germany). Antibodies and all other (radio-)materials were obtained as described previously (Müller et al., 1997a; 2002; 2005; 2008a,d;).
The nomenclature of drugs and targets follows Alexander et al. (2009).
Results
Adiposome-derived GPI-anchored CD73 is transferred to plasma membrane DIGs of small adipocytes
Previously, the release of adiposomes harbouring GPI-proteins from rat adipocytes had been demonstrated (Müller et al., 2009a) and shown to depend on cell size, with larger adipocytes being significantly more effective than smaller ones (Müller et al., 2009b). As a model system for the elucidation of the putative physiological function of microvesicles and exosomes released from mammalian cells as intercellular carriers for GPI-proteins, the transfer of CD73 from donor to acceptor adipocytes via adiposomes was studied. For this, adiposomes prepared from metabolically labelled (with myo-[14C]inositol for the detection of CD73) and then GO-induced (for the release of adiposomes driven by H2O2, which is generated upon GO action in the incubation medium) (Müller et al., 2008c) rat adipocytes as the donor cells were incubated with small and large adipocytes as the putative acceptor cells (Figure 1). The known H2O2-driven translocation of GPI-proteins from DIGs to intracellular LD was blocked by the inhibitor of the GPI-PLC, GPI-2350 (Müller et al., 2008b,d,e;). After incubation for 2 min at 10°C, the association of CD73 with the adipocytes or SVC was analysed after their recovery from the incubation mixtures by flotation through dinonylphtalate or centrifugation, respectively (Figure 1). SVC served as control as they are commonly assumed to represent the adipose tissue vascular cell depot from which adipocyte precursor cells are recruited (Rodeheffer et al., 2008).
Figure 1.
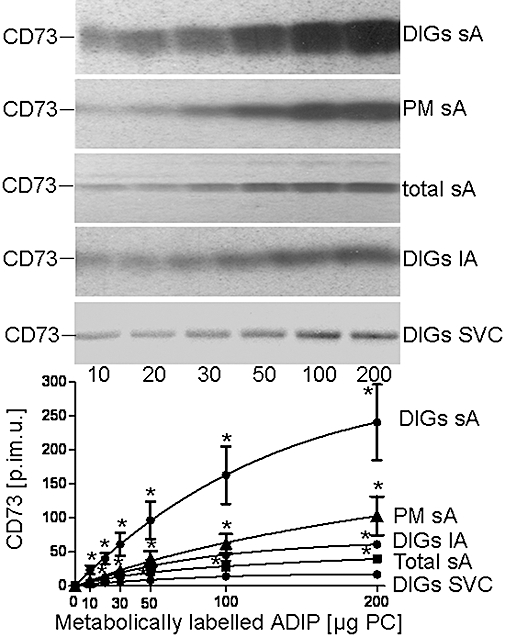
Transfer of adiposome-derived CD73 with detergent-insoluble glycolipid-enriched plasma membrane microdomains (DIGs) of various adipose tissue cells. Small adipocytes (sAs), large adipocytes (lAs) or stromal vascular cell (SVCs) were incubated (2 min, 10°C, presence of 100 µM GPI-2350) in the absence or presence of increasing amounts [according to phosphatidylcholine (PC) content] of adiposomes (ADIP) from glucose oxidase (GO)-induced adipocytes harbouring metabolically labelled CD73. The adipocytes or SVCs were recovered by flotation and subsequent centrifugation through dinonylphtalate or centrifugation, respectively. From portions of the cells, total plasma membrane (PM) or plasma membrane DIGs were prepared. Proteins of the DIGs, PMs and total small adipocytes (total sAs) were extracted, precipitated under native conditions and then analysed for CD73 by affinity purification, sodium dodecylsulfate-polyacrylamide gel electrophoresis and phosphorimaging. Representative images are shown. Quantitative evaluations of the amounts of radiolabelled CD73 [in phosphorimaging units (p.im.u.)] are given for three independent incubations and determinations in triplicate each (mean ± standard deviation). *Significantly different from DIGs from SVC.
Adiposome-derived CD73 was found associated with small adipocytes and their plasma membranes and DIGs as well as with large adipocytes and SVC. The accumulation was dependent on the amount of adiposomes and higher for DIGs compared with plasma membranes and total membranes and more pronounced for small, rather than large, adipocytes and SVC. This is compatible with the known enrichment of endogenous CD73 at DIGs of rat adipocytes (Müller et al., 2001). The association of CD73 with DIGs of small adipocytes was significantly reduced upon increasing the incubation temperature to 37°C or period to 30 min or on using adiposomes that had been pretreated with bacterial PI-PLC (Figure 2). In the last instance, the partial reduction in DIGs-associated CD73 correlated well with 50–65% loss of CD73 from the adiposomes (data not shown). This is presumably caused by (incomplete) lipolytic cleavage of the GPI anchor of CD73. Blockade of the translocation of GPI-proteins from DIGs to LD by the non-hydrolyzable AMP analogue, AMPCP, or independently by inhibition of the GPI-PLC by GPI-2350 (Müller et al., 2008b,e;) reduced the DIGs association of CD73 to similar extents (Figure 2). The amount of DIGs-associated CD73 was not significantly altered upon incubation of (small) acceptor adipocytes with GO, glimepiride or palmitate (data not shown).
Figure 2.
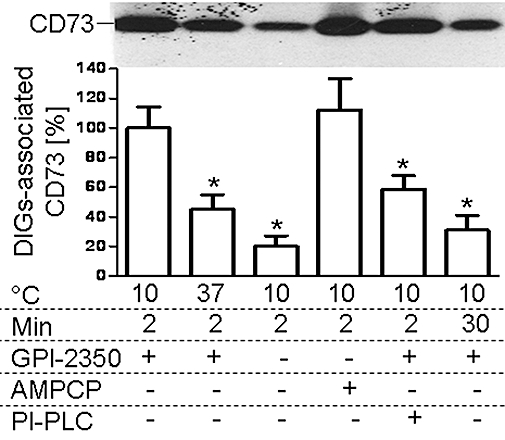
Mechanism of the transfer of adiposome-derived CD73 with detergent-insoluble, glycolipid-enriched plasma membrane microdomains (DIGs) of small adipocytes. Adiposomes from glucose oxidase (GO)-induced adipocytes [100 µg phosphatidylcholine (PC) each] harbouring metabolically labelled CD73, which had been pretreated without or with phosphatidylinositol-specific phospholipase C (PI-PLC) from Bacillus cereus (2.5 IU mL−1), were incubated (2 or 30 min, 10 or 37°C) with small adipocytes in the absence or presence of GPI-2350 (100 µM) or α,β-methlyene-ADP (AMPCP; 100 µM). After recovery of the adipocyte plasma membrane, DIGs were prepared and then extracted for protein under native conditions. Precipitated proteins were analysed for CD73 by affinity purification, sodium dodecylsulfate-polyacrylamide gel electrophoresis and phosphorimaging. Quantitative evaluations of the amount of DIGs-associated radiolabelled CD73 are given for four independent incubations and determinations in duplicate each (mean ± standard deviation) as % of the control (2 min, 10°C, presence of GPI-2350) set at 100. *Significantly different from control.
Next, the nature of the association of adiposome-derived CD73 with adipocyte DIGs was studied. Treatment with NaCl or alkaline Na2CO3 for the release of typical peripheral but not integral membrane proteins and GPI-proteins or with Triton X-100 (1%, 4°C, 1 h) for the release of integral membrane proteins from plasma membranes and GPI-proteins from areas of plasma membranes distinct from DIGs (Müller et al. 2001; 2002;) did not reduce the amount of DIGs-associated CD73 compared with control (Figure 3). However, incubation with Triton X-100 in the presence of octylglucoside or β-amidotaurocholate or bacterial PI-PLC, all known to exert efficient release of GPI-proteins from adipocyte DIGs (Müller et al., 1994a; 1997b; 2001; 2002;) led to almost complete loss of CD73 from the DIGs (Figure 3).
Figure 3.
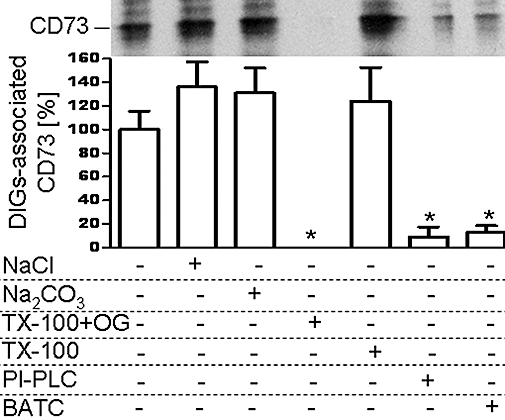
Extraction of adiposome-derived CD73 from detergent-insoluble, glycolipid-enriched plasma membrane microdomains (DIGs). Adiposomes from glucose oxidase (GO)-induced adipocytes [100 µg phosphatidylcholine (PC) each] harbouring metabolically labelled CD73 were incubated (2 min, 10°C, presence of 100 µM GPI-2350) with small adipocytes. After recovery of the adipocytes plasma membrane DIGs were prepared and then treated in the absence (control) or presence of NaCl (1 M), Na2CO3 (0.1 M, pH 11.5), TX-100 (2%) plus octylglucoside (60 mM; TX-100+OG), TX-100 (1%), phosphatidylinositol-specific phospholipase C (PI-PLC) from Bacillus cereus (2.5 IU mL−1), or β-amidotaurocholate (BATC; 4%). The DIGs were recovered from the incubation mixtures by centrifugation (100 000× g, 1 h, 4°C) and then washed twice with adiposome buffer and then extracted for protein under native conditions. Precipitated proteins were analysed for CD73 by affinity purification, sodium dodecylsulfate-polyacrylamide gel electrophoresis and phosphorimaging. Representative images are shown. Quantitative evaluations of the amount of DIGs-associated radiolabelled CD73 are shown for three independent incubations and determinations in quadruplicate each (mean ± standard deviation) as % of the control (no additions) set at 100. *Significantly different from control.
Together these findings suggest that CD73 is transferred from adiposomes to plasma membrane DIGs of acceptor adipocytes and upon interference with its subsequent translocation to LD (by low temperature, short time, blockade of the GPI-PLC, presence of AMPCP) accumulates at DIGs. The transfer depends on the presence of the intact GPI anchor as: (i) CD73 accumulated at DIGs was metabolically labelled with the GPI anchor constituent myo-inositol; and (ii) accumulation of CD73 at DIGs was prevented by treatment of the adiposomes or DIGs with bacterial PI-PLC.
Adiposome-derived GPI-anchored CD73 is translocated to LDs of small adipocytes
Recently, the translocation of endogenous Gce1 and CD73 from plasma membrane DIGs to intracellular LDs in rat adipocytes upon their induction with H2O2, glimepiride or palmitate has been reported (Müller et al., 2008a–c,e). To study the putative translocation of adiposome-derived CD73 after its transfer to plasma membrane DIGs of acceptor cells, small and large adipocytes as well as SVC were incubated with adiposomes from GO-induced adipocytes harbouring metabolically labelled CD73. LDs were prepared from the recovered adipocytes and analysed for CD73 (Figure 4, upper panel). In the absence of GPI-2350 and at 37°C, which favor the translocation of endogenous GPI-proteins from DIGs to LD, CD73 was found associated with LD of small and large adipocytes and SVC. The association was dependent on the amout of adiposomes added and more efficient with small than large adipocytes or SVC. Alternatively, LD association of CD73 was followed by assaying the LD from GO-induced small adipocytes upon their incubation with (unlabelled) adiposomes for 5′-nucleotidase (Figure 4, lower panel). Adiposome-derived 5′-nucleotidase activity (calculated as the difference between incubation with and without adiposomes) associated with the LD from both non-induced and GO-induced adipocytes increased with increasing amounts of adiposomes from non-induced and, more pronouncedly, GO-induced adipocytes. The association of adiposome-derived metabolically labelled CD73 with LD was time-dependent and significantly stimulated by GO, glimepiride and palmitate in this order of declining potency (Figure 5) with half maximal effective concentration (EC50) values of 0.25–0.33 U mL−1, 5.3–7.7 µM and 0.5–0.7 mM, respectively (Figure 6).
Figure 4.
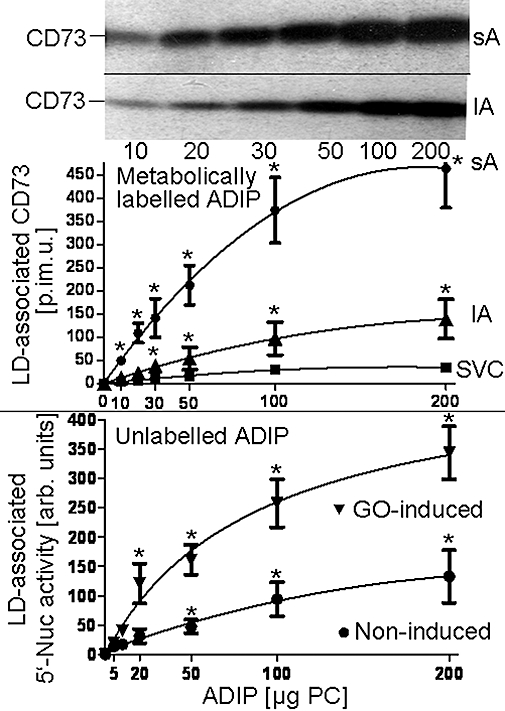
Translocation of adiposome-derived CD73 with lipid droplets (LDs) of adipose tissue cells. Increasing amounts of adiposomes [ADIPs; according to phosphatidylcholine (PC) content] harbouring metabolically labelled CD73 or unlabelled adiposomes from glucose oxidase (GO)-induced (0.5 U mL−1) or non-induced adipocytes were incubated (1 h, 37°C) with small adipocytes (sAs), large adipocytes (lAs) or stromal vascular cell (SVCs) in the presence of GO (1 U mL−1). After recovery of the adipocytes or SVC by flotation and subsequent centrifugation through dinonylphtalate or centrifugation, respectively, LD were prepared and then extracted for protein under native conditions. Precipitated proteins from incubations with metabolically labelled adiposomes were analysed for CD73 by affinity purification, sodium dodecylsulfate-polyacrylamide gel electrophoresis and phosphorimaging (upper panel). Representative images are shown. Proteins from incubations with unlabelled adiposomes were analysed for 5′-nucleotidase activity (5′-Nuc) (lower panel). Quantitative evaluations of the amount of LD-associated radiolabelled CD73 (in phosphorimaging units = p.im.u.) (upper panel) or of the LD-associated 5′-nucleotidase activity (in arbitrary units) (lower panel) are given for 4 independent incubations and determinations in duplicate each (mean ± standard deviation). *Significantly different from SVC or absence of adiposomes.
Figure 5.
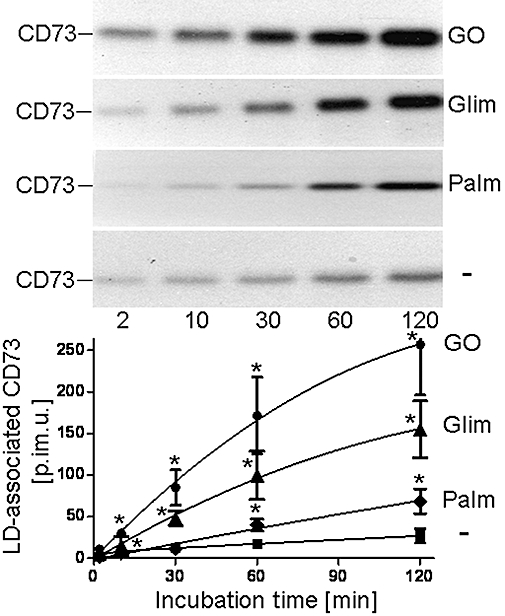
Time course of the translocation of adiposome-derived CD73 with lipid droplets (LD) of small adipocytes. Adiposomes from glucose oxidase (GO)-induced adipocytes [100 µg phosphatidylcholine (PC) each] harbouring metabolically labelled CD73 were incubated (37°C) with small adipocytes in the absence (non-induced, squares) or presence of palmitate (1 mM, Palm, diamonds), glimepiride (20 µM, Glim, triangles) or GO (1 U mL−1, circles) for increasing periods of time. After recovery of the adipocytes LD were prepared and extracted for protein under native conditions. Precipitated proteins were analysed for CD73 by affinity purification, sodium dodecylsulfate-polyacrylamide gel electrophoresis and phosphorimaging. Representative images are shown. Quantitative evaluations of the amounts of LD-associated radiolabelled CD73 (in phosphorimaging units = p.im.u.) are given for three independent incubations and determinations in duplicate each (mean ± standard deviation). *Significantly different from non-induced state.
Figure 6.
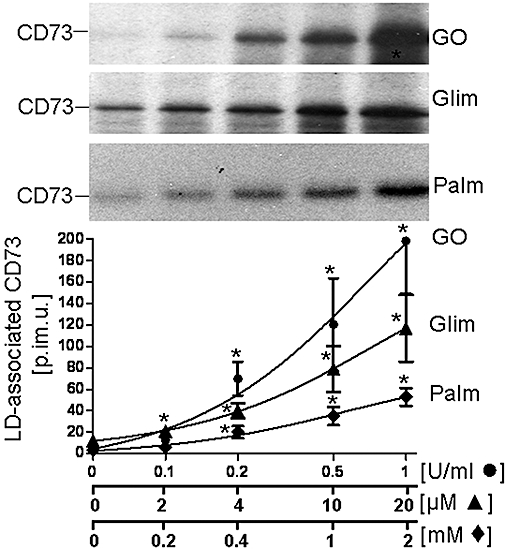
Concentration-dependent induction of the translocation of adiposome-derived CD73 with lipid droplets (LDs) of small adipocytes. Adiposomes from glucose oxidase (GO)-induced adipocytes [100 µg phosphatidylcholine (PC) each] harbouring metabolically labelled CD73 were incubated (1 h, 37°C) with small adipocytes in the absence (non-induced) or presence of increasing concentrations of palmitate (Palm), glimepiride (Glim) or GO. After recovery of the adipocytes, LD were prepared and extracted for protein under native conditions. Precipitated proteins were analysed for CD73 by affinity purification, sodium dodecylsulfate-polyacrylamide gel electrophoresis and phosphorimaging. Representative images are shown. Quantitative evaluations of the amounts of LD-associated radiolabelled CD73 (in phosphorimaging units = p.im.u.) are given for four independent incubations and determinations in triplicate each (mean ± standard deviation). *Significantly different from non-induced.
Next, the molecular mechanism of the translocation of adiposome-derived CD73 to LD upon its transfer to DIGs of acceptor adipocytes was studied using different modes of blockade of endogenous GPI-protein translocation (Müller et al., 2008b,c,e;), such as low temperature, disruption of DIGs in course of cholesterol depletion with m-βCD or insertion of the non-physiological ceramide analogue, l-t-LacCer, inhibition of the GPI-PLC by GPI-2350 and conformational stabilization of CD73 by AMPCP. Incubation of acceptor adipocytes with metabolically labelled adiposomes at 10°C or in the presence of m-βCD, β-D-lactosyl-N-octanoyl-L-threo-sphingosine (l-t-LacCer), GPI-2350 or AMPCP significantly reduced the amounts of LD-associated CD73 compared with 37°C in the absence of m-βCD or presence of m-βCD preloaded with cholesterol (m-βCD-chol) (Figure 7).
Figure 7.
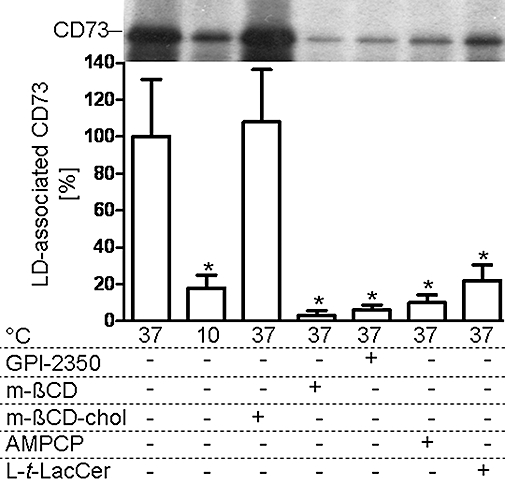
Mechanism of the translocation of adiposome-derived CD73 with lipid droplets (LDs) of small adipocytes. Adiposomes from glucose oxidase (GO)-induced adipocytes [100 µg phosphatidylcholine (PC) each] harbouring metabolically labelled CD73 were incubated (1 h, 10 or 37°C) with small adipocytes and GO (1 U mL−1) in the absence (control) or presence of GPI-2350 (100 µM), α,β-methlyene-ADP (AMPCP; 100 µM), methyl-β-cyclodextrin (m-βCD; 3.8 mM), m-βCD bound to cholesterol (m-βCD-chol, 1.9 mM m-βCD plus 1.9 mM cholesterol) or β-D-lactosyl-N-octanoyl-L-threo-sphingosine (l-t-LacCer) (50 µM). After recovery of the adipocytes, LDs were prepared and extracted for protein under native conditions. Precipitated proteins were analysed for CD73 by affinity purification, sodium dodecylsulfate-polyacrylamide gel electrophoresis and phosphorimaging. Representative images are shown. Quantitative evaluations of the amounts of LD-associated radiolabelled CD73 are shown for two independent incubations and determinations in triplicate each (mean ± standard deviation) as % of the control (37°C, no additions) set at 100. *Significantly different from control.
Next, the nature of the association of adiposome-derived CD73 with the intracellular LD was studied. On basis of the apparent translocation of adiposome-derived CD73 from DIGs to LD of acceptor adipocytes, LD prepared from small adipocytes that had been incubated with adiposomes harbouring metabolically labelled CD73 were treated with the same methods effective with DIGs (see Figure 3). NaCl, alkaline Na2CO3 and m-βCD preloaded with cholesterol (m-βCD-chol) did not significantly alter the amount of adiposome-derived CD73 found associated with the LD (Figure 8, upper panel) or found released from the LD into the infranatant upon their flotation (Figure 8, lower panel) compared with control. In contrast, m-βCD alone or SDS led to almost complete loss of adiposome-derived CD73 from the LD and, in parallel, release into the lower layer. Following treatment with NaNO2, which is known to cause cleavage of GPI anchors by deamination of their non-acetylated glucosamine constituent (Müller et al., 1997b), CD73 metabolically labelled at the inositol constituent of its GPI anchor (Figure 8) was detected neither with the LD (upper panel) nor lower layer (lower panel). This is compatible with removal of the radiolabelled myo-inositol from the GPI anchor by nitrous acid deamination and consequent release of the unlabelled CD73 protein moiety from the LD into the lower layer. Together the findings suggest that adiposome-derived CD73 with intact GPI anchor from (GO-induced) adipocytes (as the donor cells) upon transfer to plasma membrane DIGs of (preferably) small adipocytes (as the acceptor cells) is translocated to cytoplasmic LD. This process is controlled by the H2O2-, glimepiride- and palmitate-induced GPI-PLC and leads to elevated 5′-nucleotidase activity at the LD surface zone.
Figure 8.
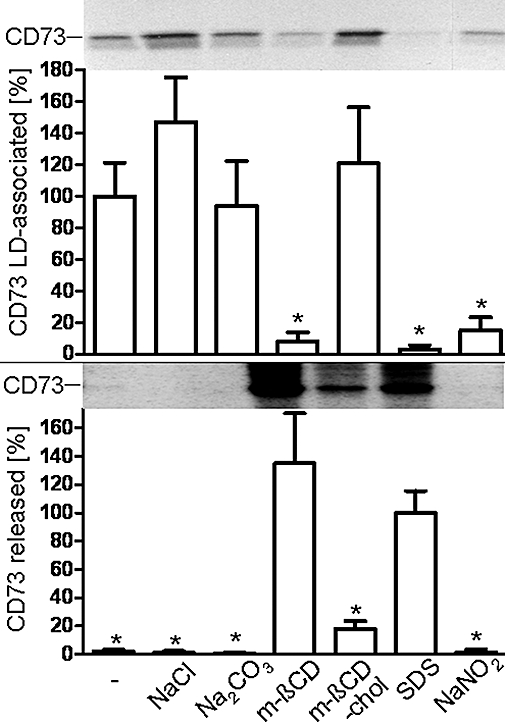
Extraction of adiposome-derived CD73 from lipid droplets (LDs). Adiposomes from glucose oxidase (GO)-induced adipocytes [200 µg phosphatidylcholine (PC) each] harbouring metabolically labelled CD73 were incubated (1 h, 37°C) with small adipocytes in the presence of GO (1 U mL−1). After recovery of the adipocytes by flotation and subsequent centrifugation through dinonylphtalate, LD were prepared and then treated in the absence (control) or presence of NaCl (1 M), Na2CO3 (0.1 M, pH 11.5), methyl-β-cyclodextrin (m-βCD; 3.8 mM), m-βCD bound to cholesterol (m-βCD-chol, 1.9 mM m-βCD plus 1.9 mM cholesterol), sodium dodecyl sulphate (SDS; 1%) or NaNO2 (0.25 M). After flotation (2000× g, 5 min, 24°C) the LD were collected from the top, washed twice with adiposome buffer and then extracted for protein under native conditions (upper panel). Proteins contained in the layer below the LD were precipitated under native conditions (lower panel). Precipitated proteins from the LD and lower layer were analysed for CD73 by affinity purification, sodium dodecylsulfate-polyacrylamide gel electrophoresis and phosphorimaging. Representative images are shown. Quantitative evaluations of the amount of radiolabelled LD-associated (upper panel) and released (lower panel) CD73 are shown for two independent incubations and determinations in triplicate each (mean ± standard deviation) as % of the control or SDS treatment set at 100, each. *Significantly different from control (upper panel) or from SDS treatment (lower panel), respectively.
The transfer of CD73 from adiposomes to plasma membranes and its subsequent translocation to LD of acceptor adipocytes was confirmed independently of the method of cell fractionation. For this, the localization of CD73 after incubation of small acceptor adipocytes with adiposomes from GO-induced donor adipocytes was analysed by in/on-cell Western blotting. This was accomplished by adsorption of the adipocytes to the wells of microtiter plates, incubation with adiposomes and then fixation under maintenance of cellular integrity. To allow access of antibodies against perilipin A, a typical LD-associated PAT protein, and against CD73 to the cytoplasm, including the LD (in-cell Western), or only to the cell surface (on-cell Western), the adipocytes were permeabilized with Triton X-100 or left untreated, respectively (Figure 9). LD, which almost completely fill the cytoplasm of rat adipocytes, were labelled with anti-perilipin A antibodies by in-cell Western. In contrast, very weak perilipin-A signals were visible by on-cell Western, only. This may be due to the known expression of minor amounts of perilipin at the plasma membrane of adipocytes (Aboulaich et al., 2006). This low amount of perilipin-A at the cell surface of intact adipocytes was not significantly increased on induction of the adipocytes with palmitate, glimepiride and GO or incubation with adiposomes. In contrast, CD73 was clearly detected during on-cell and, at a slightly elevated level, during in-cell Western blotting (Figure 9). Importantly, induction with glimepiride and GO significantly reduced the amounts of both endogenous (incubation in the absence of adiposomes) and adiposome-derived CD73 at the cell surface of intact adipocytes, but did not affect total CD73 as visualized by in-cell Western blots with permeabilized adipocytes. This correlated well with the corresponding increases of the difference between total (and roughly constant) CD73 as measured for permeabilized cells by in-cell Western and CD73 at the cell surface of intact cells according to on-cell Western, which represents an indirect measurement of the amount of LD-associated CD73. The increases are therefore compatible with translocation of CD73 from plasma membrane DIGs to cytoplasmic LD in response to palmitate, glimepiride and GO. Upon incubation with adiposomes, the amounts of CD73 were significantly upregulated in both intact and permeabilized adipocytes (Figure 9). By calculation, the amount of CD73 at the LD was found to increase upon incubation with adiposomes and to be stimulated by GO and glimepiride in correlation to its loss from the plasma membranes (Figure 9). The apparent correlation between the appearance of both endogenous and adiposome-derived CD73 at LD and their loss at the cell surface is compatible with the constitutive transfer of CD73 from adiposomes to plasma membranes and its subsequent signal-induced translocation to cytoplasmic LD of acceptor adipocytes and thereby confirms the conclusion drawn from the cell fraction experiments.
Figure 9.
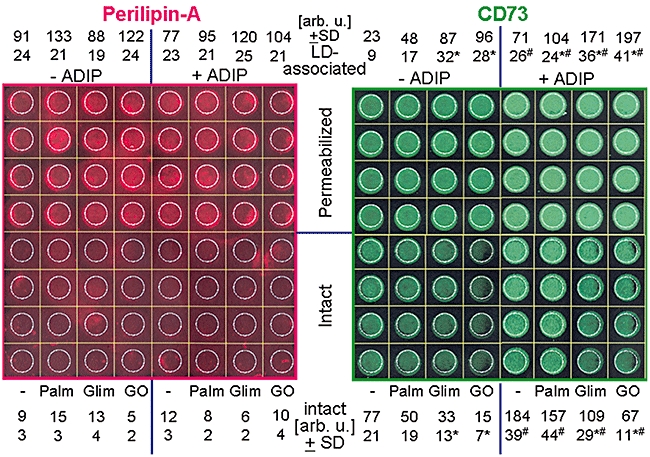
Demonstration of the transfer to plasma membranes and translocation to lipid droplets (LDs) of adiposome-derived CD73 in intact adipocytes. Small rat adipocytes (25 000 per well) were adsorbed to the wells of a collagen-coated 96-well plate (Corning Schiphol-Rijk, The Netherlands 3603), induced in the absence or presence of palmitate (1 mM, Palm), glimepiride (20 µM, Glim) and GO (1 U mL−1) and subsequently incubated (1 h, 37°C) in the absence or presence of adiposomes (ADIPs) from glucose oxidase (GO)-induced adipocytes [50 µg phosphatidylcholine (PC) each]. After several cycles of washing with adipocyte buffer, the cells were fixed and then permeabilized and washed or left intact. The subsequent in/on-cell Western blotting was performed by using rat anti-perilipin-A and rabbit anti-CD73 antibodies and infrared dye-labelled goat anti-rat IgG and goat anti-rabbit IgG, respectively, and fluorescent imaging at 700 and 800 nm. Quantitative evaluations of the amount of perilipin-A and CD73 accessible for the antibodies in intact (i.e. at the cell surface) and permeabilized (i.e. total cellular) were measured in the regions encircled in white. The values are given for intact adipocytes only, for four independent incubations in arbitrary units (mean ± standard deviation) set at 100, each, for the uninduced permeabilized adipocytes in the absence of ADIP. The amounts of intracellular perilipin-A and CD73 (i.e. LD-associated, predominantly) were calculated as the differences between the corresponding values for permeabilized and intact adipocytes. *Significantly different from non-induced. #Significantly different from absence of adiposomes.
Adiposomes harbouring GPI-anchored CD73 stimulate lipid synthesis in small adipocytes
Endogenous Gce1 and CD73 have previously been shown to mediate regulation of lipid metabolism in rat adipocytes after their H2O2-, glimepiride- or palmitate-induced translocation from plasma membrane DIGs to LD (Müller et al., 2008a,b,c,d,e;). To test for a similar role of adiposome-derived CD73 after its transfer to DIGs and translocation to LD, small acceptor adipocytes were assayed for stimulation of esterification upon incubation with adiposomes from GO-induced adipocytes under conditions compatible with GPI-protein translocation from DIGs to LD (i.e. 37°C, absence of GPI-2350). Basal and induced (by half-maximally effective concentrations of GO, glimepiride or palmitate in the absence of adiposomes) incorporation of [1-14C]palmitate into total acylglycerols increased with the amounts of adiposomes (according to PC content) and was higher with GO, glimepiride and palmitate compared with the non-induced control (in that order of declining potency) at each amount of adiposomes (Figure 10, upper panel). The increase in GO-, glimepiride- and palmitate-induced esterification stimulation exerted by maximally effective amounts of adiposomes was more pronounced for small adipocytes (set at 100%) compared with large ones (29–36%) and SVC (7–11%). Thus adiposomes improve responsiveness of, preferentially, small adipocytes to stimulation of esterification by GO, glimepiride and palmitate.
Figure 10.
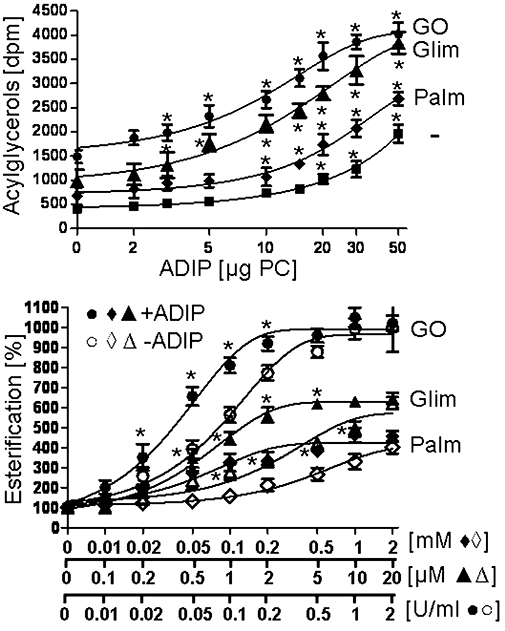
Stimulation of the basal and glucose oxidase (GO)-, glimepiride- and palmitate-induced esterification by adiposomes. Small adipocytes were incubated (2 h, 37°C) without or with increasing amounts of adiposomes [ADIPs; according to phosphatidylcholine (PC) content] from GO-induced (0.5 U mL−1) adipocytes (upper panel) in the absence (non-induced) or presence of GO (0.15 µ mL−1), glimepiride (2 µM, Glim) or palmitate (0.3 mM, Palm) or (lower panel) without or with adiposomes (30 µg PC each) from GO-induced adipocytes in the absence (non-induced) or presence of increasing concentrations of GO, glimepiride (Glim) or palmitate (Palm). The adipocytes were recovered by flotation (500× g, 2 min, 37°C). Portions of the adipocytes were assayed for esterification by the addition of [1-14C]palmitate and further incubation (90 min, 37°C). Upper panel: Quantitative evaluations of esterification are given as the amount of total radiolabelled acylglycerols for five independent incubations and determinations in quadruplicate each (mean ± standard deviation). Lower panel: Quantitative evaluations of esterification stimulation by GO, glimepiride and palmitate (after correction for the effects of adiposomes in the absence of GO, glimepiride or palmitate) are given as % of the amount of total radiolabelled acylglycerols for four independent incubations and determinations in duplicate each (mean ± standard deviation) set at 100 for the absence of GO, glimepiride or palmitate and ADIP. *Significantly different from absence of adiposomes.
Moreover, adiposomes increased the sensitivity of small adipocytes for GO-, glimepiride- and palmitate-induced esterification stimulation. This was revealed by the significant left-ward shifts of the concentration-response curves for stimulation of esterification by GO, glimepiride and palmitate in the presence of maximally effective amounts of adiposomes compared with their absence resulting in considerable reductions of the EC50 values (GO, from 0.10 to 0.05 U mL−1; glimepiride, from 3.0 to 0.5 µM; palmitate, from 0.45 to 0.11 mM). The maximal responses elicited by maximally effective concentrations of GO, glimepiride and palmitate were not upregulated by adiposomes (Figure 10, lower panel).
Finally, the requirement of GPI-anchored and enzymatically active CD73 as a component of the adiposomes for their stimulatory effect on the GO-, glimepiride- and palmitate-induced esterification stimulation was studied. For this, adiposomes were used that had been prepared from non-induced or GO-induced adipocytes or pretreated with bacterial PI-PLC or adsorbed to AMP-Sepharose beads prior to their incubation with small adipocytes (Figure 11). As expected, the esterification stimulation induced by half-maximally effective concentrations of GO, glimepiride and palmitate (with regard to the absence of ADIP) was further increased in response to maximally effective amounts of adiposomes from GO-induced adipocytes (set at 100% in Figure 11). This difference between the presence and absence of adiposomes was significantly diminished with adiposomes from non-induced adipocytes or adiposomes harbouring cleaved GPI anchors (the residual adiposome effects left after the PI-PLC pretreatment may be explained by incomplete GPI anchor cleavage as already observed above; see Figure 3) or adiposomes lacking CD73 (upon removal of adiposomes harbouring CD73 from total adiposomes by adsorption to AMP-Sepharose beads) (Figure 11). Thus, adiposomes exert both enhancing and sensitizing effects on GO-, glimepiride- and palmitate-induced esterification stimulation in (preferably small) rat adipocytes, which critically depend on the expression of CD73 with intact GPI anchor on the adiposomes.
Figure 11.
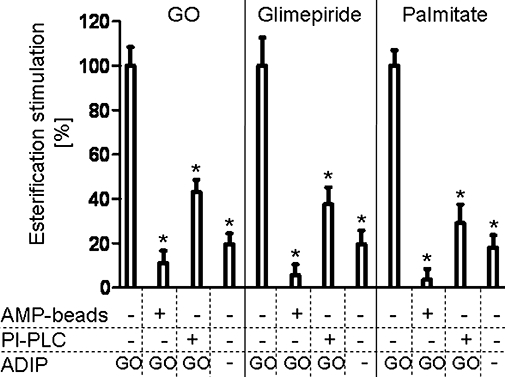
Mechanism of the stimulation of the glucose oxidase (GO)-, glimepiride- and palmitate-induced esterification by adiposomes. Small adipocytes were incubated (2 h, 37°C) in the presence of GO (0.15 U mL−1), glimepiride (2 µM) or palmitate (0.3 mM) without or with adiposomes [ADIP; 30 µg phosphatidylcholine (PC) each] from GO-induced (0.5 U mL−1, GO) or non-induced (–) adipocytes, which had been pretreated without or with phosphatidylinositol-specific phospholipase C (PI-PLC) from Bacillus cereus (2.5 IU mL−1), in the absence or presence of AMP-Sepharose beads (AMP beads; see Materials and Methods). After centrifugation (500× g, 2 min, 20°C) of the incubation mixtures, the floating adipocytes and the lower layers were recovered and then incubated together (2 h, 37°C). Thereafter, the adipocytes were recovered by flotation (2500× g, 2 min, 20°C) and then assayed for esterification by the addition of [1-14C]palmitate and further incubation (90 min, 37°C). Quantitative evaluations of esterification stimulation by adiposomes (after correction for the effects of GO, glimepiride or palmitate without adiposomes) are given as % of the amount of total radiolabelled acylglycerols for two independent incubations and determinations in quadruplicate, each (mean ± standard deviation), set at 100 for adipocytes, which had been incubated with GO-induced adiposomes in the absence of PI-PLC and AMP-Sepharose beads, each (controls). *Significantly different from control.
Discussion
Recent findings unequivocally demonstrated the release of microvesicles and exosomes, collectively called adiposomes, from primary and cultured rodent adipocytes (Aoki et al., 2007; Müller et al., 2008a,b;). However, they left open the physiological function of adiposomes that may be related to horizontal information transfer from donor to acceptor adipocytes. The present studies provide the first evidence that adiposomes operate as intercellular vehicles or carriers of signalling information.
Thus, adiposomes apparently interact with acceptor adipocytes as revealed by the transfer of the GPI-protein CD73 from adiposomes to plasma membrane DIGs (Figure 1). This became evident in adipocytes under conditions of blocked GPI-protein translocation from DIGs to LD. This interaction seems to be specific as it is saturable, more pronounced with a subpopulation of adipose tissue cells, that is, small adipocytes compared with large adipocytes and SVC as acceptor cells, more efficient with DIGs than total plasma membranes (Figure 1), temperature-sensitive and rapid (Figure 2). The interaction of adiposomes with acceptor adipocytes leads to the incorporation of their constituent GPI-protein CD73 into the phospholipid bilayer of plasma membrane DIGs, which requires intact DIGs and GPI anchors (Figure 3). It remains open whether the transfer involves direct binding of the adiposomes to and subsequent fusion with the DIGs (e.g. receptor-mediated or non-nspecific) or shuttling of (certain subsets of) the GPI-proteins and adiposome components via soluble intermediates (e.g. phospholipid micelles or soluble carrier proteins). So far, the adiposome constituent GPI-protein, Gce1, has been found to accompany CD73 during its transfer from adiposomes to DIGs and subsequent translocation to LD in acceptor adipocytes (G. Müller et al., unpubl. data).
Following the transfer from adiposomes to plasma membrane DIGs of acceptor adipocytes, CD73 was translocated from the DIGs to cytoplasmic LD as revealed by the precursor-product relationship between the DIGs-associated and the LD-associated CD73. The association of CD73 with both DIGs and LD depended on the amount of adiposomes incubated with the adipocytes (Figures 1 and 4). The association of CD73 with both DIGs and LD was more efficient with small adipocytes than large ones and SVC (Figures 1 and 4). The association of CD73 with both DIGs and LD depended on their structural integrity (Figures 3 and 7). However, the sensitivity toward cholesterol depletion apparently differs between DIGs and LD. Comparable amounts of CD73 are found associated with DIGs and LD upon transfer from identical amounts of adiposomes (Figures 1 and 4). This may be explained by high efficacy of the GPI-protein translocation and/or its incomplete blockade during assaying DIGs association and thus partial loss of the DIGs-associated CD73. The association of CD73 with both DIGs (data not shown) and LD (Figure 4) is considerably lower with adiposomes released from non-induced compared with GO-induced donor adipocytes. This difference may rely on the observed relative depletion of CD73 (vs. the phospholipid constituents) in the former compared with the latter adiposomes (Müller et al., 2009a). Alternatively, the transfer and/or translocation efficacy may be lower for adiposomes from non-induced compared with GO-induced adipocytes, which could be related to the reported differences in their lipid and protein composition (Aoki et al., 2007; Müller et al., 2009a). The association of CD73 with both DIGs and LD is of integral nature and depends on the intact GPI anchor (Figures 3 and 8). Together, the precursor-product relationship between DIGs- and LD-associated CD73 strongly argues for DIGs functioning as the source of the adiposome-derived CD73 for translocation to LD.
The translocation of ADIP-derived CD73 from DIGs to LD was stimulated by H2O2, glimepiride and palmitate (Figure 5) with the same rank order of potency (Figure 6) and sensitivity toward inhibitors (Figure 7) as endogenous CD73. This argues for the engagement of identical molecular mechanisms, which may encompass vesicular trafficking, intraendosomal membrane transport and remodelling of the cortical cytoskeleton (Keller et al., 1992; Cauwenberghs et al., 2006; Trajkovic et al., 2008). The sequential transfer to DIGs and translocation to LD of ADIP-derived CD73 leads to upregulation of fatty acid esterification into lipids in the acceptor adipocytes (Figure 10). The causal relationship between these processes was suggested by the similar dependencies on the amount of adiposomes (Figures 1, 4 and 10), the identical ranking orders for the efficacy as acceptor cell (small adipocytes > large adipocytes > SVC; Figures 1, 4 and data not shown) and the identical ranking orders and similar EC50 values for their induction by H2O2, glimepiride and palmitate (Figures 6 and 10). Importantly, the EC50 values are at the upper range of the total serum concentrations of free fatty acids during starvation with 0.5–1.5 mM and of glimepiride during antidiabetic therapy with 0.1–0.7 µM (Müller, 2005). Additional evidence for the involvement of LD-associated CD73 in esterification stimulation by adiposomes in acceptor adipocytes is provided by its partial to up to complete blockade by different strategies for interference with CD73 translocation to LD (Figures 2 and 7), such as the use of adiposomes released from non-induced compared with GO-induced adipocytes (Figure 11), the use of adiposomes with lipolytically cleaved GPI anchors (Figure 11), the removal of CD73-harbouring adiposomes (Figure 11) or the disruption of DIGs (data not shown). Together these findings suggest that the transfer to DIGs and translocation to LD of ADIP-derived CD73 leads to upregulation of esterification.
The presented studies demonstrate that a GPI-protein, CD73, transferred via adiposomes, to plasma membrane DIGs of small adipocytes, and translocation into the cell to the surface of cytoplasmic LD, can operate as a intercellular carrier, within the adipose tissue, of signalling information for the stimulation of esterification. Within the adipose tissue, this chain of events may enable the transfer of information about the lipid-laden and esterification states from large adipocytes, acting preferentially as adiposome donors to small adipocytes, acting preferentially as adiposome acceptors. The small adipocytes are thereby forced to increase LD formation and to take over the burden of lipid loading. The putative shift in the working load from large to small adipocytes may be of particular importance during exposure of adipose tissue to excess of fatty acids (palmitate) or reactive oxygen species (H2O2) as well as to high therapeutic concentrations of glimepiride (Müller, 2005). These situations are typical for starvation, obesity and type II diabetes (Crescimanno et al., 1989; Gan and Watts, 2008). The present findings with rat adipocytes argue for the possibility of paracrine or endocrine communication between other mammalian donor and acceptor cells of the same or different tissues by certain GPI-proteins. A prerequisite for this type of intercellular communication is their transfer by subpopulations of microvesicles and/or exosomes via the interstitial spaces or the blood.
Previously, the transfer of GPI-proteins onto the plasma membrane of acceptor cells had been observed upon physical contact with donor cells expressing these GPI-proteins at their surface (Zhang et al., 1992; Kooyman et al., 1995; van den Berg et al., 1995; Anderson et al., 1996; Dunn et al., 1996; Fritzsching et al., 2002) or incubation with the purified GPI-proteins presented in detergent micelles or reconstituted into liposomes (Medof et al., 1984; Mchugh et al., 1995; Civenni et al., 1998; Premkumar et al., 2001). However, only in some cases have the assumed enzymic, adhesion, receptor or signalling functions of the transferred GPI-proteins been confirmed. In those cases, the functions were related to cell surface localization of the GPI-proteins and their coupling to extracellular mechanisms at the acceptor cells. These seemed to be mediated, at least in part, by receptor binding and the extracellular assembly of multimolecular signaling complexes (Gasser and Schifferli, 2004). In contrast, cytoplasmic localization and effects on the intracellular physiology and metabolism of the acceptor cells have not been reported for transferred GPI-proteins so far, but may underlie the well-known transfer of information from donor to acceptor cells via microvesicles and exosomes, such as during blood coagulation and tumor growth (Wolf, 1967; Poste and Nicolson, 1980). For instance, in human glioma cells, only a small fraction exhibiting a transformed phenotype was found to express the truncated epidermal growth factor receptor, EGFRvIII, necessary for the tumor to grow. This discrepancy was resolved by the finding that EGFRvIII-harbouring microvesicles were shed into the circulation and transferred this receptor from one cell to another without the need for direct cell-to-cell contact (Al-Nedawi et al., 2008).
Recent studies have linked microvesicle- and exosome-mediated horizontal information transfer to many physiological processes, such as inflammation and angiogenesis (Denzer et al., 2000; Stoorvogel et al., 2002; Freyssinet, 2003; Gould et al., 2003; Fevrier and Raposo, 2004; Johnstone 2006; Keller et al., 2006; Piccin et al., 2007; Pap et al., 2009). However, the underlying molecular mechanisms and components remained less well characterized. So far it seems clear that the vesicles do not interact with just any cell they come into contact with but rather only with cells that they recognize specifically (Lösche et al., 2004; Pluskota et al., 2008). The interaction is followed by either the fusion of the vesicles with the plasma membrane of the acceptor cell or the endocytic uptake of the vesicles (Cauwenberghs et al., 2006; Trajkovic et al., 2008). This leads to discharge of the vesicle content into the cytosol either directly or upon membrane fusion of the endocytosed vesicles and the segregating endosomes. Alternatively, non-vesicular mechanisms involving the transient formation of bicellular structures, which are created by the fusion of the extracellular and cytoplasmic leaflets of the plasma membrane, are conceivable for the escape of components from microvesicles and exosomes, such as adiposome-derived GPI-proteins, from DIGs into the cytoplasm of acceptor cells. This mechanism has been proposed for the (glyco)protein quality control in the endoplasmic reticulum membrane (Ploegh, 2007). It remains to be elucidated which mechanisms and components play a role in the transfer of GPI-proteins from microvesicles/exosomes into the cytoplasm of acceptor cells.
In conclusion, in response to certain physiological and pharmacological signals microvesicles and exosomes are released from donor adipocytes that transfer their constituent GPI-protein CD73 to the plasma membrane DIGs and cytoplasmic LD of acceptor adipocytes. The resulting stimulation of esterification argues for communication by GPI-proteins between adipose tissue cells. Thereby they represent an attractive model system for the analysis of the physiological relevance of the microvesicle- and exosome-mediated transfer of GPI-proteins between cells, in general.
Glossary
Abbreviations:
- AMPCP
α,β-methlyene-ADP
- DIG
detergent-insoluble glycolipid-enriched plasma membrane microdomain
- GO
glucose oxidase
- (GPI-)PLC
(glycosylphosphatidylinositol-specific) phospholipase C
- GPI-protein
glycosylphosphatidylinositol-anchored protein
- LD
lipid droplet
- l-t-LacCer
β-D-lactosyl-N-octanoyl-L-threo-sphingosine
- MES
4-morpholineethanesulfonic acid
- m-βCD
methyl-β-cyclodextrin
- PC
phosphatidylcholine
- SVC
stromal vascular cell
Conflict of interest
All authors are employed by Sanofi-Aventis Germany GmbH Corporation.
References
- Aboulaich N, Vener AV, Stralfors P. Hormonal control of reversible translocation of perilipin B to the plasma membrane in primary human adipocytes. J Biol Chem. 2006;281:11446–11449. doi: 10.1074/jbc.C500461200. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 4th edn. Br J Pharmacol. 2009;158(Suppl 1):S1–S254. doi: 10.1111/j.1476-5381.2009.00499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Nedawi K, Meehan B, Micallef J, Lhotak V, May L, Guha A, et al. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat Cell Biol. 2008;10:619–624. doi: 10.1038/ncb1725. [DOI] [PubMed] [Google Scholar]
- Anderson SM, Yu G, Giattina M, Miller JL. Intercellular transfer of a glycosylphosphatidylinositol (GPI)-linked protein: release and uptake of CD4-GPI from recombinant adeno-associated virus-transduced HeLa cells. Proc Natl Acad Sci USA. 1996;93:5894–5898. doi: 10.1073/pnas.93.12.5894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki N, Jin-no S, Nakagawa Y, Asai N, Arakawa E, Tamura N, et al. Identification and characterization of microvesicles secreted by 3T3-L1 adipocytes: redox- and hormone-dependent induction of milk fat globule-epidermal growth factor 8-associated microvesicles. Endocrinology. 2007;148:3850–3862. doi: 10.1210/en.2006-1479. [DOI] [PubMed] [Google Scholar]
- van den Berg CW, Cinek T, Hallett MB, Horejsi V, Morgan BP. Exogenous glycosyl phosphatidylinositol-anchored CD59 associates with kinases in membrane clusters on U937 cells and becomes Ca(2+) signaling competent. J Cell Biol. 1995;131:669–677. doi: 10.1083/jcb.131.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D, London L. Functions of lipid rafts in biological membranes. Annu Rev Cell Dev Biol. 1998;14:111–136. doi: 10.1146/annurev.cellbio.14.1.111. [DOI] [PubMed] [Google Scholar]
- Bütikofer P, Kuypers FA, Xu CM, Chiu DT, Lubin B. Enrichment of two glycosyl-phosphatidylinositol-anchored proteins, acetylcholinesterase and decay accelerating factor, in vesicles released from human red blood cells. Blood. 1989;74:1481–1485. [PubMed] [Google Scholar]
- Cauwenberghs S, Feijge MA, Harper AG, Sage SO, Curvers J, Heemskerk JW. Shedding of procoagulant microparticles from unstimulated platelets by integrin-mediated destabilization of actin cytoskeleton. FEBS Lett. 2006;580:5313–5320. doi: 10.1016/j.febslet.2006.08.082. [DOI] [PubMed] [Google Scholar]
- Civenni G, Test ST, Brodbeck U, Bütikofer P. In vitro incorporation of GPI-anchored proteins into human erythrocytes and their fate in the membrane. Blood. 1998;9:11784–11792. [PubMed] [Google Scholar]
- Cocucci E, Racchetti G, Meldolesi J. Shedding microvesicles: artefacts no more. Trends Cell Biol. 2009;19:43–51. doi: 10.1016/j.tcb.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Crescimanno M, Armata MG, Rausa L, Gueli MC, Nicotra C, D'Alessandro N. Cardiac peroxisomal enzymes and starvation. Free Radic Res Commun. 1989;7:67–72. doi: 10.3109/10715768909087925. [DOI] [PubMed] [Google Scholar]
- Cushman SW, Salans LB. Determination of adipose cell size and number in suspensions of isolated rat and human adipose cells. J Lipid Res. 1978;19:269–273. [PubMed] [Google Scholar]
- Del Conde I, Shrimpton CN, Thiagarajan P, Lopez JA. Tissue-factor-bearing microvesicles arise from lipid rafts and fuse with activated platelets to initiate coagulation. Blood. 2005;106:1604–1611. doi: 10.1182/blood-2004-03-1095. [DOI] [PubMed] [Google Scholar]
- Denzer K, Kleijmeer MJ, Heijnen HF, Stoorvogel W, Geuze HJ. Exosome: from internal vesicle of the multivesicular body to intercellular signaling device. J Cell Sci. 2000;113:3365–3374. doi: 10.1242/jcs.113.19.3365. [DOI] [PubMed] [Google Scholar]
- Ducharme NA, Bickel PE. Lipid droplets in lipogenesis and lipolysis. Endocrinology. 2008;149:942–949. doi: 10.1210/en.2007-1713. [DOI] [PubMed] [Google Scholar]
- Dunn DE, Yu J, Nagarajan S, Devetten M, Weichold FF, Medof ME, et al. A knock-out model of paroxysmal nocturnal hemoglobinuria: pig-a(-) hematopoiesis is reconstituted following intercellular transfer of GPI-anchored proteins. Proc Natl Acad Sci USA. 1996;93:7938–7943. doi: 10.1073/pnas.93.15.7938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fevrier B, Raposo G. Exosomes: endosomal-derived vesicles shipping extracellular messages. Curr Opin Cell Biol. 2004;16:415–421. doi: 10.1016/j.ceb.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Freyssinet J-M. Cellular microparticles: what are they bad or good for? J Thromb Haemost. 2003;1:1655–1662. doi: 10.1046/j.1538-7836.2003.00309.x. [DOI] [PubMed] [Google Scholar]
- Fritzsching B, Schwer B, Kartenbeck J, Pedal A, Horejsi V, Ott M. Release and intercellular transfer of cell surface CD81 via microparticles. J Immunol. 2002;169:5531–5537. doi: 10.4049/jimmunol.169.10.5531. [DOI] [PubMed] [Google Scholar]
- Fujimoto T, Ohsaki Y, Cheng J, Suzuki M, Shinohara Y. Lipid droplets: a classic organelle with new outfits. Histochem Cell Biol. 2008;130:263–279. doi: 10.1007/s00418-008-0449-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan SK, Watts GF. Is adipose tissue lipolysis always an adaptove response to starvation?: implications for non-alcoholic fatty liver disease. Clin Sci. 2008;114:543–545. doi: 10.1042/CS20070461. [DOI] [PubMed] [Google Scholar]
- de Gassart A, Geminard C, Fevrier B, Raposo G, Vidal M. Lipid raft-associated protein sorting in exosomes. Blood. 2003;102:4336–4344. doi: 10.1182/blood-2003-03-0871. [DOI] [PubMed] [Google Scholar]
- Gasser O, Schifferli JA. Activated polymorphonuclear neutrophils disseminate anti-inflammatory by ectocytosis. Blood. 2004;104:2543–2548. doi: 10.1182/blood-2004-01-0361. [DOI] [PubMed] [Google Scholar]
- Gould SJ, Booth AM, Hildreth JE. The Trojan exosome hypothesis. Proc Natl Acad Sci USA. 2003;100:10592–10597. doi: 10.1073/pnas.1831413100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding C, Heuser J, Stahl P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J Cell Biol. 1983;97:329–339. doi: 10.1083/jcb.97.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris TJ, Siu CH. Reciprocal raft-receptor interactions and the assembly of adhesion complexes. Bioessays. 2002;24:996–1003. doi: 10.1002/bies.10172. [DOI] [PubMed] [Google Scholar]
- Heijnen HF, Schiel AE, Fijnheer R, Geuze HJ, Sixma JJ. Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood. 1999;94:3791–3799. [PubMed] [Google Scholar]
- Ikezawa H. Glyosylphosphatidylinositol (GPI)-anchored proteins. Biol Pharm Bull. 2002;25:409–417. doi: 10.1248/bpb.25.409. [DOI] [PubMed] [Google Scholar]
- Johnstone RM. Exosomes biological significance: a concise review. Blood Cells Mol Dis. 2006;36:315–321. doi: 10.1016/j.bcmd.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation: association of plasma membrane activities with released vesicles (exosomes) J Biol Chem. 1987;262:9412–9420. [PubMed] [Google Scholar]
- Karagogeos D. Neural GPI-anchored cell adhesion molecules. Front Biosci. 2003;8:s1304–s1320. doi: 10.2741/1214. [DOI] [PubMed] [Google Scholar]
- Keller GA, Siegel MW, Caras IW. Endocytosis of glycophospholipid-anchored and transmembrane forms of CD4 by different endocytic pathways. EMBO J. 1992;11:863–874. doi: 10.1002/j.1460-2075.1992.tb05124.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller S, Sanderson MP, Stoeck A, Altevogt P. Exosomes: from biogenesis and secretion to biological function. Immunol Lett. 2006;107:102–108. doi: 10.1016/j.imlet.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Kooyman DL, Byrne GW, Logan JS. Glycosyl Phosphatidylinositol Anchor. Exp Nephrol. 1998;6:148–151. doi: 10.1159/000020516. [DOI] [PubMed] [Google Scholar]
- Kooyman DL, Byrne GW, McClellan S, Nielsen D, Tone M, Waldmann H, et al. In vivo transfer of GPI-linked complement restriction factors from erythrocytes to the endothelium. Science. 1995;269:89–92. doi: 10.1126/science.7541557. [DOI] [PubMed] [Google Scholar]
- Long KE, Yomtovian R, Kida M, Knez JJ, Medof ME. Time-dependent loss of surface complement regulatory activity during storage of donor blood. Transfusion. 1993;33:294–300. doi: 10.1046/j.1537-2995.1993.33493242635.x. [DOI] [PubMed] [Google Scholar]
- Lösche W, Scholz T, Temmler U, Oberle V, Claus RA. Platelet-derived microvesicles transfer tissue factor to monocytes but not to neutrophils. Platelets. 2004;15:109–115. doi: 10.1080/09537100310001649885. [DOI] [PubMed] [Google Scholar]
- McHugh RS, Ahmed SN, Wang YC, Sell KW, Selvavaj P. Construction, purification, and functional incorporation on tumor cells of glycolipid-anchored human B7-1 (CD80) Proc Natl Acad Sci USA. 1995;92:8059–8063. doi: 10.1073/pnas.92.17.8059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medof ME, Kinoshita T, Nussenzweig V. Inhibition of complement activation on the surface of cells after incorporation of decay-accelerating factor (DAF) into their membranes. J Exp Med. 1984;160:1558–1578. doi: 10.1084/jem.160.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medof ME, Nagarajan S, Tykocinski ML. Cell-surface engineering with GPI-anchored proteins. FASEB J. 1996;10:574–586. doi: 10.1096/fasebj.10.5.8621057. [DOI] [PubMed] [Google Scholar]
- Müller G. The mode of action of glimepiride – beyond insulin secretion. Curr Med Chem. 2005;5:499–518. [Google Scholar]
- Müller G, Frick W. Signalling via caveolin: involvement in the cross-talk between phosphoinositolglycans and insulin. Cell Mol Life Sci. 1999;56:945–970. doi: 10.1007/s000180050485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller G, Petry S. Triacylglycerol, storage and mobilization of human. In: Meyers A, editor. Encyclopedia in Biochemistry and Molecular Biology. Weinheim: Wiley VCH; 2005. pp. 621–704. Vol. 14. [Google Scholar]
- Müller G, Wied S. The sulfonylurea drug, glimepiride, stimulates glucose transport, glucose transporter translocation, and dephosphorylation in insulin-resistant rat adipocytes in vitro. Diabetes. 1993;42:1852–1867. doi: 10.2337/diab.42.12.1852. [DOI] [PubMed] [Google Scholar]
- Müller G, Korndörfer A, Saar K, Karbe-Thönges B, Fasold H, Müllner S. 4'-Amino-benzamido-taurocholic acid selectively solubilizes glycosyl-phosphatidylinositol-anchored membrane proteins and improves lipolytic cleavage of their membrane anchors by specific phospholipases. Arch Biochem Biophys. 1994a;309:329–340. doi: 10.1006/abbi.1994.1121. [DOI] [PubMed] [Google Scholar]
- Müller G, Wied S, Wetekam E-M, Crecelius A, Unkelbach A, Pünter J. Stimulation of glucose utilization in 3T3 adipocytes and rat diaphragm in vitro by the sulfonylureas, glimepiride and glibenclamide, is correlated with modulations of the cAMP regulatory cascade. Biochem Pharmacol. 1994b;48:985–996. doi: 10.1016/0006-2952(94)90369-7. [DOI] [PubMed] [Google Scholar]
- Müller G, Ertl J, Gerl M, Preibisch G. Leptin impairs metabolic actions of insulin in isolated rat adipocytes. J Biol Chem. 1997a;272:10585–10593. doi: 10.1074/jbc.272.16.10585. [DOI] [PubMed] [Google Scholar]
- Müller G, Wied S, Crecelius A, Kessler A, Eckel J. Phosphoinositolglycan-peptides from yeast potently induce metabolic insulin actions in isolated rat adipocytes, cardiomyocytes and diaphragms. Endocrinology. 1997b;138:3459–3475. doi: 10.1210/endo.138.8.5308. [DOI] [PubMed] [Google Scholar]
- Müller G, Jung C, Wied S, Welte S, Jordan H, Frick W. Redistribution of glycolipid raft domain components induces insulin-mimetic signaling in rat adipocytes. Mol Cell Biol. 2001;21:4553–4567. doi: 10.1128/MCB.21.14.4553-4567.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller G, Hanekop N, Wied S, Frick W. Cholesterol depletion blocks redistribution of lipid raft components and insulin-mimetic signaling by glimepiride and phosphoinositolglycans in rat adipocytes. Mol Med. 2002;8:120–136. [PMC free article] [PubMed] [Google Scholar]
- Müller G, Schulz A, Wied S, Frick W. Regulation of lipid raft proteins by glimepiride- and insulin-induced glycosylphosphatidylinositol-specific phospholipase C in rat adipocytes. Biochem Pharmacol. 2005;69:761–780. doi: 10.1016/j.bcp.2004.11.014. [DOI] [PubMed] [Google Scholar]
- Müller G, Over S, Wied S, Frick W. Association of (c)AMP-degrading glycosylphosphatidylinositol-anchored proteins with lipid droplets is induced by palmitate, H2O2 and the sulfonylurea drug, glimepiride, in rat adipocytes. Biochem. 2008a;47:1274–1287. doi: 10.1021/bi7022915. [DOI] [PubMed] [Google Scholar]
- Müller G, Wied S, Jung C, Over S. Translocation of glycosylphosphatidylinositol-anchored proteins to lipid droplets and inhibition of lipolysis in rat adipocytes is mediated by reactive oxygen species. Br J Pharmacol. 2008b;154:901–913. [Google Scholar]
- Müller G, Wied S, Jung C, Straub J. Coordinated regulation of esterification and lipolysis by palmitate, H2O2 and the anti-diabetic sulfonylurea drug, glimepiride, in rat adipocytes. Eur J Pharmacol. 2008c;597:6–18. doi: 10.1016/j.ejphar.2008.08.034. [DOI] [PubMed] [Google Scholar]
- Müller G, Wied S, Over S, Frick W. Inhibition of lipolysis by palmitate, H2O2 and the sulfonylurea drug, glimepiride, in rat adipocytes depends on cAMP degradation by lipid droplets. Biochem. 2008d;47:1259–1273. doi: 10.1021/bi701413t. [DOI] [PubMed] [Google Scholar]
- Müller G, Wied S, Walz N, Jung C. Translocation of glycosylphosphatidylinositol-anchored proteins from plasma membrane microdomains to lipid droplets in rat adipocytes is induced by palmitate, H2O2 and the sulfonylurea drug, glimepiride. Mol Pharmacol. 2008e;73:1513–1529. doi: 10.1124/mol.107.043935. [DOI] [PubMed] [Google Scholar]
- Müller G, Jung C, Straub J, Wied S. Induced release of membrane vesicles and exosomes from rat adipocytes containing lipid droplet, lipid raft and glycosylphosphatidylinositol-anchored proteins. Cell Signal. 2009a;21:324–338. doi: 10.1016/j.cellsig.2008.10.021. [DOI] [PubMed] [Google Scholar]
- Müller G, Jung C, Wied S, Biemer-Daub G. Induced translocation of glycosylphosphatidylinositol-anchored proteins from lipid droplets to adiposomes in rat adipocytes. Br J Pharmacol. 2009b;158:749–770. doi: 10.1111/j.1476-5381.2009.00360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosjean O, Briolay A, Roux B. Mammalian GPI proteins: sorting, membrane residence and functions. Biochim Biophys Acta. 1997;1331:153–186. doi: 10.1016/s0304-4157(97)00005-1. [DOI] [PubMed] [Google Scholar]
- Pan BT, Johnstone RM. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell. 1983;33:967–978. doi: 10.1016/0092-8674(83)90040-5. [DOI] [PubMed] [Google Scholar]
- Pap E, Pallinger E, Pasztoi M, Falus A. Highlights of a new type of intercellular communication: microvesicle-based information transfer. Inflamm Res. 2009;58:1–8. doi: 10.1007/s00011-008-8210-7. [DOI] [PubMed] [Google Scholar]
- Piccin A, Murphy WG, Smith OP. Circulating microparticles: pathophysiology and clinical implications. Blood Rev. 2007;21:157–171. doi: 10.1016/j.blre.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Ploegh HL. A lipid-based model for the creation of an escape hatch from the endoplasmic reticulum. Nature. 2007;448:435–438. doi: 10.1038/nature06004. [DOI] [PubMed] [Google Scholar]
- Pluskota E, Woody NM, Szpak D, Ballantyne CM, Soloviev DA, Simon DI, et al. Expression, activation, and function of integrin αMβ2 (Mac-1) on neutrophil-derived microparticles. Blood. 2008;112:2327–2335. doi: 10.1182/blood-2007-12-127183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poste G, Nicolson GL. Arrest and metastasis of blood-borne tumor cells are modified by fusion of plasma membrane vesicles from highly metastatic cells. Proc Natl Acad Sci USA. 1980;77:399–403. doi: 10.1073/pnas.77.1.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premkumar DR, Fukuoka Y, Sevlever D, Brunschwig E, Rosenberry TL, Tykocinski ML, et al. Properties of exogenously added GPI-anchored proteins following their incorporation into cells. J Cell Biochem. 2001;82:234–245. doi: 10.1002/jcb.1154. [DOI] [PubMed] [Google Scholar]
- Rodbell M. Metabolism of isolated fat cells. I. Effects of hormones on glucose metabolism and lipolysis. J Biol Chem. 1964;239:375–380. [PubMed] [Google Scholar]
- Rodeheffer MS, Birsoy K, Friedman JM. Identification of white adipocyte progenitor cells in vivo. Cell. 2008;135:240–249. doi: 10.1016/j.cell.2008.09.036. [DOI] [PubMed] [Google Scholar]
- Rooney IA, Atkinson JP, Krul ES, Schonfeld G, Polakoski K, Saffitz JE, et al. Physiologic relevance of the membrane attack complex inhibitory protein CD59 in human seminal plasma: CD59 is present on extracellular organelles (prostasomes), binds cell membranes, and inhibits complement-mediated lysis. J Exp Med. 1993;177:1409–1420. doi: 10.1084/jem.177.5.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloand EM, Maciejewski JP, Dunn D, Moss J, Brewer B, Kirby M, et al. Correction of the PNH defect by GPI-anchored protein transfer. Blood. 1998;92:4439–4445. [PubMed] [Google Scholar]
- Stoorvogel W, Kleijmeer MJ, Geuze H, Raposo G. The biogenesis and functions of exosomes. Traffic. 2002;3:321–330. doi: 10.1034/j.1600-0854.2002.30502.x. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Okumura Y. GPI-linked proteins do not transfer spontaneously from erythrocytes to liposomes. New aspects of reorganization of the cell membrane. Biochem. 2000;39:9477–9485. doi: 10.1021/bi000113v. [DOI] [PubMed] [Google Scholar]
- Thery C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;3:569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319:1244–1247. doi: 10.1126/science.1153124. [DOI] [PubMed] [Google Scholar]
- Varma R, Mayor S. GPI-anchored proteins are organized in submicron domains at the cell surface. Nature. 1998;394:798–801. doi: 10.1038/29563. [DOI] [PubMed] [Google Scholar]
- Wolf P. The nature and significance of platelet products in human plasma. Br J Haematol. 1967;13:269–288. doi: 10.1111/j.1365-2141.1967.tb08741.x. [DOI] [PubMed] [Google Scholar]
- Zhang F, Schmidt WG, Hou Y, Williams AF, Jacobson K. Spontaneous incorporation of the glycosyl-phosphatidylinositol-linked protein Thy-1 into cell membranes. Proc Natl Acad Sci USA. 1992;89:5231–5235. doi: 10.1073/pnas.89.12.5231. [DOI] [PMC free article] [PubMed] [Google Scholar]


