Abstract
Background and purpose:
The epithelial sodium channel (ENaC) regulates airway mucosal hydration and mucus clearance. The lack of such regulation in cystic fibrosis patients leads to dessication of the airway lumen, resulting in mucostasis that establishes the environment for infections. Osmotic agents and negative ENaC regulators can be used to restore mucosal hydration. We aimed to assess whether: (i) osmotically driven fluid flux into the rat lung could be quantified in vivo by magnetic resonance imaging (MRI); and (ii) the MRI signals could be modulated through the regulation of ENaC function.
Experimental approach:
Lung images from spontaneously breathing rats were acquired following intra-tracheal (i.t.) administration of physiological or hypertonic saline (HS). Compounds known to modulate the ENaC function were given i.t. prior to saline. Volumes of fluid signals were quantified on the images.
Key results:
A tonicity-dependent increase in lung fluid was demonstrated following HS administration. Pretreatment with the ENaC blockers, amiloride or 552-02, resulted in an enhancement of HS-induced lung fluid signals, which were detectable for up to 4 h, consistent with a role for ENaC in fluid clearance. Aprotinin, a serine protease inhibitor that attenuates ENaC function, likewise enhanced the HS-induced increase in lung fluid signal, while α1-anti-trypsin was without significant effect.
Conclusions and implications:
Proton MRI provides a non-invasive technique for studying modulators of lung fluid hydration in rat lung in vivo. The pharmacological sensitivity of MRI-detected fluid signals is consistent with ENaC-mediated fluid reabsorption after HS. This target-related readout may be used to characterize new ENaC modulators.
Keywords: amiloride, aquaporin, cystic fibrosis, cystic fibrosis transmembrane regulator, epithelial sodium channel, imaging, lung, magnetic resonance imaging, molecular imaging, mucus, mucus clearance
Introduction
In human airways, epithelial sodium channel (ENaC)-mediated Na+ absorption plays a key role in the regulation of mucosal hydration and as such contributes directly to an effective mucus clearance (Boucher, 2007). The central regulatory function that ENaC plays in the maintenance of airway mucus clearance can be observed in cystic fibrosis (CF), where ENaC-mediated Na+ hyperabsorption in the absence of normal anion secretion is widely believed to lead to the dessication of the airway lumen that results in mucostasis establishing the environment for chronic respiratory infections and overt inflammation (Boucher, 2007).
Approaches to restore mucosal hydration in the CF airway include the inhalation of osmotic agents, in addition to modulation of ion transport function. It has been recently demonstrated that inhaled hypertonic saline (HS) can improve lung function and reduce exacerbation frequency in CF patients (Donaldson et al., 2006; Elkins et al., 2006), through a mechanism that probably involves an increase in airway hydration (Tarran et al., 2001). Negative regulation of ENaC function in the airways represents another therapeutic opportunity for the treatment of CF and other conditions associated with impaired mucus clearance (Knowles et al., 1981; Hirsh, 2002). Initial studies indicated that inhalation of the ENaC blocker, amiloride, enhances mucociliary clearance and improves lung function in CF patients (App et al., 1990; Knowles et al., 1990). However, subsequent studies failed to validate the positive benefits on lung function. The lack of robust clinical benefit with inhaled amiloride has been ascribed to the poor potency of the compound and a suboptimal pharmacokinetic profile (Bowler et al., 1995; Hofmann et al., 1997). As such, the identification of novel negative regulators of ENaC function, more suited to inhaled delivery, has been explored to further test the concept clinically (Hirsh et al., 2008). Moreover, the combination of inhaled ENaC blockers with inhaled osmolytes has been predicted to be additive in terms of an enhanced hydration of the airway mucosa. To this end, in vitro data obtained with primary human airway epithelial cultures (non-CF) demonstrated that amiloride prolonged the airway surface liquid (ASL) volume response to the mucosal addition of NaCl (Tarran et al., 2001). However, a recent clinical study in CF patients reported a paradoxical negative impact of amiloride on the benefits obtained with inhaled HS (Donaldson et al., 2006). In vitro studies implied that amiloride could block an osmotically driven flux of fluid onto the mucosa of primary CF bronchial epithelia, that was in direct contrast to the earlier report in non-CF airway epithelia (Tarran et al., 2001). Future clinical studies with alternative ENaC blockers are needed to assess whether they can be used in combination with HS.
The aims of the present study were, in the first place, to assess whether magnetic resonance imaging (MRI) could be used to quantify levels of lung hydration in vivo in anaesthetized rats and, specifically, to assess whether an osmotically driven flux of fluid could be observed. Proton MRI in spontaneously breathing animals has been shown earlier to be well suited to quantify fluid signals in the small rodent lung in several models of pulmonary inflammation (Beckmann et al., 2001; 2002; Bléet al., 2008; Karmouty-Quintana et al., 2008). Secondly, we asked whether osmotically induced lung fluid signals could be modulated through the regulation of ENaC function in the airway. Pharmacological agents at doses previously demonstrated to attenuate ENaC function in the airways of guinea-pigs (Coote et al., 2008) were administered prior to HS or physiological saline (PS) by intra-tracheal (i.t.) instillation. In addition to blocking ENaC, amiloride has also been reported to modulate the activity of a variety of additional proteins including acid sensing ion channels, Na+/Ca2+ exchangers and Na+/H+ exchangers (see Kleyman and Cragoe, 1988;Simchowitz et al., 1992; Kemp and Kim, 2004 for reviews). To this end, the effects of an alternative and more potent ENaC blocker, 552-02, and the channel-activating protease (CAP) inhibitor, aprotinin, were also studied to confirm that the results obtained were an ENaC-mediated effect. The results obtained were consistent with a significant role for ENaC in the regulation of the dynamics of HS-induced fluid clearance quantified by MRI in the whole lung.
Methods
Experiments were performed with the approval of the Veterinary Authority of the City of Basel (license number 1989).
Animals
Male Brown Norway (BN) rats (n= 116 animals), weighing 270–300 g, were supplied by IFFA CREDO (L'Arbresle, France). Upon arrival animals were kept at an ambient temperature of 22 ± 2°C under a 12 h normal phase light-dark cycle and fed NAFAG® pellets (Nahr- und Futtermittel AG, Gossau, Switzerland) for at least 1 week before the start of the experiments. Drinking water was freely available.
Intra-tracheal administration of substances
For substance administration, rats were anaesthetized (2% isoflurane; Abbott, Cham, Switzerland) and then suspended at approximately a 45° angle by the two front upper teeth using a rubber band attached to a metal support. A laryngoscope was used to lift the lower jaw and keep the mouth open and the tongue displaced, allowing a clear view of the tracheal opening. A curved cannula with a diameter of 1.6 mm attached to a 1 mL syringe was then inserted into the trachea at the level above the carina. Immediately after insertion of the cannula, 0.2 mL of fluid was sprayed into the trachea.
Experimental protocols
In the first series of experiments, the fluid signals induced by the i.t. administration of saline (0.9% (n= 8 rats), 1.5% (n= 4 rats), 3% (n= 4 rats) and 6% NaCl (n= 4 rats), 0.2 mL) were quantified using MRI. Measurements were carried out at baseline (at least 3 h before saline) and at 30 min, 1 h and 4 h after saline instillation.
In a separate series of studies, the ENaC blocking compounds were administered 20 min before HS (1.5% w/v, 0.2 mL). The ENaC blockers (amiloride, 552-02) or vehicle (5% dextrose, 0.2 mL, n= 6 animals) were administered to the rats by i.t. instillation, as described above. Amiloride was given at doses of 0.03, 0.3 or 3 mg·kg−1 (n= 6 rats per group), while 552-02 was administered at doses of 0.1, 1, 10 or 100 µg·kg−1 (n= 6 rats per group). The effects of aprotinin (1 µg·kg−1, n= 6 rats) and α1-antitrypsin (0.77 mg·kg−1, n= 6 rats) were also examined using an identical paradigm, except that these compounds were administered 2 h before HS. MRI was performed at baseline (at least 3 h before substance application) and at time points 30 min, 1 h and 4 h after dosing with HS. Chemicals were purchased from Sigma-Aldrich (Buchs, Switzerland) with the exception of 552-02 that was produced in-house.
As this is a functional assessment in which anaesthesia may influence the rate of fluid clearance, care was taken to have reproducible experimental conditions concerning level (always kept at 2% isoflurane) and duration of anaesthesia. Induction time was 10 min for each animal in all instances. In the experiments detailed in Figures 1–3, anaesthesia was maintained from the time of compound administration to the 1 h time point (duration of anaesthesia 1 h 40 min including induction time). Animals were then were removed from the magnet and allowed to regain consciousness before being re-anaesthetized for the 4 h acquisition time point (duration of anaesthesia 30 min). In total, rats were anaesthetized for 2 h 10 min, with one interruption. For the antitrypsin and aprotinine experiments, the rats were anaesthetized with 2% isoflurane for 10 min to enable i.t.administration of vehicle or protease inhibitor and then again to enable administration of the saline and the imaging sessions, with induction time kept to 10 min in all instances. In total, the time the rats were anaesthetized for this series of experiments was 2 h 30 min, with three interruptions.
Figure 1.
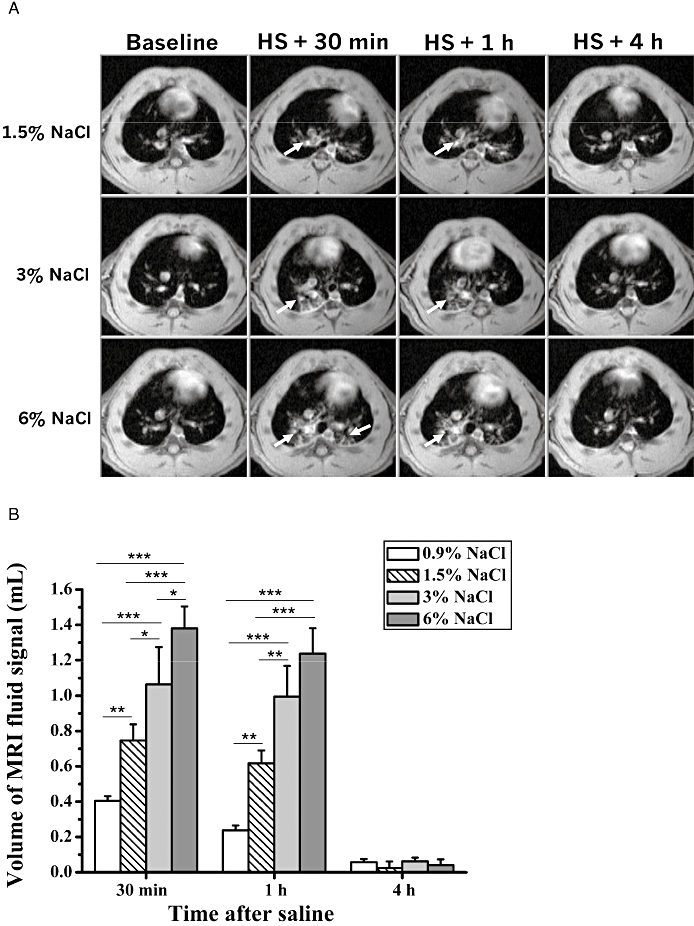
(A) Axial MR images of three rats acquired before, and at 30 min, 1 h and 4 h after i.t. administration of saline (0.2 mL) of different tonicities. The arrows indicate the fluid signals detected by MRI at the different time points. (B) Corresponding volumes (means ± SEM, n= 8 rats for physiological saline (PS, 0.9% NaCl; n= 4 animals per group for the higher tonicities) of fluid signals evaluated from the MR images. The levels of significance *0.01 ≤P < 0.05, **0.001 ≤P < 0.01 and ***P < 0.001 correspond to extended anova comparisons. HS, hypertonic saline; MRI, magnetic resonance imaging.
Figure 3.
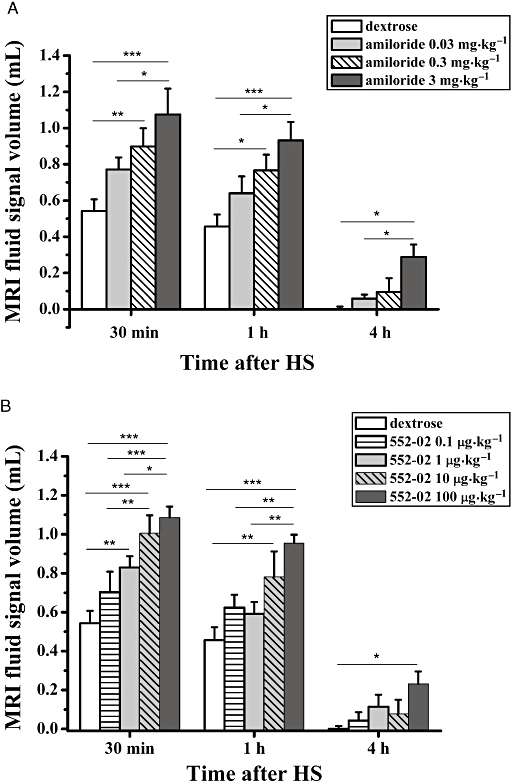
Volumes (means ± SEM, n= 6 animals per group) of fluid signals detected by MRI in the lungs of BN rats, at 30 min, 1 h and 4 h following i.t. administration of HS (1.5% NaCl, 0.2 mL). Rats had received either vehicle (dextrose 5%, 0.2 mL) (A) amiloride or (B) 552-02 at the specified dose (0.2 mL solution volume), 20 min before saline. The levels of significance *0.01 ≤P < 0.05, **0.001 ≤P < 0.01 and ***P < 0.001 correspond to extended anova comparisons. HS, hypertonic saline; MRI, magnetic resonance imaging.
Doses of compounds used here were selected based on their effects on tracheal potential difference (TPD) assessments in vivo in guinea-pigs for the study of ENaC function in the airways (Coote et al., 2008).
All drug and molecular target nomenclature conforms with the British Journal of Pharmacology's Guide to Receptors and Channels (Alexander et al., 2008).
MRI
Measurements were carried out with a Biospec 47/40 spectrometer (Bruker Medical Systems, Ettlingen, Germany) operating at 4.7 T and equipped with an actively shielded gradient system capable of generating a gradient of 200 mT·m−1. The operational software of the scanner was Paravision (Bruker). During MRI signal acquisitions, rats were placed in supine position in a cradle made of Plexiglas. Body temperature was maintained at 37 ± 1°C using warm air. Anaesthesia was maintained with 2% isoflurane, in a mixture of O2/N2O (2:1), administered via a nose cone. All measurements were performed on spontaneously breathing animals, and neither cardiac nor respiratory triggering was applied.
For detection of fluid signals a gradient-echo sequence with the following parameters was applied (Beckmann et al., 2001): repetition time, 5.6 ms; echo time, 2.7 ms; flip angle of the excitation pulse, approximately 15°; field-of-view, 6 × 6 cm2; matrix size, 256x128; and slice thickness, 1.5 mm. A single slice image was obtained by computing the two-dimensional Fourier transformation of the averaged signal from 45 individual image acquisitions and interpolating the data set to 256 × 256 pixels. There was an interval of 530 ms between individual image acquisitions, resulting in a total acquisition time of 59 s for a single slice. The entire lung was covered by 18 consecutive axial slices.
The volume of fluid signals was quantified using a semi-automatic segmentation procedure implemented in the IDL (Interactive Data Language Research Systems, Boulder, CO, USA) environment (version 5.1) on a Linux system. The procedure has been extensively described previously (Beckmann et al., 2001; 2002; Bléet al., 2008). Segmentation parameters were the same for all images analysed, chosen to segment regions corresponding to high intensity signals. Because the signals from fluid and vessels were of comparable intensities, the volume corresponding to the vessels was assessed on baseline images and then subtracted from the volumes determined on post-treatment images (parsed data).
Statistics
Differential signal volumes, obtained by subtracting, for each animal, the baseline signal volume from the post-saline values, were analysed by SYSTAT Version 12 (Systat Software, Inc., San José, CA, USA). For the analyses of multiple acquisition data, we used an extension of anova, called ‘Mixed model analysis’ or ‘anova with random effects’ to take into account the longitudinal structure of the data. For multiple comparisons a Bonferroni correction followed the anova.
Results
HS enhanced lung fluid volumes in a tonicity-dependent manner
Figure 1A shows representative axial magnetic resonance (MR) images of the chest of BN rats, acquired at various time points with respect to i.t. administration of saline of different tonicities. Although only one image is depicted per time point, 18 sequential slices covering the whole lungs of a rat were acquired at each time point. Compared with baseline, noticeable fluid signals were present in the lungs during the first hour after saline (arrows). As summarized in Figure 1B, saline induced a tonicity-dependent increase in lung fluid volume. Four hours after administration of saline fluid signals had returned to baseline levels. No histological evidence of increased perivascular oedema or mucus release was found after HS or PS administration at this time point (data not shown). The tonicity of 1.5% NaCl was then selected for the ensuing experiments.
Effects of ENaC blockers on HS-induced lung fluid
An initial study using a single high dose of amiloride (3 mg·kg−1 i.t.), that was predicted to be supramaximal in terms of ENaC block in the airways (Coote et al., 2008), induced a significant enhancement of the lung fluid volume above that of the vehicle-treated control group (Figure 2). Of note, the signal volumes were higher than the theoretical 0.4 mL one might expect from the total amount of fluid administered i.t. This enhanced fluid volume was observed at both 30 and 60 min following HS administration and remained elevated for at least 4 h, a time when the vehicle/HS-treated group had returned to baseline levels. Subsequent dose–response studies using either amiloride (Figure 3A) or 552-02 (Figure 3B), a recently described, potent ENaC blocker (Hirsh et al., 2008), confirmed the initial observations of the enhanced lung fluid volumes. These data suggest that 552-02 is approximately 30-fold more potent than amiloride in vivo, consistent with previous reports in rodent airways (Coote et al., 2008). Once it had been established that the reference ENaC blocker, amiloride, dose-dependently increased osmotically driven fluid volumes in the lung (as detected by MRI) further compounds were tested to validate the model.
Figure 2.
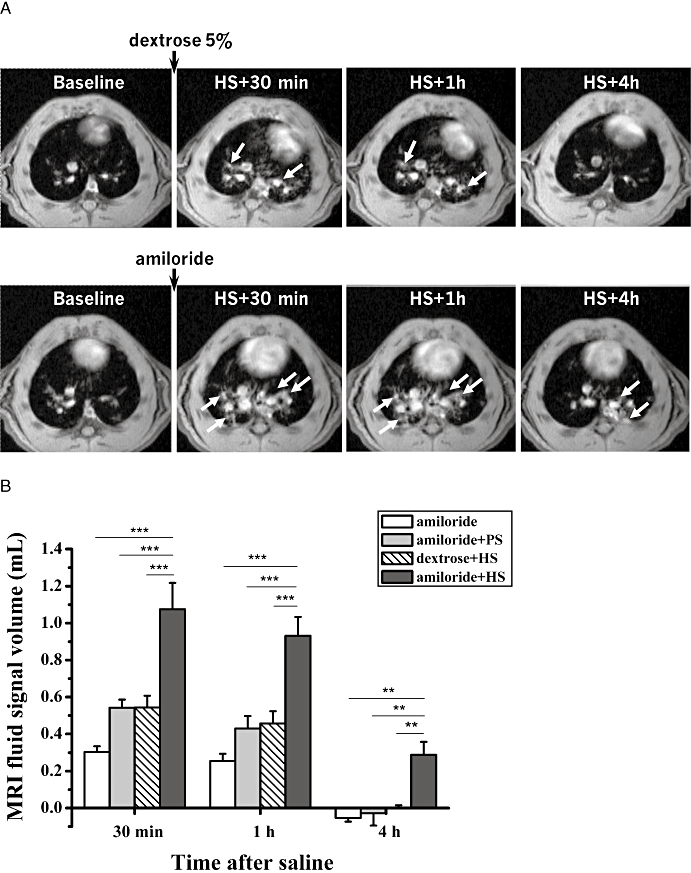
(A) Axial MR images of two BN rats acquired 1 day before (baseline) and at 30 min, 1 h and 4 h following i.t. administration of HS (1.5% NaCl, 0.2 mL) as a spray. One animal (lower row) had been pretreated with amiloride (3 mg·kg−1 i.t., 0.2 mL) 20 min before HS administration, while the other rat (upper row) had received vehicle (dextrose 5%, 0.2 mL) at the same time point. Fluid signals (arrows) following HS were more prominent and of longer duration for the animal pretreated with amiloride. (B) Volumes (means ± SEM, n= 6 animals per group) of fluid signals in the lungs evaluated from the MR images acquired at 30 min, 1 h and 4 h following i.t. administration of HS (1.5% NaCl, 0.2 mL) or PS (0.9% NaCl, 0.2 mL). Rats had received either amiloride (3 mg·kg−1, 0.2 mL) or vehicle (dextrose 5%, 0.2 mL) 20 min before saline. For amiloride administered alone, the first MRI acquisition took place at 50 min following the compound. The levels of significance **0.001 ≤P < 0.01 and ***P < 0.001 correspond to extended anova comparisons. HS, hypertonic saline; MRI, magnetic resonance imaging; PS, physiological saline.
Effects of aprotinin and α1-antitrypsin on HS-induced lung fluid
In the first hour after HS, the lungs of rats that had received aprotinin (1 µg·kg−1) 2 h prior to HS presented similar fluid signal volumes (Figure 4) to those detected by MRI following pretreatment with amiloride (3 mg·kg−1) (Figure 3A) or 552-02 (100 µg·kg−1) (Figure 3B). Signals in the lungs of rats pretreated with aprotinin were detectable 6 h after HS. Application of α1-antitrypsin 2 h before HS resulted in significantly smaller fluid responses following saline, comparable to those obtained after pretreatment with the vehicle (Figure 4).
Figure 4.
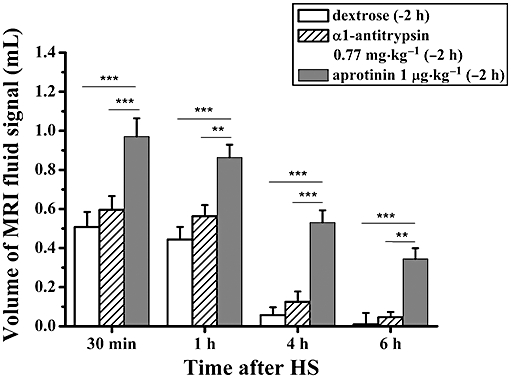
Volumes (means ± SEM, n= 6 animals per group) of fluid signals detected by MRI in the lungs of BN rats, at 30 min, 1 h, 4 h and 6 h following i.t. administration of HS (1.5% NaCl, 0.2 mL). Animals were pretreated (−2 h) with either vehicle (dextrose 5%, 0.2 mL), α1-antitrypsin (0.77 mg·kg−1, 0.2 mL) or aprotinin (1 µg·kg−1, 0.2 mL). Levels of significance **0.001 ≤P < 0.01 and ***P < 0.001 correspond to extended anova comparisons. HS, hypertonic saline; MRI, magnetic resonance imaging.
Discussion
There is strong evidence to link airway mucosal hydration with mucus clearance in human diseases, most notably in CF (Boucher, 2007). Modulators of airway epithelial ion transport processes therefore represent an interesting approach to enhance mucosal hydration and thereby promote mucus clearance. The aim of the present work was to develop a rat model that would be suitable to characterize airway hydration in vivo and specifically the effects of HS and negative regulators of ENaC function in the airways. The choice for MRI resided on the fact that the technique in its very essence detects the distribution of water in tissue. Due to this inherent characteristic, proton MRI has been shown earlier to be well-suited to quantify non-invasively inflammation-related fluid signals in the lungs of rats and mice in several models of pulmonary inflammation (Beckmann et al., 2001; 2002; Bléet al., 2008; Karmouty-Quintana et al., 2008).
An initial series of experiments demonstrated the ability of MRI to quantify osmotically driven fluid flux into the lungs of healthy animals. Following i.t. administration of saline, tonicity-dependent increases in lung fluid signals detected by MRI were apparent. The increase in lung fluid volume was transient and had recovered by 4 h to baseline levels, presumably through absorption of fluid or by mucociliary clearance. It has been previously demonstrated that mucosal fluid can be transported together with the overlying mucus gel (Forteza et al., 2001). These data are therefore consistent with a model of HS inducing an osmotically driven fluid flux into the lungs (Tarran et al., 2001). However, the spatial resolution of the MR images does not enable the precise location of the fluid signals to be determined. Nevertheless, the diffuse appearance of the MRI signals obtained following HS administration are in contrast to the bright and continuous MRI signals that are observed to correlate with perivascular oedema in lung inflammation models in the rat (Beckmann et al., 2001; Tigani et al., 2003; Karmouty-Quintana et al., 2008). Furthermore, the HS-induced fluid signals were rapidly resolved within 4 h of dosing, whereas pulmonary oedema has consistently taken days to resolve in other rodent models evaluated by MRI. Together, these observations are consistent with osmotically induced fluid being located within the airway lumen.
In terms of the magnitude of the MRI signals, the administration of 0.2 mL of 0.9% saline induced a fluid signal of greater magnitude than would have been expected from the volume of fluid added alone, at both the 30 and 60 min time points (Figure 1B), suggesting that the administration of fluid may induce either a secretory response or inhibit absorption that could further contribute to the fluid signal. In addition, a systematic error in the evaluation of fluid signals cannot be excluded and this would lead to an overall overestimation of their volumes with our method. At the higher concentrations of saline, the fluid signal was somewhat smaller than would have been predicted. This may reflect a rapid clearance of a large fluid volume during the initial 30 min following HS administration. Consistent with this idea, pre-dosing of rats with amiloride before HS, induced a significant enhancement of the lung fluid signal, suggesting that amiloride was slowing an early absorptive process.
In order to investigate the possibility that this amiloride-sensitive, potentially absorptive pathway is mediated by ENaC, a series of pharmacological studies were performed to assess the potency of established ENaC blockers, together with the sensitivity of the phenotype to serine protease inhibitors. Amiloride and 552-02 (direct ENaC blockers) both enhanced the lung fluid signal in response to HS in a dose-dependent manner. These studies indicated that 552-02 was approximately 30 times more potent than amiloride under in vivo conditions, an observation that is consistent with TPD measurements in guinea-pigs (Coote et al., 2008) and in agreement with the pharmacology of ENaC (Coote et al., 2008; Hirsh et al., 2008).
To further validate that the enhanced lung fluid signals were as a consequence of reduced ENaC-mediated absorption, we next evaluated the effects of the Kunitz-type macromolecular serine protease inhibitor, aprotinin, and the serpin, α1-antitrypsin. Whereas aprotinin has been previously demonstrated to attenuate ENaC activity in human bronchial epithelial (HBE) cells derived from both normal and CF airways, α1-antitrypsin was without effect (Bridges et al., 2001; Donaldson et al., 2002). A model for CAP regulation of ENaC function in the airways proposes that ENaC is inserted into the apical membrane of the epithelial cells in an inactive state, and that an interaction with a CAP is required for the activation of the channels (Planès and Caughey, 2007). Thus, in the presence of a CAP inhibitor such as aprotinin, ENaC would not become activated upon insertion into the plasma membrane resulting in a steady decline in epithelial ENaC-mediated Na+ transport as the active channel is internalized. In vitro, HBE cell studies have indicated a maximal effect of Kunitz-type inhibitors on ENaC by approximately 90 min after their addition (Bridges et al., 2001). Two hours was therefore selected as the time point for assessing CAP inhibitory activity in vivo in the rat, analogous to TPD assessments performed in guinea-pigs (Coote et al., 2008). At this time, aprotinin potently enhanced the MRI signals detected after HS to a similar degree to that observed with the direct ENaC blockers. In contrast, α1-antitrypsin had no effect on the MRI signals, a result that is consistent with the lack of in vitro effects of this protease inhibitor on human airway epithelium (Bridges et al., 2001; Donaldson et al., 2002). Taken together, these data support both the proposed role of CAP in the regulation of ENaC function and help validate the proposal that the MRI detected fluid signals are located at the apical side of the airway epithelium.
Recent data have indicated the clinical benefit of nebulized HS in CF lung disease, with a proposed mechanism involving sustained increase in ASL volume (Tarran et al., 2001; 2006;). However, a paradoxical effect was reported by Donaldson et al. (2006) when amiloride was administered together with HS to CF patients. Rather than improving lung function, amiloride negated the beneficial effect of HS on the measured mucus clearance. To account for this paradoxical observation in that amiloride suppressed the beneficial effect of HS, it has been postulated that amiloride-inhibitable aquaporin (AQP) water channels in CF airway epithelia modulate ASL volume (Donaldson et al., 2006). Further experiments using several sensitive methods to assess osmotic water permeabilities reported that amiloride did not inhibit water permeability in non-CF or CF airway epithelia, AQP-transfected Fisher rat thyroid cells, or the intact lung (Levin et al., 2006). The results in the present study using rats with normal cystic fibrosis transmembrane regulator (CFTR) function, likewise show no evidence of an amiloride-induced block of osmotically driven fluid flux.
In our experiments, animals breathed spontaneously throughout the experiment, i.e. no artificial ventilation was used. Anaesthesia decreased the breathing and the heart rate. These changes did not affect the fluid determination by imaging, as MRI acquisitions were performed without gating. On the other hand, as anaesthesia may have influenced (reduced) the rate of fluid absorption, particular care was taken about having identical timings in the experiments with the ENaC blockers and with the serine protease inhibitors.
In summary, we have demonstrated that proton MRI non-invasively provides quantitative information on osmotically driven fluid influx into the airways of spontaneously breathing rats. The results obtained here for amiloride, 552-02, aprotinin and α1-antitrypsin suggest that the dynamics of the fluid signals detected by MRI reflected ENaC activity. In other words, ENaC-related information was derived using MRI without the administration of any specific imaging probe. In the context of in vivo molecular imaging techniques of interest for pharmacological research (Rudin and Weissleder, 2003; Ripoll et al., 2008; Willmann et al., 2008), target-related information is usually obtained by the administration of target-specific agents following their proper validation. Instead, in the present work pharmacological agents known to act upon the ENaC function were used to modulate the fluid dynamics in the rat lung as assessed by MRI. This target-related readout may thus be used to characterize new modulators of the activity of this sodium channel. Adaptation of the protocol to animal models of pathology, e.g. to lipopolysaccharide-challenged rats (Beckmann et al., 2002) or to CFTR-deficient mice (Allard et al., 2006), could be of interest in view of studies of mucus dynamics. Finally, it is conceivable that the present model has translational potential to clinical studies of lung MRI involving the use of ENaC blockers.
Acknowledgments
N.B. received an award from the 3R Research Foundation, Muensingen, Switzerland (Project 82-02).
Glossary
Abbreviations:
- 552-02
N-(3,5-diamino-6-chloropyrazine-2-carbonyl)-N′-4-[4-(2,3-dihydroxypropoxy)phenyl] butyl-guanidine
- AQP
aquaporin
- CAP
channel-activating protease
- CF
cystic fibrosis
- CFTR
cystic fibrosis transmembrane regulator
- ENaC
epithelial sodium channel
- HS
hypertonic saline
- MR
magnetic resonance
- MRI
magnetic resonance imaging
- PS
physiological saline
- TPD
tracheal potential difference
Conflict of interest disclosure
The authors had full responsibility for the conduct of the trial, had full access to all the data and controlled the decision to publish.
References
- Alexander SPH, Mathie A, Peters JA. Guide to receptors and channels (GRAC) Br J Pharmacol. 2008;153(Suppl. 2):S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allard JB, Poynter ME, Marr KA, Cohn L, Rincon M, Whittaker LA. Aspergillus fumigatus generates an enhanced Th2-biased immune response in mice with defective cystic fibrosis transmembrane conductance regulator. J Immunol. 2006;177:5186–5194. doi: 10.4049/jimmunol.177.8.5186. [DOI] [PubMed] [Google Scholar]
- App EM, King M, Helfesrieder R, Köhler D, Matthys H. Acute and long-term amiloride inhalation in cystic fibrosis lung disease. A rational approach to cystic fibrosis therapy. Am Rev Respir Dis. 1990;141:605–612. doi: 10.1164/ajrccm/141.3.605. [DOI] [PubMed] [Google Scholar]
- Beckmann N, Tigani B, Ekatodramis D, Borer R, Mazzoni L, Fozard JR. Pulmonary edema induced by allergen challenge in the rat: noninvasive assessment by magnetic resonance imaging. Magn Reson Med. 2001;45:88–95. doi: 10.1002/1522-2594(200101)45:1<88::aid-mrm1013>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Beckmann N, Tigani B, Sugar R, Jackson AD, Jones G, Mazzoni L, et al. Noninvasive detection of endotoxin-induced mucus hypersecretion in rat lung by MRI. Am J Physiol Lung Cell Mol Physiol. 2002;283:L22–L30. doi: 10.1152/ajplung.00373.2001. [DOI] [PubMed] [Google Scholar]
- Blé FX, Cannet C, Zurbruegg S, Karmouty-Quintana H, Bergmann R, Frossard N, et al. Allergen-induced lung inflammation in actively sensitized mice assessed with MR imaging. Radiology. 2008;248:834–843. doi: 10.1148/radiol.2482071452. [DOI] [PubMed] [Google Scholar]
- Boucher RC. Airway surface dehydration in cystic fibrosis: pathogenesis and therapy. Annu Rev Med. 2007;58:157–170. doi: 10.1146/annurev.med.58.071905.105316. [DOI] [PubMed] [Google Scholar]
- Bowler IM, Kelman B, Worthington D, Littlewood JM, Watson A, Conway SP, et al. Nebulised amiloride in respiratory exacerbations of cystic fibrosis: a randomised controlled trial. Arch Dis Child. 1995;73:427–430. doi: 10.1136/adc.73.5.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges RJ, Newton BB, Pilewski JM, Devor DC, Poll CT, Hall RL. Na+ transport in normal and CF human bronchial epithelial cells is inhibited by BAY 39-9437. Am J Physiol Lung Cell Mol Physiol. 2001;281:L16–L23. doi: 10.1152/ajplung.2001.281.1.L16. [DOI] [PubMed] [Google Scholar]
- Coote KJ, Atherton H, Young A, Sugar R, Burrows R, Smith NJ, et al. The guinea-pig tracheal potential difference as an in vivo model for the study of epithelial sodium channel function in the airways. Br J Pharmacol. 2008;155:1025–1033. doi: 10.1038/bjp.2008.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson SH, Bennett WD, Zeman KL, Knowles MR, Tarran R, Boucher RC. Mucus clearance and lung function in cystic fibrosis with hypertonic saline. N Engl J Med. 2006;354:241–250. doi: 10.1056/NEJMoa043891. [DOI] [PubMed] [Google Scholar]
- Donaldson SH, Hirsh A, Li DC, Holloway G, Chao J, Boucher RC, et al. Regulation of the epithelial sodium channel by serine proteases in human airways. J Biol Chem. 2002;277:8338–8345. doi: 10.1074/jbc.M105044200. [DOI] [PubMed] [Google Scholar]
- Elkins MR, Robinson M, Rose BR, Harbour C, Moriarty CP, Marks GB, et al. A controlled trial of long-term inhaled hypertonic saline in patients with cystic fibrosis. N Engl J Med. 2006;354:229–240. doi: 10.1056/NEJMoa043900. [DOI] [PubMed] [Google Scholar]
- Forteza R, Lieb T, Aoki T, Savani RC, Conner GE, Salathe M. Hyaluronan serves a novel role in airway mucosal host defense. FASEB J. 2001;15:2179–2186. doi: 10.1096/fj.01-0036com. [DOI] [PubMed] [Google Scholar]
- Hirsh AJ. Altering airway surface liquid volume: inhalation therapy with amiloride and hyperosmotic agents. Adv Drug Deliv Rev. 2002;54:1445–1462. doi: 10.1016/s0169-409x(02)00161-8. [DOI] [PubMed] [Google Scholar]
- Hirsh AJ, Zhang J, Zamurs A, Fleegle J, Thelin WR, Caldwell RA, et al. Pharmacological properties of N-(3,5-diamino-6-chloropyrazine-2-carbonyl)-N′-4-[4-(2,3-dihydroxypropoxy)phenyl]butyl-guanidine methanesulfonate (552-02), a novel epithelial sodium channel blocker with potential clinical efficacy for cystic fibrosis lung disease. J Pharmacol Exp Ther. 2008;325:77–88. doi: 10.1124/jpet.107.130443. [DOI] [PubMed] [Google Scholar]
- Hofmann T, Senier I, Bittner P, Hüls G, Schwandt HJ, Lindemann H. Aerosolized amiloride: dose effect on nasal bioelectric properties, pharmacokinetics, and effect on sputum expectoration in patients with cystic fibrosis. J Aerosol Med. 1997;10:147–158. doi: 10.1089/jam.1997.10.147. [DOI] [PubMed] [Google Scholar]
- Karmouty-Quintana H, Blé FX, Cannet C, Zurbruegg S, Fozard JR, Page CP, et al. In vivo pharmacological evaluation of compound 48/80-induced airways oedema by MRI. Br J Pharmacol. 2008;154:1063–1072. doi: 10.1038/bjp.2008.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp PJ, Kim KJ. Spectrum of ion channels in alveolar epithelial cells: implications for alveolar fluid balance. Am J Physiol Lung Cell Mol Physiol. 2004;287:L460–L464. doi: 10.1152/ajplung.00191.2004. [DOI] [PubMed] [Google Scholar]
- Kleyman TR, Cragoe EJ., Jr Amiloride and its analogs as tools in the study of ion transport. J Membr Biol. 1988;105:1–21. doi: 10.1007/BF01871102. [DOI] [PubMed] [Google Scholar]
- Knowles MR, Church NL, Waltner WE, Yankaskas JR, Gilligan P, King M, et al. A pilot study of aerosolized amiloride for the treatment of lung disease in cystic fibrosis. N Engl J Med. 1990;322:1189–1194. doi: 10.1056/NEJM199004263221704. [DOI] [PubMed] [Google Scholar]
- Knowles M, Gatzy J, Boucher R. Increased bioelectric potential difference across respiratory epithelia in cystic fibrosis. N Engl J Med. 1981;305:1489–1495. doi: 10.1056/NEJM198112173052502. [DOI] [PubMed] [Google Scholar]
- Levin MH, Sullivan S, Nielson D, Yang B, Finkbeiner WE, Verkman AS. Hypertonic saline therapy in cystic fibrosis: evidence against the proposed mechanism involving aquaporins. J Biol Chem. 2006;281:25803–25812. doi: 10.1074/jbc.M604332200. [DOI] [PubMed] [Google Scholar]
- Planès C, Caughey GH. Regulation of the epithelial Na+ channel by peptidases. Curr Top Dev Biol. 2007;78:23–46. doi: 10.1016/S0070-2153(06)78002-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripoll J, Ntziachristos V, Cannet C, Babin AL, Kneuer R, Gremlich HU, et al. Investigating pharmacology in vivo using magnetic resonance and optical imaging. Drugs R D. 2008;9:277–306. doi: 10.2165/00126839-200809050-00001. [DOI] [PubMed] [Google Scholar]
- Rudin M, Weissleder R. Molecular imaging in drug discovery and development. Nat Rev Drug Discov. 2003;3:123–131. doi: 10.1038/nrd1007. [DOI] [PubMed] [Google Scholar]
- Simchowitz L, Kleyman TR, Cragoe EJ., Jr . An overview of the structure-activity relationships in the amiloride series. In: Cragoe EJ Jr, Kleyman TR, Simchowitz L, editors. Amiloride and Its Analogs: Unique Cation Transport Inhibitors. Weinheim: Wiley-VCH; 1992. pp. 9–24. [Google Scholar]
- Tarran R, Grubb BR, Parsons D, Picher M, Hirsh AJ, Davis CW, et al. The CF salt controversy: in vivo observations and therapeutic approaches. Mol Cell. 2001;8:149–158. doi: 10.1016/s1097-2765(01)00286-6. [DOI] [PubMed] [Google Scholar]
- Tarran R, Trout L, Donaldson SH, Boucher RC. Soluble mediators, not cilia, determine airway surface liquid volume in normal and cystic fibrosis superficial airway epithelia. J Gen Physiol. 2006;127:591–604. doi: 10.1085/jgp.200509468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tigani B, Cannet C, Zurbrugg S, Schaeublin E, Mazzoni L, Fozard JR, et al. Resolution of the oedema associated with allergic pulmonary inflammation in rats assessed noninvasively by magnetic resonance imaging. Br J Pharmacol. 2003;140:239–246. doi: 10.1038/sj.bjp.0705429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willmann JK, van Bruggen N, Dinkelborg LM, Gambhir SS. Molecular imaging in drug development. Nat Rev Drug Discov. 2008;7:591–607. doi: 10.1038/nrd2290. [DOI] [PubMed] [Google Scholar]


