Abstract
Hyperthermia is probably the most widely known acute adverse event that can follow ingestion of 3,4-methylenedioxymethamphetamine (MDMA, ecstasy) by recreational users. The effect of MDMA on body temperature is complex because the drug has actions on all three major monoamine neurotransmitters [5-hydroxytryptamine (5-HT), dopamine and noradrenaline], both by amine release and by direct receptor activation. Hyperthermia and hypothermia can be induced in laboratory animals by MDMA, depending on the ambient temperature, and involve both central thermoregulation and peripheral changes in blood flow and thermogenesis. Acute 5-HT release is not directly responsible for hyperthermia, but 5-HT receptors are involved in modulating the hyperthermic response. Impairing 5-HT function with a neurotoxic dose of MDMA or p-chlorophenylalanine alters the subsequent MDMA-induced hyperthermic response. MDMA also releases dopamine, and evidence suggests that this transmitter is involved in both the hyperthermic and hypothermic effects of MDMA in rats. The noradrenergic system is also involved in the hyperthermic response to MDMA. MDMA activates central α2A-adrenoceptors and peripheral α1-adrenoceptors to produce cutaneous vasoconstriction to restrict heat loss, and β3-adrenoceptors in brown adipose tissue to increase heat generation. The hyperthermia occurring in recreational users of MDMA can be fatal, but data reviewed here indicate that it is unlikely that any single pharmaceutical agent will be effective in reversing the hyperthermia, so careful body cooling remains the principal clinical approach. Crucially, educating recreational users about the potential dangers of hyperthermia and the control of ambient temperature should remain key approaches to prevent this potentially fatal problem.
Keywords: 3,4-methylenedioxymethamphetamine; 3,4-methylenedioxyethamphetamine; 3,4-methylenedioxyamphetamine; hyperthermia; hypothermia; 5-hydroxytrypamine; dopamine; noradrenaline; thermoregulation
Introduction
Hyperthermia is an acute adverse event that can follow ingestion of 3,4-methylenedioxymethamphetamine (MDMA) by recreational users. The problem was first reported over 20 years ago as emergency rooms found they were admitting young persons who had taken MDMA while they were present at dance clubs or parties, and especially at ‘raves’, an environment where the ambient temperature tends to be high and there is excessive physical exertion during dancing. These persons sometimes presented with a range of medical problems including rhabdomyolysis, myoglobinuria, renal failure, liver damage and disseminated intravascular coagulopathy. These are problems also seen in persons suffering from heatstroke (Kalant, 2001), and have also been reported to occur in persons who have taken high doses of other amphetamines such as methamphetamine, amphetamine and 3,4-methylenedioxyethamphetamine (MDEA or ‘eve’) (Ginsberg et al., 1970; Kendrick et al., 1977; Tehan, 1993). 3,4-Methylenedioxyamphetamine (MDA or ‘love’), as well as being a drug of abuse in its own right, is a metabolite both of MDMA (de la Torre et al., 2000; Cole and Sumnall, 2003b) and MDEA (Ensslin et al., 1996).
Hyperthermia can also be induced in laboratory animals by administration of MDMA, and it has been associated with the long-term neurotoxic degeneration of 5-hydroxytryptamine (5-HT) nerve endings that can occur in the forebrain following administration of the drug. This association is indicated by the fact that the degree of neurotoxic damage is related to the severity of the hyperthermic response. While neurotoxicity can occur after MDMA in the absence of a hyperthermic response, there is nevertheless, in general, a close correlation between the temperature response and degree of neurotoxic damage (Malberg and Seiden, 1998). Furthermore, the damage induced by a neurotoxic dose of MDMA can be attenuated by placing the animals in a low ambient temperature (Schmidt et al., 1990; Broening et al., 1995), and exacerbated by placing the animals in a room with an elevated ambient temperature (Broening et al., 1995; Sanchez et al., 2004).
The ability of MDMA to induce neurotoxic damage in the brain of the recreational user remains contentious (Green, 2004). However, even if it does not occur there are good reasons to try and minimize the hyperthermia occurring in any recreational user because it is associated with other severe acute clinical problems and can be fatal. Current treatment relies on the use of dantrolene or trying to reduce the body temperature quickly by the use of ice packs.
Over the last few years, there have been a series of studies made in experimental animals to try and understand better the pharmacology of the hyperthermic response. This review focuses on the role of the major monoamine neurotransmitters (5-HT, dopamine and noradrenaline) in the body temperature altering the action of MDMA. Receptor nomenclature in this paper conforms to the British Journal of Pharmacology's Guide to Receptors and Channels (Alexander et al., 2008).
Physiology of temperature control
Body temperature is closely regulated because changes in body temperature affect cellular function, and relatively small increases in body temperature are particularly dangerous in causing protein denaturation (Roti Roti, 2008) and pathological changes in nerve function (Sharma and Hoopes, 2003). Core body temperature is maintained as a balance between heat generation and heat loss. Increased heat generation and diminished heat loss help maintain body temperature in a cold environment. Heat production can be increased by increased skeletal muscle tone and activity, by shivering and by non-shivering thermogenesis (increase in cellular metabolism involving adrenergic and thyroid hormones), and heat loss is reduced by skin vasoconstriction, warm clothing and postural changes to reduce exposed surface area. In response to a warm environment, heat generation is decreased, and heat loss is increased by skin vasodilatation, sweating and removing clothes. The transfer of heat between the body and the environment involves four mechanisms: radiation (transfer of infrared radiation energy, from warmer to cooler objects); conduction (transfer of heat to objects, air or water, from warmer to cooler); convection (warm air currents rising from the skin to aid conduction, or by the effects of fans or wind); and evaporation (passive or obligatory evaporation in the airways and skin, and active evaporation by sweating). When the ambient temperature is above body temperature, heat loss relies on evaporation by sweating, but at high air humidity, sweat does not evaporate, and this may be of relevance to the ‘rave’ environment. Some differences between rodents and humans in thermoregulation should be noted. Rodent tail is a major organ of temperature regulation, so that increased tail blood flow and panting increase heat loss, whereas piloerection (to trap air for insulation) reduces heat loss, and the presence of brown fat allows more marked increases in heat generation than occurs in humans. Autonomic control of temperature involves mainly control of cutaneous blood flow, modulation of heat generation and sweating.
MDMA-induced temperature changes in rats
Many studies have reported that when rats are housed in normal ambient room temperatures (20–22°C), their body temperature is elevated by MDMA administration in a dose-dependent manner. This has been reported to occur in several strains by many investigators (see Green et al., 2003). However, a hypothermic response has also been reported to occur in rats housed at this temperature (Malberg and Seiden, 1998; Malpass et al., 1999; Daws et al., 2000; Bexis and Docherty, 2006). In the study of Bexis and Docherty (2006), both MDMA and MDEA produced only hypothermia in rats housed at 22°C, whereas MDA produced a transient hypothermia followed by hyperthermia.
At higher room temperatures (e.g. 30°C), hyperthermia is always seen, and hyperthermia induced in the rats is greater than that seen in rats housed in normal conditions (Green et al., 2005).
In contrast, when MDMA is given to rats housed in low-room temperature conditions, its effect is to lower body temperature. Dafters (1994) observed dose-dependent hypothermia in rats administered with MDMA when housed at 11°C, and subsequently reported hypothermia to occur when the rats were housed at 17°C (Dafters, 1995). Green et al. (2005) also observed hypothermia in rats housed at 15°C. This suggests that the switching point for inducing hypothermia or hyperthermia occurs at a room temperature of around 19–22°C, which is not far below normal ambient conditions for rats. Broening et al. (1995) noted that rats aged 40 and 70 days showed hypothermia and hyperthermia, respectively, at low and high room temperatures, but that MDMA did not influence body temperature in 10 day rats. MDMA is not the only amphetamine compound to produce a temperature response that is dependent on ambient temperature. The simpler congener amphetamine also produces, respectively, hyperthermia and hypothermia when rats are housed, respectively, in warm or cool ambient temperature room conditions (Yehuda and Wurtman, 1972a,b).
MDMA-induced temperature changes in guinea pigs and mice
While MDMA does induce hyperthermia in guinea pigs, it does so only after the first dose, with a subsequent dose 6 h later having no effect. Further dosing at day 2 was also without effect in body temperature (Saadat et al., 2004).
Most investigations on MDMA-induced body temperature effects in mice have also generally used repeat dosing, and the responses obtained have indicated that the response obtained is very dependent on the strain being examined. MDMA induced dose-dependent hyperthermia in C57BL/6J (Miller and O'Callaghan, 1994, Johnson et al., 2000; 2002b; Sanchez et al., 2003; Bexis and Docherty, 2008), NIH/Swiss (Colado et al., 2001) and Charles River mice (Carvalho et al., 2002), but hypothermia in BALB/c mice (Johnson et al., 2002a). Swiss-Webster mice had a biphasic response to repeat doses of MDMA, with hypothermia being the major effect when 10 mg·kg−1 was injected, but hyperthermia followed by hypothermia being observed when doses of 30 mg·kg−1 were given (O'Shea et al., 2001). In C57BL/6J mice, a biphasic hypothermia followed by hyperthermia can be revealed by either α2- or α1-adrenoceptor blockade (Bexis and Docherty, 2005; 2008;) (see below).
Effect of housing conditions on the temperature response of rodents to MDMA
Part of the explanation for these apparent contradictory results on the temperature response of rodents to MDMA when they are housed at normal ambient room temperature conditions (in addition to strain differences) is that the way they are housed influences the temperature response. It was observed many years ago that mice that were grouped together experienced a greater hyperthermic response following amphetamine than those housed singly (sometimes referred to as ‘aggregation toxicity’: Gunn and Gurd, 1940; Chance, 1946), and this phenomenon has been observed to occur in mice following MDMA (Fantegrossi et al., 2003). While the effect has not been formally reported to occur in rats, it is generally accepted by investigators to also occur in that species.
Even the cage housing can influence the hyperthermic response since Gordon and Fogelson (1994) observed a greater body temperature increase in rats given MDMA and housed in an acrylic floored cage than one with a grid type of flooring. This suggests that heat loss plays a major role in determining the body temperature following MDMA.
Effect of rat strain and MDMA metabolism on the MDMA-induced hyperthermic effect in rats
A recent study by Green et al. (2009) which used published data examined the relationship between dose administered and plasma concentration in rats, and found that data on the peak plasma concentration obtained from several rat strains could be pooled as there was no clear difference between values obtained at several dose values. This even included data obtained from dark agouti (DA) rats, although the data used were only those from male DA rats. Female DA rats are CYP2D6 deficient and are therefore considered a model of the poor metabolizer phenotype as they metabolize debrisoquine more slowly than either male DA rats or other rat strains (Al-Dabbagh et al., 1981). Because MDMA is demethylenated by CYP2D6 in humans (Tucker et al., 1994), the female DA rat has been examined as a model of the poor metabolizer phenotype. Following the same dose of MDMA to male and female DA rats, the females had higher peak temperature response and greater peak plasma MDMA concentration, suggesting that they did metabolize MDMA more slowly than males, and that it was MDMA that induces hyperthermia rather than a metabolite (Colado et al., 1995).
In humans, there is an increased gradient in the dose-versus-plasma-concentration plot as the dose increases, with a fourfold increase in plasma concentration with only a twofold increase in dose from 1 to 2 mg·kg−1. This is because there is mechanism-based inhibition of MDMA metabolism, which means that MDMA inhibits CYP2D6, one of its major metabolizing enzymes (Tucker et al., 1994; de la Torre et al., 2000). This inhibition can occur within 1 h (Yang et al., 2006). Whether this inhibition also occurs in rats is less clear. Baumann et al. (2009) have recently published data on low-dose MDMA administration, which suggests auto-inhibition can take place. However, the study by Green et al. (2009) using data obtained by others over a wide dose range (and one encompassing dose given in many studies on hyperthermia) indicate any inhibition is modest as there was an approximately linear relationship between dose and plasma concentration of the drug. This is perhaps not surprising because rats metabolize debrisoquine (and therefore presumably also MDMA) using several P450 enzymes, including CYP2D1, in addition to CYP2D6 (Matsunaga et al., 1989).
A final point regarding ‘translation’ of rodent data to human events is that of dose. The data of Green et al. (2009) demonstrated that at low dose of 2 mg·kg−1 in humans produced a peak plasma concentration that was only achieved by giving approximately 7 mg·kg−1 to rats. This seems a reasonable projection given that a 100 mg MDMA dose (1.4 mg·kg−1) provokes a 0.6°C oral temperature rise in humans (Farréet al., 2007), and a similar increase in rectal temperature is seen following an approximate fourfold higher i.p. dose (5 mg·kg−1) to rats (Colado et al., 1995). Baumann et al. (2009) reported similar plasma concentrations in both rats and humans at a dose of 1.4 mg·kg−1. The data of Green et al. (2009) also indicated little difference at these low doses, but that the relationship broke down at higher doses due to mechanism-based inhibition of MDMA metabolism and the dose ratio required to produce similar plasma levels in rats to humans increased markedly. While a group of recreational users had median plasma concentrations in the region of those produced in rats by dosing at approximately 2 mg·kg−1 (see Irvine et al., 2006), some values were much higher, and replicating in rats the plasma concentration seen in humans suffering a severe toxic response may require dosing rats with up to five times that dose.
Role of 5-HT in the acute MDMA-induced hyperthermic response in rats and mice
Direct effect of MDMA-induced 5-HT release on hyperthermia
Because MDMA produces a major acute release of 5-HT from nerve endings in the brain, it has often been assumed that the hyperthermia results from the release of this amine (Shankaran and Gudelsky, 1999), particularly as administration of tryptophan and a monoamine oxidase inhibitor also markedly increases 5-HT release and induces hyperthermia (Grahame-Smith, 1971). However, there is now considerable evidence to suggest that 5-HT plays little or no role in the acute hyperthermic response. Pretreatment with a variety of selective and non-selective 5-HT receptor antagonists was found to have no effect on MDMA-induced hyperthermia (Mechan et al., 2002). It has also been observed that pretreatment with either of the 5-HT uptake inhibitors fluoxetine (Schmidt et al., 1990; Berger et al., 1992; Malberg et al., 1996) or citalopram (Piper et al., 2008) has no effect on MDMA-induced hyperthermia. Crucially, Mechan et al. (2002) showed with the use of in vivo microdialysis that fluoxetine almost totally inhibited MDMA-induced 5-HT release in the brain, but that the hyperthermic response in the same animals was the same as that seen in rats pretreated with saline. Recently, Rodsiri et al. (2008) also failed to find any correlation between the hyperthermic response and the increase in extracellular 5-HT in rats given low doses of MDMA.
The role of 5-HT in the temperature effects following MDMA administration to mice is less clear. In a study in Swiss-Webster mice by O'Shea et al. (2001), there seemed to be no correlation between the temperature change and the change in cerebral 5-HT concentration. However, fluoxetine did block the MDMA-induced hyperthermia.
Effect of 5-HT receptor function on the hyperthermic response
While microdialysis studies have failed to indicate that the hyperthermic effect of MDMA is the direct result of the acute release of 5-HT by the drug, there is substantial evidence to suggest that 5-HT receptor subtype function can modulate the temperature response. The problem, however, is interpreting the data, for several important reasons.
A major problem is the promiscuous nature of the 5-HT release on its receptors. It is reasonable to propose that released 5-HT is acting on many of its receptor subtypes including 5-HT1A, 5-HT1B, 5-HT2A, 5-HT2B, 5-HT2C, 5-HT3 and 5-HT4 (Gudelsky and Yamamoto, 2008). Agonists at several of these receptors are known to alter body temperature. For example, 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT) is normally hypothermic in both rats and mice (Goodwin and Green, 1985; Goodwin et al., 1985; Hjorth, 1985), although the effect is probably pre-synaptic in mice (Goodwin et al., 1985), but post-synaptic in rats (Bill et al., 1991). In contrast, 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI), a selective 5-HT2A agonist, and m-chlorophenylpiperazine (m-CPP) and 6-chloro-2(1-piperazinyl)-pyrazine (MK-212), selective agonists of 5-HT2C receptors, induce hyperthermia (Gudelsky et al., 1986; Aulakh et al., 1995; Mazzola-Pomietto et al., 1997). While there have been several studies that have attempted to dissect the receptors responsible for inducing the temperature changes following MDMA, absolute conclusions are difficult due to the lack of total receptor subtype selectivity of any antagonist used. For example, while ketanserin is an effective 5-HT2A antagonist, it is also an effective adrenoceptor antagonist. Consequently, while several reports have implicated 5-HT1A, 5-HT2A and 5-HT2C receptors as having a role in inducing MDMA-induced temperature changes (Gudelsky et al., 1986; Mechan et al., 2002; Herin et al., 2005; Gudelsky and Yamamoto, 2007), an action via adrenoceptors cannot be discounted.
A further complication is that alterations in 5-HT function can influence dopamine release, and if, as suggested below, dopamine release has a key role in inducing both hypothermia and hyperthermia, then 5-HT being released onto its receptors will influence the temperature changes indirectly. However, the way that 5-HT and dopamine can interact is clearly brain region specific. For example, Leggio et al. (2009) reported that prefrontal cortex 5-HT2C receptors facilitate dopamine release in the accumbens, while both Di Matteo et al. (2008) and Alex and Pehek (2007) suggest that stimulation of most 5-HT receptor subtypes generally induces dopamine release, although the 5-HT2C receptor mediates an inhibitory effect on release.
One group of studies that does provide clear data on the role of 5-HT receptor subtypes on induction of MDMA-induced temperature changes are those on thermogenesis. Thermogenesis in brown adipose tissue has been shown to be increased by DOI, the 5-HT2A receptor agonist, but decreased by 8-OH-DPAT the 5-HT1A receptor agonist, thereby influencing heat production (Ootsuka and Blessing, 2006). The same group subsequently found that MDMA administration to rats in a cool environment inhibited brown adipose tissue thermogenesis (and tail artery vasoconstriction), an effect antagonized by the 5-HT1A antagonist WAY 100635, thereby strongly implicating the 5-HT1A receptor in this response. The dopamine D2 receptor antagonist potentiated the WAY100635 effect, indicating a role for dopamine (Rusyniak et al., 2008). The atypical neuroleptic clozapine, a drug which has antagonistic actions at several 5-HT receptor subtypes, as well as dopamine receptors, was also an effective compound in reversing MDMA-induced hyperthermia and peripheral vasoconstriction in rats and rabbits (Blessing et al., 2003), and reversed brown tissue thermogenesis induced by MDMA (Blessing et al., 2006).
However, in ligand binding studies, the 5-HT2A-receptor antagonists clozapine and risperidone show high affinity for α1A-adrenoceptors (pKi values of 8–9) and moderate affinity for α2A-adrenoceptors (pKi values of 6–7) (Nasrallah, 2008), and similar affinities for clozapine but higher affinities for risperidone, were reported by Morimoto et al. (2002). Functionally, clozapine causes urinary incontinence by α1A-adrenoceptor block (Fuller et al. 1996) and blocks α2-adrenoceptor-mediated inhibition of renin release (Pettinger et al., 1976). The doses of clozapine (0.1–5 mg·kg−1) and risperidone (0.5 mg·kg−1) employed in the MDMA studies (Blessing et al., 2003; Shioda et al., 2008) should produce marked α1-adrenoceptor antagonism, and this action should be considered as a possible mode of action in reversal of MDMA-induced cutaneous vasoconstriction.
Role of dopamine in the acute MDMA-induced hyperthermic response in rats and mice
MDMA not only produces a major acute release of 5-HT in the brain, it also induces a major release of the neurotransmitter dopamine (see Green et al., 2003), and there is now reasonable evidence to suggest that it is the release of this transmitter that is central to both the hyperthermic and hypothermic effects of MDMA in rats. In rats kept in normal ambient temperature conditions, the hyperthermic response following MDMA was unaffected by the dopamine D2 receptor antagonist remoxipride, but was dose-dependently antagonized by the dopamine D1 receptor antagonist SCH23390 (Mechan et al., 2002). In contrast, it was found that the MDMA-induced hypothermia was blocked by remoxipride, but not by SCH23390 in rats housed in cool ambient temperature conditions (Green et al., 2005).
The pharmacology of the hyperthermic and hypothermic response following MDMA may be indicative of ‘warm’ and ‘cold’ thermosensors (Bligh, 1979), responding preferentially to dopamine D1 and D2 receptors activated by MDMA-induced dopamine release, or the primacy of pre-synaptic and post-synaptic dopamine receptor function (Hjorth and Carlsson, 1987) at different ambient temperatures. This latter explanation is supported by the fact that dopamine agonists have opposite body temperature effects in normal rats and those pretreated with reserpine to inhibit pre-synaptic dopamine function (Verma and Kulkarni, 1993).
The involvement of dopamine in mice is not clear. Pretreatment with the selective dopamine uptake inhibitor GBR 12909 did not alter temperature response of mice to MDMA, suggesting that MDMA is not displacing dopamine from nerve endings to induce the hyperthermia (O'Shea et al., 2001).
Studies on binge dosing of MDMA and body temperature in rats
Over the last few years, the pattern of MDMA ingestion in humans has often involved repeated drug administration over a single short episode, which is referred to as ‘binge dosing’ (Hammersley et al., 1999; Topp et al., 1999; Winstock et al., 2001; Parrott, 2005). It is claimed that binge use of MDMA boosts its subjective effects and sustains the actions of the drug over time (Parrott, 2005). However, binge dosing is probably also employed by recreational users to divide the total dose and presumably therefore hopefully lessen the chance of an acute adverse event that could follow ingestion of a single high dose. Such hopes, however, fail to take account of the pharmacokinetics of the drug, particularly the half-life and metabolism of the compound.
Several studies have been conducted on the effects of binge-dosing schedules to rats that examined the body temperature response in animals housed in both normal and warm ambient room conditions. Repeated doses (three doses of 2, 4 or 6 mg·kg−1 given at 3 h intervals produced a linear (r = 0.95) dose-dependent increase in hyperthermia (Figure 1). The peak response seen after the third of the 2 mg·kg−1 doses (total dose 6 mg·kg−1) was significantly greater than that seen after a single 5 mg·kg−1 dose, which suggests that binge dosing does not confer greater safety in terms of avoiding the hyperthermic response. The situation became progressively worse at higher doses (Green et al., 2004). When the rats were housed at 30°C, the situation worsened further because a single MDMA dose of 5 mg·kg−1 produced a greater hyperthermic response than that seen in animals housed at 20°C, but the peak response seen in animals treated with 3 × 2 mg·kg−1 was now more than twice that observed in rats given 5 mg·kg−1 when housed at 30°C, and over five times greater than that seen in rats housed at 20°C (Figure 1; Green et al., 2004).
Figure 1.
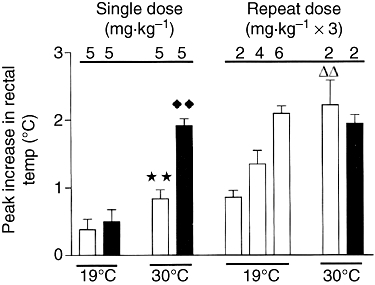
The peak increase in rectal temperature after repeated challenge doses of MDMA following subtraction of the appropriate saline-injected control value. The rats were treated with either saline (open bars) or a neurotoxic dose of MDMA (12.5 mg·kg−1; closed bars) 5 weeks earlier. Challenge doses of MDMA (5 mg·kg−1) were administered to rats housed at either 19 or 30°C as shown. ★★, different from saline-injected group at 19°C; P < 0.01; ♦♦, different from saline-injected group at 19°C, P < 0.01; rr, different from group given 2 mg·kg−1 when housed at 19°C, P < 0.01. Analysed by anova plus Bonferroni correction for ‘t’ test. Results shown as mean ± SEM with n = 6 per group. Reproduced from Green et al. (2004) with permission of Springer-Verlag.
These data suggest that humans who binge dose are not protecting themselves from the possibility of minimizing the chance of suffering an acute hyperthermic adverse event; indeed, bingeing may increase the possibility, particularly in warm room conditions. Pharmacokinetic studies suggest the problem of binge dosing may be even worse in humans than in rats. The study of Green et al. (2009), using published data, examined the relationship between dose administered and plasma concentration in rats and humans. While there was an approximately linear relationship between dose and plasma concentration of the drug, in humans there was an increased gradient in the slope as the dose increases with a fourfold increase in plasma concentration with only a twofold increase in dose from 1 to 2 mg·kg−1 because of mechanism-based inhibition of MDMA metabolism (see earlier). Because evidence suggests that it is exposure to MDMA that produces the acute adverse event of hyperthermia, then this auto-inhibition could have severe consequences. Binge dosing produced an additive effect in terms of the peak hyperthermia (Green et al., 2004). In contrast, because the first dose of MDMA in humans inhibits metabolism within an hour (Yang et al., 2006), further dosing is likely to induce a greater than additive temperature response. Consequently, binge dosing experiments in rats may prove to be a poor model as they probably underestimate the potential problem in humans.
Effect of decreased brain 5-HT function on MDMA-induced hyperthermia
High or repeated doses of MDMA to rats result in selective long-term neurotoxic damage to 5-HT nerve endings in the forebrain (see Green et al., 2003). There is some evidence that similar changes may occur in the brains of some recreational users of the drug, but unequivocal evidence is lacking and opinions of investigators are divided (Green et al., 2003; Green, 2004; Gouzoulis-Mayfrank and Daumann, 2009; Selvaraj et al., 2009). It is beyond the scope of this review to examine such evidence. However, it is of interest to examine studies that have investigated the consequences of such a prior lesion on the hyperthermic response of rats following further ingestion of the drug, to determine what the effect of neurotoxicity might be. Such studies would also show what might be the effect any loss of brain 5-HT concentration induced by the MDMA-induced acute release of neuronal 5-HT and inhibition of tryptophan hydroxylase (see Green et al., 2003).
The first evidence that a loss of 5-HT in the brain resulted in impaired thermoregulation was the study of Dafters and Lynch (1998), which found that a prior neurotoxic dose of MDMA resulted in rats showing a sustained hyperthermic response following exposure to a 60 min period of high ambient room temperature when compared to the response seen in non-lesioned rats. This observation was confirmed and extended by Mechan et al. (2001) who reported that, when compared to non-lesioned rats, MDMA-lesioned animals displayed a more rapid rise in rectal temperature when placed in a high ambient temperature room, and a slower decrease to normal values when they were then placed back in a normal ambient temperature room. This suggested that heavy recreational users of MDMA might (if the drug does produce a sustained decrease in 5-HT function in the human brain) have a problem in thermoregulating in high ambient room temperature conditions. Consequently, further studies were conducted to see what effect further MDMA dosing had in animals with a prior neurotoxic lesion of 5-HT neurones.
Two studies found that a low dose of MDMA produced a similar hyperthermic response in rats previously given a neurotoxic dose of MDMA to that seen in saline-pretreated control animals when the rats were present in normal ambient room temperature conditions (Beveridge et al., 2004; Green et al., 2004). These results contrast with a study by Shankaran and Gudelsky (1999) who reported an inhibited hyperthermic response to the MDMA challenge dose in lesioned rats. However, the degree of 5-HT loss was greater (45%) in that study than that achieved in the studies of Beveridge et al. (2004) and Green et al. (2004). When the lesioned rats were given a subsequent dose of MDMA while housed in a warm room (30°C), the hyperthermic response was significantly greater than that seen in control rats, and the return to pretreatment values slower, consistent with the problems in thermoregulation seen by Mechan et al. (2001).
These data suggest that intact brain 5-HT function is required in rats for effective thermoregulation when they are present in warm conditions and challenged by a low dose of MDMA. This interpretation was strengthened by a later study which found that rats present in 30°C room conditions and pretreated with the tryptophan hydroxylase inhibitor p-chlorophenylalanine (PCPA) to deplete the brain 5-HT concentration also had a prolonged hyperthermic response following injection of a low dose of MDMA. A similar prolongation of the response was seen following pretreatment with the non-selective 5-HT antagonist methysergide and the 5-HT1A antagonist WAY100635 (Saadat et al., 2005). Giacchino et al. (1983) had previously shown that PCPA impaired the ability of rats to lose temperature in elevated temperature room conditions.
Together, these results suggest that a decrease in 5-HT function normally acting at 5-HT1A receptors leads to an impaired ability of rats to lose heat in high ambient temperatures. This problem is expressed strongly when such animals are administered a hyperthermia-inducing dose of MDMA, and suggest that if heavy recreational users of MDMA may be a more risk than light users of experiencing a severe hyperthermic reaction, if they take the drug in hot conditions (such as a dance club).
The major mechanism by which rats lose heat is by vasodilation of the tail veins (Grant, 1963; Romanovsky et al., 2002). This leads to an increase in tail temperature. When rats are given MDMA, the rectal temperature rises, but the tail temperature does not (Mechan et al., 2002). This suggests that MDMA has interfered with this normal heat loss mechanism, probably by inducing peripheral vasoconstriction (Gordon et al., 1991; Pedersen and Blessing, 2001).
The results of both Dafters and Lynch (1998) and Mechan et al. (2001) outlined above suggested that rats with a prior neurotoxic lesion of 5-HT nerve endings induced by MDMA administration had a problem in losing body heat when exposed to high ambient room temperatures, and then returned to normal room conditions. This problem was also noted when lesioned rats were administered a dose of MDMA when present in warm room conditions, but not when housed at normal ambient temperature (Green et al., 2004). Again, it appears that the problem is associated with a defect in the functioning of the major heat loss organ in the rat, namely the tail; in this case, the effect of the prior neurotoxic lesion. It is noteworthy that PCPA administration increases mortality in rats exposed to high ambient temperatures (Reid et al., 1968; Cronin, 1976) because this supports the notion that intact 5-HT function is required for effective heat loss mechanisms to occur in the rat. Consequently, administration of MDMA to rats with a prior neurotoxic lesion when housed at 30°C is faced with a double problem, impaired heat loss ability because of the acute effect of the MDMA, and impaired thermoregulation because of decreased 5-HT function in the brain. This is exactly what is seen when the tail temperature is examined (Green et al., 2005).
If heavy recreational users of MDMA do have impaired 5-HT functions in the brain and ingest further doses when present in hot rooms, then they may be more at risk of a hyperthermic crisis. However, humans, unlike rats, can cool themselves by shedding clothing. Nevertheless, in a possibly analogous situation, rats given MDMA and exposed to high ambient temperature conditions for 30 min then choose, when offered, a cooler environment in order to correct their hyperthermia (Jaehne et al., 2007). A prior neurotoxic lesion produced by repeated MDMA administration resulted in this cooler environment-seeking behaviour being disrupted (Jaehne et al., 2008). Finally, it should be noted that the human set point for thermoneutrality is at an ambient temperature of around 32°C (see for example. Srámek et al., 2000), which is far higher than the rat. These facts should mitigate against hyperthermia being a major problem for most human recreational users unless they are present in high ambient temperature room conditions.
Adrenergic mechanisms involved in MDMA-induced changes in body temperature
Although much of the research into the adverse actions of amphetamine derivatives has focused on 5-hydroxytryptaminergic and dopaminergic systems, there is also evidence for the involvement of the noradrenergic system, particularly in terms of peripheral actions.
Users of MDMA are reported to have elevated plasma catecholamine levels, which may be due to noradrenergic hyperactivity and may be linked to cardiovascular complications (Stuerenburg et al., 2002). The ‘exchange diffusion model’ was postulated to explain the combined uptake inhibition and neurotransmitter-releasing action of amphetamine derivatives (Fischer and Cho, 1979; Crespi et al., 1997). They are thought to compete with the neurotransmitter for the transporter, are transported into nerve terminals and the carrier functions in reverse to release neurotransmitter, although some results question a simple exchange diffusion hypothesis (Sitte et al., 2001). Hence, indirect release of neurotransmitters by amphetamine derivatives may be linked to their action at transporters. MDMA also inhibits MAO (Leonardi and Azmitia, 1994) to block metabolism of noradrenaline.
Actions at the monoamine transporters
MDMA was first reported to cause release of [3H]5-HT from synaptosomes (Nichols et al., 1982). Battaglia et al. (1988) found that the affinity of MDMA for uptake sites was 5-HT > noradrenaline > dopamine, and affinities of MDA were comparable to those for MDMA. MDA also inhibits noradrenaline uptake with higher potency than dopamine uptake (Johnson et al., 1991).
The potency order in causing release of 5-HT in rat striatal slices was MDA > MDMA > MDEA (Schmidt, 1987), but the potency order in releasing dopamine from striatal slices (Schmidt, 1987) and synaptosomes (O'Loinsigh et al., 2001) was MDMA > MDA > MDEA.
MDMA also induces release of noradrenaline from striatal slices, and this is blocked by the noradrenaline transporter inhibitor desipramine (Fitzgerald and Reid, 1993). MDMA releases noradrenaline with a similar potency as for 5-HT release and a greater potency than for dopamine release (Johnson et al., 1991; Rothman et al., 2001). MDEA is less potent in inhibiting uptake of NA in the left ventricle when compared to MDA and MDMA (Cleary and Docherty, 2003). This may suggest an order of potency of MDA = MDMA > MDEA. Examination of Table 1 reveals that MDEA had the lowest affinity for all three transporters.
Table 1.
Relative affinities/potencies of MDMA, MDA and MDEA at adrenoceptors and monoamine transporters
| Site | Response | Order of potency/affinity | Reference |
|---|---|---|---|
| α1A | Binding | MDEA > MDA > MDMA | Bexis and Docherty, 2006 |
| α1A | Contraction | MDA > MDMA > MDEA+ | Bexis and Docherty, 2006 |
| α1D | Contraction | MDA > MDMA > MDEA+ | Bexis and Docherty, 2006 |
| α2A | Pre-synaptic | MDMA > MDA > MDEA | Bexis and Docherty, 2006 |
| α2A | Binding | MDMA > MDA > MDEA | Bexis and Docherty, 2006 |
| SERT | 5-HT release | MDA > MDMA > MDEA | Schmidt, 1987 |
| DAT | DA release | MDMA > MDA > MDEA | Schmidt, 1987 |
| NET | NA release | MDMA = MDA > MDEA | Cleary and Docherty, 2003 |
MDEA acts as partial agonist/antagonist.
Abbreviations: binding, ligand binding studies; DAT, dopamine transporter, NET, noradrenaline transporter; pre-synaptic, pre-synaptic/pre-junctional inhibition of nerve-evoked contraction; SERT, serotonin transporter.
Actions at adrenoceptors
The amphetamine derivatives have been shown to have indirect sympathomimetic actions to potentiate the actions of noradrenaline (Fitzgerald and Reid, 1994; Al-Sahli et al., 2001; Cleary et al., 2002a) by competitive blockade of the noradrenaline transporter (Al-Sahli et al., 2001; Cleary and Docherty, 2003), and the linked ability to displace noradrenaline from peripheral noradrenergic nerve terminals (Fitzgerald and Reid, 1994; Lavelle et al., 1999) and adrenal gland (O'Cain et al., 2000), as well as direct receptor activation.
Effects of amphetamine derivatives at adrenoceptors in vivo may be a mixture of direct and indirect actions. Indirect actions would not be dependent on receptor subtype selectivity of the amphetamine derivative.
α1-Adrenoceptors
In comparison to MDMA (and MDA), MDEA has significantly higher affinity for α1A- and lower affinity for α2A-adrenoceptors, but all had similar affinities at α2B- and α2C-adrenoceptors (Bexis and Docherty, 2006). However, despite this higher affinity for α1A-adrenoceptors, MDEA had low potency at producing contractions of rat vas deferens (predominantly α1A), suggesting low efficacy, and failed to contract rat aorta (predominantly α1D), acting as an antagonist. Hence, the potency order for α1-adrenoceptor agonism was MDA > MDMA > MDEA. (Bexis and Docherty, 2006) (see Table 1).
The major α1-adrenoceptor-mediated actions involve vasoconstriction, causing a general rise in blood pressure and reducing cutaneous blood flow. In addition, coronary arterial contraction may increase the risk of myocardial infarction, as has been reported for cocaine (Mittleman et al., 1999).
However, although MDMA has affinity for α1-adrenoceptors, and produces contractions in tissues with α1-adrenoceptors, there is evidence that at least some of these responses are resistant to α1-adrenoceptor antagonists, and indeed other classical antagonists. Actions of MDMA and other amphetamine derivatives not involving adrenergic or 5-hydroxytryptaminergic receptors, may involve trace amine receptors (see Broadley, 2010).
In humans, MDMA, MDEA and MDA cause increases in arterial pressure (Gouzoulis et al., 1993; Vollenweider et al., 1998; Hegadoren et al., 1999), and increases in both systolic and diastolic pressure occur in conscious rats (Bexis and Docherty, 2006). MDMA has been shown to have actions as an agonist at both α1- and α2-adrenoceptors, in addition to 5-HT2 receptors, to raise blood pressure in the anaesthetized rat, and MDA produced larger pressor responses, but MDEA failed to raise blood pressure in anaesthetized (McDaid and Docherty, 2001) or conscious rats (Bexis and Docherty, 2006). The blood pressure actions of these agents in the rat are consistent with their actions at α1-adrenoceptors: MDA is more potent than MDMA as an agonist, and MDEA with low efficacy or acting as an antagonist. MDA has overall more marked cardiovascular, temperature and locomotor actions than the other agents (Bexis and Docherty, 2006). Urinary retention reported with MDMA (McCann et al., 1996) may also involve peripheral α1-adrenoceptor-mediated actions.
In the mouse, the α1-adrenoceptor antagonist prazosin revealed an early significant hypothermia following MDMA, presumably because of blockade of vasoconstriction (Bexis and Docherty, 2008) (see Figure 2). In further studies, it has been demonstrated that both α1A- and α1D-adrenoceptors are involved in the initial hyperthermia to MDMA in mice, and combined block of both receptors is necessary to remove the full α1-adrenoceptor-mediated hyperthermic component and reveal a hypothermia (Bexis and Docherty, 2008) (see Figure 2).
Figure 2.
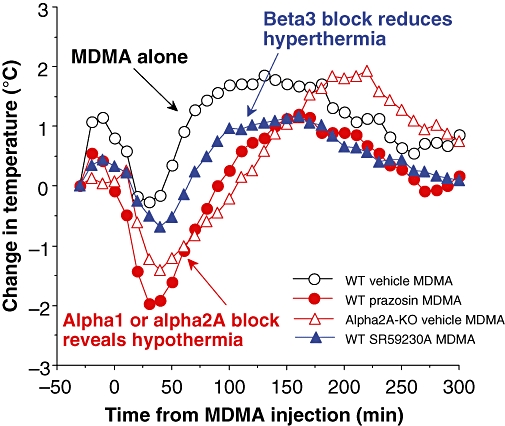
Summary of adrenergic components to the temperature response to MDMA in mice. The results shown are mean experimental data taken from Bexis and Docherty (2006; 2008; 2009;), but for simplicity error bars have been omitted. Antagonist drugs or vehicle are injected 30 min before MDMA (20 mg·kg−1) injected at time zero. Knockout (or blockade) of α2A-adrenoceptors, or blockade of α1-adrenoceptors with prazosin, reveals an initial hypothermia to MDMA. Blockade of β3-adrenoceptors with SR 59230A (low dose) reduces the maximum hyperthermia to MDMA. We interpret these results as follows: (i) α1-adrenoceptor-mediated cutaneous vasoconstriction mediates a component of the hyperthermic response to MDMA, masking a hypothermic component; (ii) α2-adrenoceptors in the CNS may be involved in this cutaneous vasoconstriction; and (iii) β3-adrenoceptors (and possibly α1-adrenoceptors) in adipose tissue mediate a component of heat generation.
Indeed, peripheral effects of MDMA at α1-adrenoceptors could explain a major component of its temperature actions, namely cutaneous vasoconstriction. Cutaneous vasoconstriction has been shown to contribute to the induction by MDMA of hyperthermia, although this has been previously reported to be largely mediated by central sympathetic activation involving serotonergic systems (Gordon et al., 1991; Pedersen and Blessing, 2001; Blessing et al., 2003). Such an effect may also explain the dependency of the temperature effects of MDMA on ambient temperature. At low ambient temperatures, cutaneous vasoconstriction is already marked so that MDMA produces little further vasoconstriction and early centrally mediated hypothermic actions of MDMA predominate. At high ambient temperatures, cutaneous dilatation has occurred; allowing a marked vasoconstrictor component to the actions of MDMA, and hyperthermia predominates. Hence, α-adrenoceptor-mediated peripheral vasoconstrictor actions of MDMA modulate central hypo- and hyperthermic components. In addition, there may be an α1-adrenoceptor component of heat generation from brown fat.
α2-Adrenoceptors
α2-Adrenoceptors mediate pre- and post-synaptic actions of noradrenaline both in the central and peripheral nervous systems. α2-Adrenoceptors have been separated into three subtypes, α2A-, α2B and α2C (Bylund et al., 1994; Docherty, 1998), with α2A and α2C subtypes predominating in the central nervous system (Philipp et al., 2002).
α2-Adrenoceptor agonists such as clonidine produce hypothermia in wild-type (WT) mice, and hypothermia involves α2A-adrenoceptors because it is absent in α2A-KO mice (Hunter et al., 1997; Zarrindast et al., 2003; Bexis and Docherty, 2005). The hypothermic response elicited by clonidine is thought to be primarily mediated by activation of post-synaptic α2A-adrenoceptors in the pre-optic area of the hypothalamus (Myers et al., 1987). α2A-Adrenoceptors are also involved in the MDMA-induced hyperthermia. In the presence of the α2A-adrenoceptor antagonist BRL44408, the monophasic hyperthermic response produced by MDMA in WT mice became a biphasic response with an initial hypothermia followed by a small increase in body temperature (Bexis and Docherty, 2005) (see Figure 2). Similarly, when α2A-KO mice were injected with MDMA, a biphasic response was seen, hypothermia followed by hyperthermia (Bexis and Docherty, 2005) (see Figure 2). The results are surprising because α2A-adrenoceptors are involved in producing hypothermia.
As well as being found post-synaptically where they mediate hypothermia (Myers et al., 1987), α2-adrenoceptors are also found pre-synaptically as inhibitory receptors regulating release of noradrenaline (autoreceptors) and other neurotransmitters, such as dopamine and 5-HT (heteroceptors), in the central and peripheral nervous systems (Philipp et al., 2002, Brede et al., 2003). Because the monoaminergic systems are interconnected and can influence each other, it could be suggested that under the conditions of increased extracellular levels of the three monoamines produced by MDMA, concomitant activation of the pre-synaptic α2A-adrenoceptor results in a component of the hyperthermic response. In the absence of α2A-adrenoceptors, this component of the hyperthermia is absent, and the resultant changes in levels of dopamine, 5-HT and possibly other neurotransmitters such as GABA, leads to the hypothermic component seen in α2A-KO mice.
In addition to the predominant α2A-adrenoceptor, it has been demonstrated that α2C-adrenoceptors also function as a pre-synaptic regulator of noradrenaline release, both centrally and peripherally, but they are more prominent in sympathetic nerve endings than central adrenergic neurons (Ho et al., 1998; Philipp et al., 2002). The α2C-adrenoceptors have also been shown to be involved in inducing a hypothermic response because in the absence or over-expression of α2C-adrenoceptors, the hypothermic response to the α2-adrenoceptor agonist, dexmedetomidine, is slightly decreased (17%) or increased (12%), respectively (Sallinen et al., 1997). Indeed, α2C-adrenoceptor up-regulation has been shown to occur in α2A-KO mice to partly replace α2A-adrenoceptors prejunctionally in rat vas deferens (Ho et al., 1998; Hein et al., 1999; Cleary et al., 2002b). However, clonidine had no significant effect on temperature in α2A knock-out mice (Bexis and Docherty, 2005), suggesting that either that the α2C-adrenoceptor component is small, or that clondine has low potency at α2C-adrenoceptors. MDMA has similar affinities/potencies at α2A- and α2C-adrenoceptors in ligand binding (Lavelle et al., 1999) and functional studies (Rajamani et al., 2001), but the relative low importance of α2C-adrenoceptors in hypothermia and the lack of hypothermia to MDMA in WT animals still argue for a hyperthermic action of MDMA by α2A-adrenoceptor activation in WT mice.
Although vasoconstriction is mediated predominantly by α1-adrenoceptors, α2-adrenoceptors, particularly α2A-adrenoceptors, also contribute to systemic vasoconstriction (Docherty, 1998; Duka et al., 2000).). α2C-Adrenoceptors are present on veins (Gavin et al., 1997) and on cutaneous arteries, and have been shown to be involved particularly in cold-induced vasoconstriction (Chotani et al., 2000).
In terms of prejunctional α2A-adrenoceptor potency in rat vas deferens, the potency order agreed with the ligand binding affinity order of MDMA > MDA > MDEA (Bexis and Docherty, 2006).
MDMA also induces hyperthermia by central sympathetic activation of cutaneous vasoconstriction, reducing the ability to dissipate heat (Pedersen and Blessing, 2001).
Cardiac actions of MDMA and derivatives
Increased cardiac function can contribute to hyperthermia by increased blood pressure and flow, or be a result of hyperthermia. In humans, both MDA and MDEA increased heart rate (Gouzoulis et al., 1993; Hegadoren et al., 1999). In the anaesthetized rat, MDA elicited an initial bradycardia (McDaid and Docherty, 2001). All three agents tended to increase heart rate later, but this reached significance only for MDEA. These changes may be at least partly baroreflex responses to changes in blood pressure, given that MDA produced the largest increase in blood pressure and MDEA produced a fall. In conscious animals and humans, MDMA has been shown to produce no change in heart rate, bradycardia (at high doses) or tachycardia (O'Cain et al., 2000; Pedersen and Blessing, 2001; Badon et al., 2002; Cole and Sumnall, 2003a).
In the rat isolated right ventricle, MDMA potentiated contractions to noradrenaline, but not isoprenaline, suggesting an action at the noradrenaline transporter (Al-Sahli et al., 2001). These results suggest cardiac stimulant actions of MDMA, at least partly involving indirect sympathomimetic actions.
β3-Adrenoceptors
There are probably a complex series of physiological changes involved in the production of the hyperthermic response that follows MDMA administration to the rat. Gordon et al. (1991) examined metabolic rate, evaporative water loss and rectal temperature following injection of the drug when rats were housed in ambient temperature of 10, 20 and 30°C. When compared to saline-injected control animals, it was found that MDMA increased both the metabolic rate and evaporative water loss. These changes involve both the hypothalamic–pituitary–thyroid axis (Sprague et al., 2003) and β3-adrenoceptor activation in brown adipose tissue (Sprague et al., 2004), which induces heat generation through activation of uncoupling protein (Mills et al. 2004). The problem of hyperthermia, however, seems to be related to the fact that this heat generation is not followed by activation of heat loss mechanisms.
Studies in rat have demonstrated that in addition to α1-adrenoceptors, β3-adrenoceptors may also be involved in MDMA-induced hyperthermia (Sprague et al., 2003, Sprague et al., 2004; 2005;). In a series of studies, Sprague et al. have examined the involvement of β3-adrenoceptors in the temperature actions of MDMA. In Sprague-Dawley rats, MDMA produced increases in rectal and skeletal muscle temperatures, but thyroidectomy revealed a hypothermic response to MDMA (Sprague et al., 2003). The β-antagonist cyanopindolol attenuated the increase in skeletal muscle temperature; the α1-adrenoceptor antagonist prazosin attenuated both skeletal and rectal temperatures, but the combination of prazosin and cyanopindolol abolished the effects of MDMA on temperature (Sprague et al., 2003). The combination of α1-adrenoceptor antagonism with prazosin and β3-adrenoceptor antagonism with SR59230A, or the combined β3- and α1-adrenoceptor antagonist carvedilol (Qvigstad et al., 2005), abolished the rise in core temperature to MDMA (Sprague et al., 2004; 2005;). These studies suggest major roles for α1- and β3-adrenoceptors in the temperature actions of MDMA.
Pretreatment of mice with SR59230A (5 mg·kg−1), a concentration that has been demonstrated to prevent brown adipose tissue thermogenesis (Manara et al., 1996), altered the monophasic hyperthermic response produced by MDMA to a biphasic response, with an initial hypothermic response followed by a hyperthermic response (Bexis and Docherty, 2009). However, the hypothermic responses were similar in magnitude to the responses seen when mice were pretreated with prazosin (0.1 mg·kg−1) (Bexis and Docherty, 2008). A problem in studies of responses mediated by β3-adrenoceptors is the lack of selectivity of antagonists available. Although SR59230A has been commonly described as highly selective for β3-adrenoceptors (pA2 of 8.76: Nisoli et al., 1996), it may not be selective for human β3-adrenoceptors over other β-adrenoceptors (Vrydag and Michel, 2007). There is increasing evidence, both from functional and radioligand binding studies, to suggest that SR59230A also has antagonistic actions at α1-adrenoceptors (pKi/pKB values of 6.75–6.25: Brahmadevara et al., 2004; Leblais et al., 2004; Bexis and Docherty, 2009). Doses of SR59230A (5 mg·kg−1) would be high enough to produce marked block of α1-adrenoceptors. In the presence of a low concentration of SR59230A (0.5 mg·kg−1), the temperature response produced by MDMA in the mouse remained monophasic, but the maximum temperature reached in the delayed hyperthermia was slightly, but significantly, reduced (Bexis and Docherty, 2009), so that there is a possible small β3-adrenoceptor-mediated component to the hyperthermia to MDMA in the mouse (Bexis and Docherty, 2009) (see Figure 2). These results suggest a lesser role for β3-adrenoceptors in the hyperthermia to MDMA in the mouse (Bexis and Docherty, 2009) than the rat (Sprague et al., 2004). Putative β3-adrenoceptor antagonists should be investigated taking into consideration possible α1-adrenoceptor antagonist actions.
Locomotor activity
In addition to hyperthermia, MDMA also induces a dose-dependent increase in locomotor activity in rats. It is therefore reasonable to examine whether the hyperthermia results from increased activity. All evidence to date argues against this supposition. The increased locomotor activity is unchanged when animals are placed in low ambient room temperature conditions, which prevents hyperthermia, and also in raised room temperature conditions, which enhances hyperthermia (Dafters, 1994; 1995; O'Shea et al. 2006). The recent study of Rodsiri et al. (2008) also failed to detect any relationship between the body temperature and the simultaneously measured locomotor response following administration of two different doses of MDMA in rats housed in normal ambient temperature.
Evidence suggests that MDMA-induced hypermotility involves activation of multiple 5-HT receptors and an interaction of dopamine and 5HT (Bankson and Cunningham, 2002; Cole and Sumnall, 2003a). In a study in rats, all three amphetamine derivatives tended to cause an increase in locomotor activity, but this reached significance only for MDA (Bexis and Docherty, 2006). There is also evidence that α2A-adrenoceptors mediate inhibition of locomotion (Lähdesmäki et al., 2003), and the α2A-adrenoceptor antagonist BRL 44408 revealed locomotor actions of MDMA (Bexis and Docherty, 2006).
Jaw clenching reported with the use of MDMA (Hayner and McKinney, 1986; McCann et al., 1996) may involve α2-adrenoceptor-mediated inhibition of the jaw opening reflex (Arrue et al., 2004), presumably by a central action. Acute psychiatric complications of MDMA, including panic attacks (McCann et al., 1996), may also involve noradrenergic mechanisms and α2-adrenoceptors.
Studies on temperature effects in monkeys and humans
Relatively few studies have been published on the effects of MDMA in either monkeys or humans. However, those that have suggest that the response of both monkeys and humans may differ in some important respects from that seen in rats.
MDMA can induce hyperthermia in both rhesus monkeys (Crean et al., 2006; 2007; Taffe et al., 2006; Banks et al., 2007; Von Huben et al., 2007) and humans, both when administered at modest doses (Liechti and Vollenweider, 2000a,b; Freedman et al., 2005), as well as at doses inducing an acute toxic response (e.g. Brown and Osterloh, 1987; Dowling et al., 1987; McCann et al., 1996; Farréet al., 2007).
However, in contrast to rats where one can induce hypothermia or hyperthermia depending on the ambient room temperature conditions in which the rats are housed (see earlier), MDMA administration to monkeys (Von Huben et al., 2007) and humans (Freedman et al., 2005) results in hyperthermia in low, normal or high ambient temperature conditions. There appears to be no association in monkeys between hyperthermia and increased locomotor activity (Taffe et al., 2006; Crean et al., 2006; 2007;) which is consistent with data found in rats (see earlier). Liechti and Vollenweider (2000b) observed that neither citalopram nor haloperidol block MDMA-induced hyperthermia in humans and similar data have been reported in rats (Mechan et al., 2002; Piper et al., 2008). The 5-HT2A antagonist ketanserin has, however, been reported by Liechti et al. (2000) to attenuate MDMA-induced hyperthermia in normal volunteers.
Interestingly, it has recently been reported that core temperature can alter the pharmacokinetic parameters and metabolism of MDMA in both rats (Goni-Allo et al., 2008) and monkeys (Banks et al., 2007).
Conclusions
It was recently pointed out elsewhere (Green et al., 2009; de la Torre et al., 2009) that future studies on the physiological and psychological effects of MDMA must include much more awareness of the pharmacokinetics of MDMA in different species. Ideally, such studies should include much more information than simple exposure measurements, and include information on drug exposure, half-life, active metabolites (of which MDMA has several) and even plasma protein binding which can vary markedly between species [see Gabrielsson and Green (2009) for a full discussion of the use of quantitative pharmacology in experimental pharmacology]. The absence of such information in most studies on MDMA naturally inhibits our ability to draw many firm conclusions on the effects of MDMA on body temperature in different species. However, it is also clear from this review that the effect of MDMA on body temperature is complex because the drug has actions on all the major monoamine neurotransmitters (5-HT, dopamine and noradrenaline) both releasing the amines from nerve endings and also acting on their receptors. These neurotransmitters interact in complex ways both centrally and peripherally to control temperature with actions involving both central thermoregulation and peripheral changes in blood flow and brown adipose tissue thermogenesis. Given this information, it seems unlikely that any single pharmaceutical compound is going to be effective in treating acute MDMA-induced hyperthermia, and careful body cooling of affected persons remains, at present, the principal clinical approach. It has been suggested that atypical neuroleptics with 5-HT2A and dopamine (and indeed α1-adrenoceptor) antagonist actions such as risperidone (Shioda et al., 2008) and clozapine (Blessing et al., 2003; 2006;) could prove of value in treating potentially fatal hyperthermia in humans. However, the fact that atypical neuroleptics can also induce the neuroleptic malignant syndrome (Farver, 2003) does mean that their use should be approached with caution.
Crucially, educating recreational users of the potential dangers of hyperthermia and control of the ambient temperature at clubs and other dance parties should remain key factors in fighting this potentially fatal problem.
Glossary
Abbreviations:
- 5-HIAA
5-hydroxyindole acetic acid
- 5-HT
5-hydroxytryptamine
- 8-OH-DPAT
8-hydroxy-2-(di-n-propylamino)tetralin
- BRL44408
2-[(4,5-dihydro-1H-imidazol-2-yl)methyl]-2,3-dihydro-1-methyl-1H-isoindole
- DA
dark agouti
- DOI
1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane
- GBR 12909
1-[2-[bis-(4-fluorophenyl)methoxy]ethyl]-4-(3-phenylpropyl)piperazine dihydrochloride
- m-CPP
m-chlorophenylpiperazine
- MDA
3,4-methylenedioxyamphetamine
- MDEA
3,4-methylenedioxyethamphetamine
- MDMA
3,4-methylenedioxymethamphetamine
- MK-212
6-chloro-2(1-piperazinyl)-pyrazine
- PCPA
p-chlorophenylalanine
- SCH23390
(R)-(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrochloride
- SR59230A
1-(2-ethylphenoxy)-3-[[(1S)-1,2,3,4-tetrahydro-1-naphthalenyl]amino]-(2S)-2-propanol hydrochloride
- WAY100635
N-[2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl]-N-(2-pyridyl)cyclohexanecarboxamide
Conflict of interest
The authors state no conflict of interest.
References
- Al-Dabbagh SG, Idle JR, Smith RL. Animal modelling of human polymorphic drug oxidation – the metabolism of debrisoquine and phenacitin in inbred rat strains. J Pharm Pharmacol. 1981;33:161–164. doi: 10.1111/j.2042-7158.1981.tb13740.x. [DOI] [PubMed] [Google Scholar]
- Alex KD, Pehek EA. Pharmacologic mechanisms of serotonergic regulation of dopamine neurotransmission. Pharmacol Ther. 2007;113:296–320. doi: 10.1016/j.pharmthera.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SP, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC) Br J Pharmacol. (3rd edition) 2008;153(Suppl 2):S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Sahli W, Ahmad H, Kheradmand F, Connolly C, Docherty JR. Effects of methylenedioxymethamphetamine on noradrenaline-evoked contractions of rat right ventricle and small mesenteric artery. Eur J Pharmacol. 2001;422:169–174. doi: 10.1016/s0014-2999(01)01070-6. [DOI] [PubMed] [Google Scholar]
- Arrue A, Gómez FM, Giralt MT. Effects of 3,4-methylenedioxymethamphetamine (‘ecstasy’) on the jaw-opening reflex and on the α-adrenoceptors which regulate this reflex in the anesthetized rat. Eur J Oral Sci. 2004;112:127–133. doi: 10.1111/j.1600-0722.2004.00114.x. [DOI] [PubMed] [Google Scholar]
- Aulakh CS, Mazzola-Pomietto P, Murphy DL. Long-term antidepressant treatments alter 5-HT2A and 5-HT2C receptor-mediated hyperthermia in fawn-hooded rats. Eur J Pharmacol. 1995;282:65–70. doi: 10.1016/0014-2999(95)00279-t. [DOI] [PubMed] [Google Scholar]
- Badon LA, Hicks A, Lord K, Ogden BA, Meleg-Smith S, Varner KJ. Changes in cardiovascular responsiveness and cardiotoxicity elicited during binge administration of ecstasy. J Pharmacol Exp Ther. 2002;302:898–907. doi: 10.1124/jpet.302.3.898. [DOI] [PubMed] [Google Scholar]
- Banks ML, Sprague JE, Kisor DF, Czoty PW, Nichols DE, Nader MA. Ambient temperature effects on 3,4-methylenedioxymethamphetamine-induced thermodysregulation and pharmacokinetics in male monkeys. Drug Metab Dispos. 2007;35:1840–1845. doi: 10.1124/dmd.107.016261. [DOI] [PubMed] [Google Scholar]
- Bankson MG, Cunningham KA. Pharmacological studies of the acute effects of (+)-3,4-methylenedioxymethamphetamine on locomotor activity: role of 5-HT(1B/1D) and 5-HT(2) receptors. Neuropsychopharmacology. 2002;26:40–52. doi: 10.1016/S0893-133X(01)00345-1. [DOI] [PubMed] [Google Scholar]
- Battaglia G, Brooks BP, Kulsakdinun C, De Souza EB. Pharmacologic profile of MDMA (3,4-methylenedioxymethamphetamine) at various brain recognition sites. Eur J Pharmacol. 1988;149:159–163. doi: 10.1016/0014-2999(88)90056-8. [DOI] [PubMed] [Google Scholar]
- Baumann MH, Zolkowska D, Kim I, Scheidweiler KB, Rothman RB, Huestis MA. Effects of dose and route of administration on pharmacokinetics of (+ or −)-3,4-methylenedioxymethamphetamine in the rat. Drug Metab Dispos. 2009;37:2163–2170. doi: 10.1124/dmd.109.028506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger UV, Gu XF, Azmitia EC. The substituted amphetamines 3,4-methylenedioxymethamphetamine, methamphetamine, p-chloramphetamine and fenfluramine induce 5-hydroxytryptamine release via a common mechanism blocked by fluoxetine and cocaine. Eur J Pharmacol. 1992;215:153–160. doi: 10.1016/0014-2999(92)90023-w. [DOI] [PubMed] [Google Scholar]
- Beveridge TJ, Mechan AO, Sprakes M, Pei Q, Zetterstrom TS, Green AR, et al. Effect of 5-HT depletion by MDMA on hyperthermia and Arc mRNA induction in rat brain. Psychopharmacology. 2004;173:346–352. doi: 10.1007/s00213-003-1753-y. [DOI] [PubMed] [Google Scholar]
- Bexis S, Docherty JR. Role of α2A-adrenoceptors in the effects of MDMA on body temperature in the mouse. Br J Pharmacol. 2005;146:1–6. doi: 10.1038/sj.bjp.0706320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bexis S, Docherty JR. Effects of MDMA, MDA and MDEA on blood pressure, heart rate, locomotor activity and body temperature in the rat involve α-adrenoceptors. Br J Pharmacol. 2006;147:926–934. doi: 10.1038/sj.bjp.0706688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bexis S, Docherty JR. Role of α(1)-adrenoceptor subtypes in the effects of methylenedioxy methamphetamine (MDMA) on body temperature in the mouse. Br J Pharmacol. 2008;153:591–597. doi: 10.1038/sj.bjp.0707590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bexis S, Docherty JR. Role of α1- and β3-adrenoceptors in the modulation by SR59230A of the effects of MDMA on body temperature in the mouse. Br J Pharmacol. 2009;158:259–266. doi: 10.1111/j.1476-5381.2009.00186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bill DJ, Knight M, Forster EA, Fletcher A. Direct evidence for an important species difference in the mechanism of 8-OH-DPAT-induced hypothermia. Br J Pharmacol. 1991;103:1857–1864. doi: 10.1111/j.1476-5381.1991.tb12342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blessing WW, Seaman B, Pedersen NP, Ootsuka Y. Clozapine reverses hyperthermia and sympathetically mediated cutaneous vasoconstriction induced by 3,4-methylenedioxymethamphetamine (ecstasy) in rabbits and rats. J Neurosci. 2003;23:6385–6391. doi: 10.1523/JNEUROSCI.23-15-06385.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blessing WW, Zilm A, Ootsuka Y. Clozapine reverses increased brown adipose tissue thermogenesis induced by 3,4-methylenedioxymethamphetamine and by cold exposure in conscious rats. Neuroscience. 2006;141:2067–2073. doi: 10.1016/j.neuroscience.2006.05.050. [DOI] [PubMed] [Google Scholar]
- Bligh J. The central neurology of mammalian thermoregulation. Neuroscience. 1979;4:1213–1236. doi: 10.1016/0306-4522(79)90153-2. [DOI] [PubMed] [Google Scholar]
- Brahmadevara N, Shaw AM, MacDonald A. α1-Adrenoceptor antagonist properties of CGP 12177A and other beta-adrenoceptor ligands: evidence against beta(3)- or atypical beta-adrenoceptors in rat aorta. Br J Pharmacol. 2004;142:781–787. doi: 10.1038/sj.bjp.0705840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brede M, Nagy G, Philipp M, Sorensen JB, Lohse MJ, Hein L. Differential control of adrenal and sympathetic catecholamine release by α2-adrenoceptor subtypes. Mol Endocrinol. 2003;17:1640–1646. doi: 10.1210/me.2003-0035. [DOI] [PubMed] [Google Scholar]
- Broadley KJ. The vascular effects of trace amines and amphetamines. Pharmacol Ther. 2010;125:363–375. doi: 10.1016/j.pharmthera.2009.11.005. [DOI] [PubMed] [Google Scholar]
- Broening HW, Bowyer JF, Slikker W., Jr Age-dependent sensitivity of rats to the long-term effects of the serotonergic neurotoxicant (+/−)-3,4-methylenedioxymethamphetamine (MDMA) correlates with the magnitude of the MDMA-induced thermal response. J Pharmacol Exp Ther. 1995;275:325–333. [PubMed] [Google Scholar]
- Brown C, Osterloh J. Multiple severe complications from recreational ingestion of MDMA (‘ecstasy’) J Am Med Assoc. 1987;258:780–781. [PubMed] [Google Scholar]
- Bylund DB, Eikenberg DC, Hieble JP, Langer SZ, Lefkowitz RJ, Minneman KP, et al. International Union of Pharmacology nomenclature of adrenoceptors. Pharmacol Revs. 1994;46:121–136. [PubMed] [Google Scholar]
- Carvalho M, Carvalho F, Remião F, de Lourdes Pereira M, Pires-das-Neves R, de Lourdes Bastos M. Effect of 3,4-methylenedioxymethamphetamine (‘ecstasy’) on body temperature and liver antioxidant status in mice: influence of ambient temperature. Arch Toxicol. 2002;76:166–172. doi: 10.1007/s00204-002-0324-z. [DOI] [PubMed] [Google Scholar]
- Chance MRA. Aggregation as a factor influencing the toxicity of sympathomimetic amines in mice. J Pharmacol Exp Ther. 1946;87:214–219. [PubMed] [Google Scholar]
- Chotani MA, Flavahan S, Mitra S, Daunt D, Flavahan NA. Silent α(2C)-adrenergic receptors enable cold-induced vasoconstriction in cutaneous arteries. Am J Physiol Heart Circ Physiol. 2000;278:H1075–H1083. doi: 10.1152/ajpheart.2000.278.4.H1075. [DOI] [PubMed] [Google Scholar]
- Cleary L, Docherty JR. Actions of amphetamine derivatives and cathinone at the noradrenaline transporter. Eur J Pharmacol. 2003;476:31–34. doi: 10.1016/s0014-2999(03)02173-3. [DOI] [PubMed] [Google Scholar]
- Cleary L, Buber R, Docherty JR. Effects of amphetamine derivatives and cathinone on noradrenaline-evoked contractions of rat right ventricle. Eur J Pharmacol. 2002a;451:303–308. doi: 10.1016/s0014-2999(02)02305-1. [DOI] [PubMed] [Google Scholar]
- Cleary L, Vandeputte C, Docherty JR. Investigation of neurotransmission in vas deferens from α(2A/D)-adrenoceptor knockout mice. Br J Pharmacol. 2002b;136:857–864. doi: 10.1038/sj.bjp.0704791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colado MI, Williams JL, Green AR. The hyperthermic and neurotoxic effects of ‘ecstasy’ (MDMA) and 3,4 methylenedioxyamphetamine (MDA) in the dark agouti (DA) rat, a model of the CYP2D6 poor metabolizer phenotype. Br J Pharmacol. 1995;115:1281–1289. doi: 10.1111/j.1476-5381.1995.tb15037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colado MI, Camarero J, Mechan AO, Sanchez V, Esteban B, Elliott JM, et al. A study of the mechanisms involved in the neurotoxic action of 3,4-methylenedioxymethamphetamine (MDMA, ‘ecstasy’) on dopamine neurones in mouse brain. Br J Pharmacol. 2001;134:1711–1723. doi: 10.1038/sj.bjp.0704435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole JC, Sumnall HR. Altered states: the clinical effects of ‘ecstasy’. Pharmacol Ther. 2003a;98:35–58. doi: 10.1016/s0163-7258(03)00003-2. [DOI] [PubMed] [Google Scholar]
- Cole JC, Sumnall HR. The preclinical behavioural pharmacology of 3,4-methylenedioxymethamphetamine (MDMA) Neurosci Biobehav Revs. 2003b;27:199–217. doi: 10.1016/s0149-7634(03)00031-9. [DOI] [PubMed] [Google Scholar]
- Crean RD, Davis SA, Von Huben SN, Lay CC, Katner SN, Taffe MA. Effects of (+/−)3,4-methylenedioxymethamphetamine, (+/−)3,4-methylenedioxyamphetamine and methamphetamine on temperature and activity in rhesus macaques. Neuroscience. 2006;142:515–525. doi: 10.1016/j.neuroscience.2006.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crean RD, Davis SA, Taffe MA. Oral administration of (+/−)3,4-methylenedioxymethamphetamine and (+)methamphetamine alters temperature and activity in rhesus macaques. Pharmacol Biochem Behav. 2007;87:11–19. doi: 10.1016/j.pbb.2007.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespi D, Mennini T, Gobbi M. Carrier-dependent and Ca(2+)-dependent 5-HT and dopamine release induced by (+)-amphetamine, 3,4-methylendioxymethamphetamine, p-chloroamphetamine and (+)-fenfluramine. Br J Pharmacol. 1997;121:1735–1743. doi: 10.1038/sj.bjp.0701325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronin MJ. p-Chlorophenylalanine hyperthermia in a warm environment: reversal with 5-hydroxytryptophan. Brain Res. 1976;112:194–199. doi: 10.1016/0006-8993(76)90351-6. [DOI] [PubMed] [Google Scholar]
- Dafters RI. Effect of ambient temperature on hyperthermia and hyperkinesis induced by 3,4-methylenedioxymethamphetamine (MDMA or ‘ecstasy’) in rats. Psychopharmacology. 1994;114:505–508. doi: 10.1007/BF02249342. [DOI] [PubMed] [Google Scholar]
- Dafters RI. Hyperthermia following MDMA administration in rats: effects of ambient temperature, water consumption, and chronic dosing. Physiol Behav. 1995;58:877–882. doi: 10.1016/0031-9384(95)00136-7. [DOI] [PubMed] [Google Scholar]
- Dafters RI, Lynch E. Persistent loss of thermoregulation in the rat induced by 3,4-methylenedioxymethamphetamine (MDMA or ‘ecstasy’) but not by fenfluramine. Psychopharmacology. 1998;138:207–212. doi: 10.1007/s002130050664. [DOI] [PubMed] [Google Scholar]
- Daws LC, Irvine RJ, Callaghan PD, Toop NP, White JM, Bochner F. Differential behavioural and neurochemical effects of para-methoxyamphetamine and 3,4-methylenedioxymethamphetamine in the rat. Prog Neuropsychopharmacol Biol Psychiatry. 2000;24:955–977. doi: 10.1016/s0278-5846(00)00113-5. [DOI] [PubMed] [Google Scholar]
- Di Matteo V, Di Giovanni G, Pierucci M, Esposito E. Serotonin control of central dopaminergic function: focus on in vivo microdialysis studies. Prog Brain Res. 2008;172:7–44. doi: 10.1016/S0079-6123(08)00902-3. [DOI] [PubMed] [Google Scholar]
- Docherty JR. Subtypes of functional α1- and α2-adrenoceptors. Eur J Pharmacol. 1998;361:1–15. doi: 10.1016/s0014-2999(98)00682-7. [DOI] [PubMed] [Google Scholar]
- Dowling GP, McDonough ET, Bost RO. ‘Eve’ and ‘ecstasy’. A report of five deaths associated with the use of MDEA and MDMA. J Am Med Assoc. 1987;257:1615–1617. doi: 10.1001/jama.257.12.1615. [DOI] [PubMed] [Google Scholar]
- Duka I, Gavras I, Johns C, Handy DE, Gavras H. Role of the postsynaptic α(2)-adrenergic receptor subtypes in catecholamine-induced vasoconstriction. Gen Pharmacol. 2000;34:101–106. doi: 10.1016/s0306-3623(00)00051-3. [DOI] [PubMed] [Google Scholar]
- Ensslin HK, Maurer HH, Gouzoulis E, Hermle L, Kovar KA. Metabolism of racemic 3,4-methylenedioxyethylamphetamine in humans. Isolation, identification, quantification, and synthesis of urinary metabolites. Drug Metab Dispos. 1996;24:813–820. [PubMed] [Google Scholar]
- Fantegrossi WE, Godlewski T, Karabenick RL, Stephens JM, Ullrich T, Rice KC, et al. Pharmacological characterization of the effects of 3,4-methylenedioxymethamphetamine (‘ecstasy’) and its enantiomers on lethality, core temperature, and locomotor activity in singly housed and crowded mice. Psychopharmacology. 2003;166:202–211. doi: 10.1007/s00213-002-1261-5. [DOI] [PubMed] [Google Scholar]
- Farré M, Abanades S, Roset PN, Peiro AM, Torrens M, O'Mathuna B, et al. Pharmacological interaction between 3,4 and methylenedioxymethamphetamine (ecstasy) and paroxetine: pharmacological effects and pharmacokinetics. J Pharmacol Exp Ther. 2007;323:954–962. doi: 10.1124/jpet.107.129056. [DOI] [PubMed] [Google Scholar]
- Farver DK. Neuroleptic malignant syndrome induced by atypical antipsychotics. Expert Opin Drug Saf. 2003;2:21–35. doi: 10.1517/14740338.2.1.21. [DOI] [PubMed] [Google Scholar]
- Fischer JF, Cho AK. Chemical release of dopamine from striatal homogenates: evidence for an exchange diffusion model. J Pharmacol Exp Ther. 1979;208:203–209. [PubMed] [Google Scholar]
- Fitzgerald JL, Reid JJ. Interactions of methylenedioxymethamphetamine with monoamine transmitter release mechanisms in rat brain slices. Naunyn Schmiedebergs Arch Pharmacol. 1993;347:313–323. doi: 10.1007/BF00167451. [DOI] [PubMed] [Google Scholar]
- Fitzgerald JL, Reid JJ. Sympathomimetic actions of methylenedioxymethamphetamine in rat and rabbit isolated cardiovascular tissues. J Pharm Pharmacol. 1994;46:826–832. doi: 10.1111/j.2042-7158.1994.tb03738.x. [DOI] [PubMed] [Google Scholar]
- Freedman RR, Johanson CE, Tancer ME. Thermoregulatory effects of 3,4-methylenedioxymethamphetamine (MDMA) in humans. Psychopharmacology. 2005;183:248–256. doi: 10.1007/s00213-005-0149-6. [DOI] [PubMed] [Google Scholar]
- Fuller MA, Borovicka MC, Jaskiw GE, Simon MR, Kwon K, Konicki PE. Clozapine-induced urinary incontinence: incidence and treatment with ephedrine. J Clin Psychiatry. 1996;57:514–518. doi: 10.4088/jcp.v57n1102. [DOI] [PubMed] [Google Scholar]
- Gabrielsson J, Green AR. Quantitative pharmacology or pharmacokinetic pharmacodynamic integration should be a vital component in integrative pharmacology. J Pharmacol Exp Ther. 2009;331:1–8. doi: 10.1124/jpet.109.157172. [DOI] [PubMed] [Google Scholar]
- Gavin KT, Colgan MP, Moore D, Shanik G, Docherty JR. α2C-adrenoceptors mediate contractile responses to noradrenaline in the human saphenous vein. Naunyn Schmiedebergs Arch Pharmacol. 1997;355:406–411. doi: 10.1007/pl00004961. [DOI] [PubMed] [Google Scholar]
- Giacchino JL, Schtel ER, Horowitz JM, Horowitz BA. Effect of p-chlorophenylalanine on thermoregulation in unrestrained rats. Am J Physiol. 1983;244:R299–R302. doi: 10.1152/ajpregu.1983.244.2.R299. [DOI] [PubMed] [Google Scholar]
- Ginsberg MD, Hertzman M, Schmidt-Nowara WW. Amphetamine intoxication with coagulopathy, hyperthermia and reversible renal failure. A syndrome resmbling heatstroke. Ann Intern Med. 1970;73:81–85. doi: 10.7326/0003-4819-73-1-81. [DOI] [PubMed] [Google Scholar]
- Goni-Allo B, Mathúna BÓ, Segura M, Puerta E, Lasheras B, de la Torre R, Aguirre N. The relationship between core body temperature and 3,4-methylenedioxymethamphetamine metabolism in rats: implications for neurotoxicity. Psychopharmacology. 2008;197:263–278. doi: 10.1007/s00213-007-1027-1. [DOI] [PubMed] [Google Scholar]
- Goodwin GM, Green AR. A behavioural and biochemical study in mice and rats of putative selective agonists and antagonists for 5-HT1 and 5-HT2 receptors. Br J Pharmacol. 1985;84:743–753. doi: 10.1111/j.1476-5381.1985.tb16157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin GM, De Souza RJ, Green AR. The pharmacology of the hypothermic response in mice to 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT). A model of presynaptic 5-HT1 function. Neuropharmacology. 1985;24:1187–1194. doi: 10.1016/0028-3908(85)90153-4. [DOI] [PubMed] [Google Scholar]
- Gordon CJ, Fogelson L. Metabolic and thermoregulatory responses of the rat maintained in acrylic or wire screen cages: implications for pharmacological studies. Physiol Behav. 1994;56:73–79. doi: 10.1016/0031-9384(94)90263-1. [DOI] [PubMed] [Google Scholar]
- Gordon CJ, Watkinson WP, O'Callaghan JP, Miller DB. Effects of 3,4-methylenedioxymethamphetamine on autonomic thermoregulatory responses of the rat. Pharmacol Biochem Behav. 1991;38:339–344. doi: 10.1016/0091-3057(91)90288-d. [DOI] [PubMed] [Google Scholar]
- Gouzoulis E, von Bardeleben U, Rupp A, Kovar KA, Hermle L. Neuroendocrine and cardiovascular effects of MDE in healthy volunteers. Neuropsychopharmacology. 1993;8:187–193. doi: 10.1038/npp.1993.20. [DOI] [PubMed] [Google Scholar]
- Gouzoulis-Mayfrank E, Daumann J. Neurotoxicity of drugs of abuse – the case of methylenedioxyamphetamines (MDMA, ecstasy), and amphetamines. Dialogues Clin Neurosci. 2009;11:305–317. doi: 10.31887/DCNS.2009.11.3/egmayfrank. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grahame-Smith DG. Studies in vivo on the relationship between brain tryptophan, brain 5-HT synthesis and hyperactivivty in rats treated with a monoamine oxidase inhibitor and L-tryptophan. J Neurochem. 1971;18:1053–1066. doi: 10.1111/j.1471-4159.1971.tb12034.x. [DOI] [PubMed] [Google Scholar]
- Grant RT. Vasodilatation and body warming in the rat. J Physiol. 1963;167:311–317. doi: 10.1113/jphysiol.1963.sp007151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green AR. MDMA fact and fallacy, and the need to increase knowledge in both the scientific and popular press. Psychopharmacology. 2004;173:231–233. doi: 10.1007/s00213-004-1820-z. [DOI] [PubMed] [Google Scholar]
- Green AR, Mechan AO, Elliott JM, O'Shea E, Colado MI. The pharmacology and clinical pharmacology of 3,4-methylenedioxymethamphetamine (MDMA, ‘ecstasy’) Pharmacol Rev. 2003;55:463–508. doi: 10.1124/pr.55.3.3. [DOI] [PubMed] [Google Scholar]
- Green AR, Sanchez V, O'Shea E, Saadat KS, Elliott JM, Colado MI. Effect of ambient temperature and a prior neurotoxic dose of 3,4-methylenedioxymethamphetamine (MDMA) on the hyperthermic response of rats to a single or repeated (‘binge’ ingestion) low dose of MDMA. Psychopharmacology. 2004;173:264–269. doi: 10.1007/s00213-003-1725-2. [DOI] [PubMed] [Google Scholar]
- Green AR, O'Shea E, Saadat KS, Elliott JM, Colado MI. Studies on the effect of MDMA (‘ecstasy’) on the body temperature of rats housed at different ambient room temperatures. Br J Pharmacol. 2005;146:306–312. doi: 10.1038/sj.bjp.0706318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green AR, Gabrielsson J, Marsden CA, Fone KCF. MDMA: on the translation from rodent to human dosing. Psychopharmacology. 2009;204:375–378. doi: 10.1007/s00213-008-1453-8. [DOI] [PubMed] [Google Scholar]
- Gudelsky GA, Yamamoto BK. Actions of 3,4-methylenedioxymethamphetamine (MDMA) on cerebral dopaminergic, serotonergic and cholinergic neurons. Pharmacol Biochem Behav. 2008;90:198–207. doi: 10.1016/j.pbb.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudelsky GA, Koenig JI, Meltzer HY. Thermoregulatory responses to serotonin (5-HT) receptor stimulation in the rat. Evidence for opposing roles of 5-HT2 and 5-HT1A receptors. Neuropharmacology. 1986;25:1307–1313. doi: 10.1016/0028-3908(86)90101-2. [DOI] [PubMed] [Google Scholar]
- Gunn JA, Gurd MR. The action of some amines related to adrenaline. Cyclohexylalkylamines. J Physiol. 1940;97:453–470. doi: 10.1113/jphysiol.1940.sp003822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammersley R, Ditton J, Smith I, Short E. Patterns of ecstasy use by drug users. Br J Criminol. 1999;39:625–647. [Google Scholar]
- Hayner GN, McKinney H. MDMA. The dark side of ecstasy. J Psychoactive Drugs. 1986;18:341–347. doi: 10.1080/02791072.1986.10472367. [DOI] [PubMed] [Google Scholar]
- Hegadoren KM, Baker GB, Bourin M. 3,4-Methylenedioxy analogues of amphetamine: defining the risks to humans. Neurosci Biobehav Rev. 1999;23:539–553. doi: 10.1016/s0149-7634(98)00046-3. [DOI] [PubMed] [Google Scholar]
- Hein L, Altman JD, Kobilka BK. Two functionally distinct α2-adrenergic receptors regulate sympathetic neurotransmission. Nature. 1999;402:181–184. doi: 10.1038/46040. [DOI] [PubMed] [Google Scholar]
- Herin DV, Liu S, Ullrich T, Rice KC, Cunningham KA. Role of the serotonin 5-HT2A receptor in the hyperlocomotive and hyperthermic effects of (+)-3,4-methylenedioxymethamphetamine. Psychopharmacology. 2005;178:505–513. doi: 10.1007/s00213-004-2030-4. [DOI] [PubMed] [Google Scholar]
- Hjorth S. Hypothermia in the rat induced by the potent serotoninergic agent 8-OH-DPAT. J Neural Transm. 1985;61:131–135. doi: 10.1007/BF01253058. [DOI] [PubMed] [Google Scholar]
- Hjorth S, Carlsson A. Postsynaptic dopamine (DA) receptor stimulator properties of the putative DA autoreceptor-selective agonist BHT-920 uncovered by co-treatment with the D-1 agonist SK & F38393. Psychopharmacology. 1987;173:534–537. doi: 10.1007/BF00207249. [DOI] [PubMed] [Google Scholar]
- Ho SL, Honner V, Docherty JR. Investigation of the subtypes of α2-adrenoceptor mediating prejunctional inhibition in rat atrium and cerebral cortex. Naunyn Schmiedebergs Arch Pharmacol. 1998;357:634–639. doi: 10.1007/pl00005218. [DOI] [PubMed] [Google Scholar]
- Hunter JC, Fontana DJ, Hedley LR, Jasper JR, Lewis R, Link RE, et al. Assessment of the role of α2-adrenoceptor subtypes in the antinociceptive, sedative and hypothermic action of dexmedetomidine in transgenic mice. Br J Pharmacol. 1997;122:1339–1344. doi: 10.1038/sj.bjp.0701520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine RJ, Keane M, Felgate P, McCann UD, Callaghan PD, White JM. Plasma drug concentrations and physiological measures in ‘dance party’ participants. Neuropsychopharmacology. 2006;31:424–430. doi: 10.1038/sj.npp.1300896. [DOI] [PubMed] [Google Scholar]
- Jaehne EJ, Salem A, Irvine RJ. Pharmacological and behavioral determinants of cocaine, methamphetamine, 3,4-methylenedioxymethamphetamine, and para-methoxyamphetamine-induced hyperthermia. Psychopharmacology. 2007;194:41–52. doi: 10.1007/s00213-007-0825-9. [DOI] [PubMed] [Google Scholar]
- Jaehne EJ, Salem A, Irvine RJ. The effect of long-term repeated exposure to 3,4-methylenedioxymethamphetamine on cardiovascular and thermoregulatory changes. Psychopharmacology. 2008;201:161–170. doi: 10.1007/s00213-008-1258-9. [DOI] [PubMed] [Google Scholar]
- Johnson MP, Conarty PF, Nichols DE. [3H]monoamine releasing and uptake inhibition properties of 3,4-methylenedioxymethamphetamine and p-chloroamphetamine analogues. Eur J Pharmacol. 1991;200:9–16. doi: 10.1016/0014-2999(91)90659-e. [DOI] [PubMed] [Google Scholar]
- Johnson EA, Sharp DS, Miller DB. Restraint as a stressor in mice: against the dopaminergic neurotoxicity of d-MDMA, low body weight mitigates restraint-induced hypothermia and consequent neuroprotection. Brain Res. 2000;875:107–118. doi: 10.1016/s0006-8993(00)02601-9. [DOI] [PubMed] [Google Scholar]
- Johnson EA, O'Callaghan JP, Miller DB. Chronic treatment with supraphysiological levels of corticosterone enhances d-MDMA-induced dopaminergic neurotoxicity in the C57BL/6J female mouse. Brain Res. 2002a;933:130–138. doi: 10.1016/s0006-8993(02)02310-7. [DOI] [PubMed] [Google Scholar]
- Johnson EA, Shvedova AA, Kisin E, O'Callaghan JP, Kommineni C, Miller DB. d-MDMA during vitamin E deficiency: effects on dopaminergic neurotoxicity and hepatotoxicity. Brain Res. 2002b;933:150–163. doi: 10.1016/s0006-8993(02)02313-2. [DOI] [PubMed] [Google Scholar]
- Kalant H. The pharmacology and toxicology of ‘ecstasy’ (MDMA) and related drugs. Can Med Assoc J. 2001;165:917–928. [PMC free article] [PubMed] [Google Scholar]
- Kendrick WC, Hull AR, Knochel JP. Rhabdomolysis and shock after intravenous amphetamine administration. Ann Intern Med. 1977;86:381–387. doi: 10.7326/0003-4819-86-4-381. [DOI] [PubMed] [Google Scholar]
- Lähdesmäki J, Sallinen J, MacDonald E, Sirviö J, Scheinin M. α2-Adrenergic drug effects on brain monoamines, locomotion, and body temperature are largely abolished in mice lacking the α2A-adrenoceptor subtype. Neuropharmacology. 2003;44:882–892. doi: 10.1016/s0028-3908(03)00080-7. [DOI] [PubMed] [Google Scholar]
- Lavelle A, Honner V, Docherty JR. Investigation of the prejunctional α2-adrenoceptor mediated actions of MDMA in rat atrium and vas deferens. Br J Pharmacol. 1999;128:975–980. doi: 10.1038/sj.bjp.0702875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblais V, Pourageaud F, Ivorra MD, Guibert C, Marthan R, Muller B. Role of α-adrenergic receptors in the effect of the beta-adrenergic receptor ligands, CGP 12177, bupranolol, and SR 59230A, on the contraction of rat intrapulmonary artery. J Pharmacol Exp Ther. 2004;309:137–145. doi: 10.1124/jpet.103.061192. [DOI] [PubMed] [Google Scholar]
- Leggio GM, Cathala A, Neny M, Rouge-Pont F, Drago F, Piazza PV, et al. In vivo evidence that constitutive activity of serotonin2C receptors in the medial prefrontal cortex participates in the control of dopamine release in the rat nucleus accumbens: differential effects of inverse agonist versus antagonist. J Neurochem. 2009;111:614–623. doi: 10.1111/j.1471-4159.2009.06356.x. [DOI] [PubMed] [Google Scholar]
- Leonardi ET, Azmitia EC. MDMA (ecstasy) inhibition of MAO type A and type B: comparisons with fenfluramine and fluoxetine (Prozac) Neuropsychopharmacology. 1994;10:231–238. doi: 10.1038/npp.1994.26. [DOI] [PubMed] [Google Scholar]
- Liechti ME, Vollenweider FX. The serotonin uptake inhibitor citalopram reduces acute cardiovascular and vegetative effects of 3,4-methylenedioxymethamphetamine (‘ecstasy’) in healthy volunteers. J Psychopharmacol. 2000a;14:269–274. doi: 10.1177/026988110001400313. [DOI] [PubMed] [Google Scholar]
- Liechti ME, Vollenweider FX. Acute psychological and physiological effects of MDMA (ecstasy) after haloperidol treatment in healthy humans. Eur Neuropsychopharmacol. 2000b;10:289–295. doi: 10.1016/s0924-977x(00)00086-9. [DOI] [PubMed] [Google Scholar]
- Liechti ME, Saur MR, Gamma A, Hell D, Vollenweider FX. Psychological and physiological effects of MDMA (‘ecstasy’) after pretreatment with the 5-HT(2) antagonist ketanserin in healthy humans. Neuropsychopharmacology. 2000;23:396–404. doi: 10.1016/S0893-133X(00)00126-3. [DOI] [PubMed] [Google Scholar]
- McCann UD, Slate SO, Ricaurte GA. Adverse reactions with 3,4-methylenedioxymethamphetamine (MDMA; ‘ecstasy’. Drug Safety. 1996;15:107–115. doi: 10.2165/00002018-199615020-00003. [DOI] [PubMed] [Google Scholar]
- McDaid J, Docherty JR. Vascular actions of MDMA involve α1 and α2-adrenoceptors in the anaesthetized rat. Br J Pharmacol. 2001;133:429–437. doi: 10.1038/sj.bjp.0704094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malberg JE, Seiden LS. Small changes in ambient temperature cause large changes in 3,4-methylenedioxymethamphetamine (MDMA)-induced serotonin neurotoxicity and core body temperature in the rat. J Neurosci. 1998;18:5086–5094. doi: 10.1523/JNEUROSCI.18-13-05086.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malberg JE, Sabol KE, Seiden LS. Co-administration of MDMA with drugs that protect against MDMA neurotoxicity produces different effects on body temperature in the rat. J Pharmacol Exp Ther. 1996;278:258–267. [PubMed] [Google Scholar]
- Malpass A, White JM, Irvine RJ, Somogyi AA, Bochner F. Acute toxicity of 3,4-methylenedioxymethamphetamine (MDMA) in Sprague-Dawley and dark agouti rats. Pharmacol Biochem Behav. 1999;64:29–34. doi: 10.1016/s0091-3057(99)00116-1. [DOI] [PubMed] [Google Scholar]
- Manara L, Badone D, Baroni M, Boccardi G, Cecchi R, Croci T, et al. Functional identification of rat atypical beta-adrenoceptors by the first beta 3-selective antagonists, aryloxypropanolaminotetralins. Br J Pharmacol. 1996;117:435–442. doi: 10.1111/j.1476-5381.1996.tb15209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunaga E, Zanger UM, Hardwick JP, Gelboin HV, Meyer UA, Gonzalez FJ. The CYP2D gene subfamily: analysis of the molecular basis of the debrisoquine4-hydroxylase deficiency in DA rats. Biochem. 1989;28:7349–7355. doi: 10.1021/bi00444a030. [DOI] [PubMed] [Google Scholar]
- Mazzola-Pomietto P, Aulakh CS, Tolliver T, Murphy DL. Functional subsensitivity of 5-HT2A and 5-HT2C receptors mediating hyperthermia following acute and chronic treatment with 5-HT2A/2C receptor antagonists. Psychopharmacology. 1997;130:144–151. doi: 10.1007/s002130050222. [DOI] [PubMed] [Google Scholar]
- Mechan AO, O'Shea E, Elliott JM, Colado MI, Green AR. A neurotoxic dose of 3,4-methylenedioxymethamphetamine (MDMA; ecstasy) to rats results in a long-term defect in thermoregulation. Psychopharmacology. 2001;155:413–418. doi: 10.1007/s002130100735. [DOI] [PubMed] [Google Scholar]
- Mechan AO, Esteban B, O'shea E, Elliott JM, Colado MI, Green AR. The pharmacology of the acute hyperthermic response that follows administration of 3,4-methylenedioxymethamphetamine (MDMA, ‘ecstasy’) to rats. Br J Pharmacol. 2002;135:170–180. doi: 10.1038/sj.bjp.0704442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DB, O'Callaghan JP. Environment-, drug- and stress-induced alterations in body temperature affect the neurotoxicity of substituted amphetamines in the C57BL/6J mouse. J Pharmacol Exp Ther. 1994;270:752–760. [PubMed] [Google Scholar]
- Mills EM, Rusyniak DE, Sprague JE. The role of the sympathetic nervous system and uncoupling proteins in the thermogenesis induced by 3,4-methylenedioxymethamphetamine. J Mol Med. 2004;82:787–799. doi: 10.1007/s00109-004-0591-7. [DOI] [PubMed] [Google Scholar]
- Mittleman MA, Mintzer D, Maclure M, Tofler GH, Sherwood JB, Muller JE. Triggering of myocardial infarction by cocaine. Circulation. 1999;99:2737–2741. doi: 10.1161/01.cir.99.21.2737. [DOI] [PubMed] [Google Scholar]
- Morimoto T, Hashimoto K, Yasumatsu H, Tanaka H, Fujimura M, Kuriyama M, et al. Neuropharmacological profile of a novel potential atypical antipsychotic drug Y-931 (8-fluoro-12-(4-methylpiperazin-1-yl)- 6H-[1]benzothieno[2,3-b][1,5] benzodiazepine maleate) Neuropsychopharmacology. 2002;26:456–467. doi: 10.1016/S0893-133X(01)00368-2. [DOI] [PubMed] [Google Scholar]
- Myers RD, Beleslin DB, Rezvani AH. Hypothermia: role of α1- and α2-noradrenergic receptors in the hypothalamus of the cat. Pharmacol Biochem Behav. 1987;26:373–379. doi: 10.1016/0091-3057(87)90132-8. [DOI] [PubMed] [Google Scholar]
- Nasrallah HA. Atypical antipsychotic-induced metabolic side effects: insights from receptor-binding profiles. Mol Psychiatry. 2008;13:27–35. doi: 10.1038/sj.mp.4002066. [DOI] [PubMed] [Google Scholar]
- Nichols DE, Lloyd DH, Hoffman AJ, Nichols MB, Yim GK. Effects of certain hallucinogenic amphetamine analogues on the release of [3H]serotonin from rat brain synaptosomes. J Med Chem. 1982;25:530–535. doi: 10.1021/jm00347a010. [DOI] [PubMed] [Google Scholar]
- Nisoli E, Tonello C, Landi M, Carruba MO. Functional studies of the first selective beta 3-adrenergic receptor antagonist SR 59230A in rat brown adipocytes. Mol Pharmacol. 1996;49:7–14. [PubMed] [Google Scholar]
- O'Cain PA, Hletko SB, Ogden BA, Varner KJ. Cardiovascular and sympathetic responses and reflex changes elicited by MDMA. Physiol Behav. 2000;70:141–148. doi: 10.1016/s0031-9384(00)00235-3. [DOI] [PubMed] [Google Scholar]
- O'Loinsigh ED, Boland G, Kelly JP, O'Boyle KM. Behavioural, hyperthermic and neurotoxic effects of 3,4-methylenedioxymethamphetamine analogues in the Wistar rat. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25:621–638. doi: 10.1016/s0278-5846(00)00179-2. [DOI] [PubMed] [Google Scholar]
- Ootsuka Y, Blessing WW. Thermogenesis in brown adipose tissue: increase by 5-HT2A receptor activation and decrease by 5-HT1A receptor activation in conscious rats. Neurosci Letts. 2006;395:170–174. doi: 10.1016/j.neulet.2005.10.062. [DOI] [PubMed] [Google Scholar]
- O'Shea E, Esteban B, Camarero J, Green AR, Colado MI. Effect of GBR 12909 and fluoxetine on the acute and long term changes induced by MDMA (‘ecstasy’) on the 5-HT and dopamine concentrations in mouse brain. Neuropharmacology. 2001;40:65–74. doi: 10.1016/s0028-3908(00)00106-4. [DOI] [PubMed] [Google Scholar]
- O'Shea E, Orio L, Escobedo I, Sanchez V, Camarero J, Green AR, et al. MDMA-induced neurotoxicity: long-term effects on 5-HT biosynthesis and the influence of ambient temperature. Br J Pharmacol. 2006;148:778–785. doi: 10.1038/sj.bjp.0706783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrott AC. Chronic tolerance to recreational MDMA (3,4-methylenedioxymethamphetamine) or ecstasy. J Psychopharmacol. 2005;19:71–83. doi: 10.1177/0269881105048900. [DOI] [PubMed] [Google Scholar]
- Pedersen NP, Blessing WW. Cutaneous vasoconstriction contributes to hyperthermia induced by 3,4-methylenedioxymethamphetamine (ecstasy) in conscious rabbits. J Neurosci. 2001;21:8648–8654. doi: 10.1523/JNEUROSCI.21-21-08648.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettinger WA, Keeton TK, Campbell WB, Harper DC. Evidence for a renal alpha-adrenergic receptor inhibiting renin release. Circ Res. 1976;38:338–346. doi: 10.1161/01.res.38.5.338. [DOI] [PubMed] [Google Scholar]
- Philipp M, Brede M, Hein L. Physiological significance of α(2)-adrenergic receptor subtype diversity: one receptor is not enough. Am J Physiol Regul Integr Comp Physiol. 2002;283:R287–R295. doi: 10.1152/ajpregu.00123.2002. [DOI] [PubMed] [Google Scholar]
- Piper BJ, Fraiman JB, Owens CB, Ali SF, Meyer JS. Dissociation of the neurochemical and behavioral toxicology of MDMA (‘ecstasy’) by citalopram. Neuropsychopharmacology. 2008;33:1192–1205. doi: 10.1038/sj.npp.1301491. [DOI] [PubMed] [Google Scholar]
- Qvigstad E, Sjaastad I, Bøkenes J, Schiander I, Solberg L, Sejersted OM, et al. Carvedilol blockade of α1- and β-adrenoceptor induced inotropic responses in rats with congestive heart failure. Eur J Pharmacol. 2005;516:51–59. doi: 10.1016/j.ejphar.2005.04.027. [DOI] [PubMed] [Google Scholar]
- Rajamani K, Leong S, Lavelle A, Docherty JR. Prejunctional actions of methylenedioxymethamphetamine in vas deferens from wild-type and α(2A/D)-adrenoceptor knockout mice. Eur J Pharmacol. 2001;423:223–228. doi: 10.1016/s0014-2999(01)01118-9. [DOI] [PubMed] [Google Scholar]
- Reid WD, Volicer L, Smookler H, Beaven MA, Brodie BB. Brain amines and temperature regulation. Pharmacology. 1968;1:329–344. doi: 10.1159/000135983. [DOI] [PubMed] [Google Scholar]
- Rodsiri R, Marsden CA, Fone KCF, Green AR. Changes in activity and temperature fail to correlate with 5-HT release following repeated MDMA administration in rats. J Psychopharmacol. 2008;22:A65. [Google Scholar]
- Romanovsky AA, Ivanov AI, Shimansky YP. Ambient temperature for experiments in rats: a new method for determining the zone of thermal neutrality. J Appl Physiol. 2002;92:2667–2679. doi: 10.1152/japplphysiol.01173.2001. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH, Dersch CM, Romero DV, Rice KC, Carroll FI, et al. Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse. 2001;39:32–41. doi: 10.1002/1098-2396(20010101)39:1<32::AID-SYN5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Roti Roti JL. Cellular responses to hyperthermia (40–46 degrees C): cell killing and molecular events. Int J Hyperthermia. 2008;24:3–15. doi: 10.1080/02656730701769841. [DOI] [PubMed] [Google Scholar]
- Rusyniak DE, Ootsuka Y, Blessing WW. When administered to rats in a cold environment, 3,4-methylenedioxymethamphetamine reduces brown adipose tissue thermogenesis and increases tail blood flow: effects of pretreatment with 5-HT1A and dopamine D2 antagonists. Neuroscience. 2008;154:1619–1626. doi: 10.1016/j.neuroscience.2008.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saadat KS, Elliott JM, Colado MI, Green AR. Hyperthermic and neurotoxic effect of 3,4-methylenedioxymethamphetamine (MDMA) in guinea pigs. Psychopharmacology. 2004;173:452–453. doi: 10.1007/s00213-003-1653-1. [DOI] [PubMed] [Google Scholar]
- Saadat KS, O'Shea E, Colado MI, Elliott JM, Green AR. The role of 5-HT in the impairment of thermoregulation observed in rats administered MDMA (‘ecstasy’) when housed at high ambient temperature. Psychopharmacology. 2005;179:884–890. doi: 10.1007/s00213-004-2106-1. [DOI] [PubMed] [Google Scholar]
- Sallinen J, Link RE, Haapalinna A, Viitamaa T, Kulatunga M, Sjöholm B, et al. Genetic alteration of α2C-adrenoceptor expression in mice: influence on locomotor, hypothermic, and neurochemical effects of dexmedetomidine, a subtype-nonselective α2-adrenoceptor agonist. Mol Pharmacol. 1997;51:36–46. doi: 10.1124/mol.51.1.36. [DOI] [PubMed] [Google Scholar]
- Sanchez V, Camarero J, O'Shea E, Green AR, Colado MI. Differential effect of dietary selenium on the long-term neurotoxicity induced by MDMA in mice and rats. Neuropharmacology. 2003;44:449–461. doi: 10.1016/s0028-3908(02)00411-2. [DOI] [PubMed] [Google Scholar]
- Sanchez V, O'Shea E, Saadat KS, Elliott JM, Colado MI, Green AR. Effect of repeated (‘binge’) dosing of MDMA to rats housed at normal and high temperature on neurotoxic damage to cerebral 5-HT and dopamine neurones. J Psychopharmacol. 2004;18:412–416. doi: 10.1177/026988110401800312. [DOI] [PubMed] [Google Scholar]
- Schmidt CJ. Acute administration of methylenedioxymethamphetamine: comparison with the neurochemical effects of its N-desmethyl and N-ethyl analogs. Eur J Pharmacol. 1987;136:81–88. doi: 10.1016/0014-2999(87)90782-5. [DOI] [PubMed] [Google Scholar]
- Schmidt CJ, Black CK, Abbate GM, Taylor VL. Methylenedioxymethamphetamine-induced hyperthermia and neurotoxicity are independently mediated by 5-HT2 receptors. Brain Res. 1990;529:85–90. doi: 10.1016/0006-8993(90)90813-q. [DOI] [PubMed] [Google Scholar]
- Selvaraj S, Hoshi R, Bhagwagar Z, Murthy NV, Hinz R, Cowen P, et al. Brain serotonin transporter binding in former users of MDMA (‘ecstasy’) Br J Psychiatry. 2009;194:355–359. doi: 10.1192/bjp.bp.108.050344. [DOI] [PubMed] [Google Scholar]
- Shankaran M, Gudelsky GA. A neurotoxic regime of MDMA suppresses behavioral, thermal and neurochemical responses to subsequent MDMA administration. Psychopharmacology. 1999;147:66–72. doi: 10.1007/s002130051143. [DOI] [PubMed] [Google Scholar]
- Sharma HS, Hoopes PJ. Hyperthermia induced pathophysiology of the central nervous system. Int J Hyperthermia. 2003;19:325–354. doi: 10.1080/0265673021000054621. [DOI] [PubMed] [Google Scholar]
- Shioda K, Nisijima K, Yoshino T, Kuboshima K, Iwamura T, Yui K, et al. Risperidone attenuates and reverses hyperthermia induced by 3,4-methylenedioxymethamphetamine (MDMA) in rats. Neurotoxicology. 2008;29:1030–1036. doi: 10.1016/j.neuro.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Sitte HH, Hiptmair B, Zwach J, Pifl C, Singer EA, Scholze P. Quantitative analysis of inward and outward transport rates in cells stably expressing the cloned human serotonin transporter: inconsistencies with the hypothesis of facilitated exchange diffusion. Mol Pharmacol. 2001;59:1129–1137. doi: 10.1124/mol.59.5.1129. [DOI] [PubMed] [Google Scholar]
- Sprague JE, Banks ML, Cook VJ, Mills EM. Hypothalamic–pituitary–thyroid axis and sympathetic nervous system involvement in hyperthermia induced by 3,4-methylenedioxymethamphatamine (ecstasy) J Pharmacol Exp Ther. 2003;305:159–166. doi: 10.1124/jpet.102.044982. [DOI] [PubMed] [Google Scholar]
- Sprague JE, Brutcher RE, Mills EM, Caden D, Rusyniak DE. Attenuation of 3,4-methylenedioxymethamphatamine (MDMA, ecstasy)-induced rhabdomyolysis with α1- plus β3-adrenoceptor antagonists. Br J Pharmacol. 2004;142:667–670. doi: 10.1038/sj.bjp.0705823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprague JE, Moza P, Caden D, Rusyniak DE, Homes C, Goldstein DS, et al. Carvedilol reverses hyperthermia and attenuates rhabdomyolysis induced by 3,4-methylenedioxymethamphatamine (MDMA, ecstasy) in an animal model. Crit Care Med. 2005;33:1311–1316. doi: 10.1097/01.ccm.0000165969.29002.70. [DOI] [PubMed] [Google Scholar]
- Srámek P, Simecková M, Janský L, Savlíková J, Vybíral S. Human physiological responses to immersion into water of different temperatures. Eur J Appl Physiol. 2000;81:436–442. doi: 10.1007/s004210050065. [DOI] [PubMed] [Google Scholar]
- Stuerenburg HJ, Petersen K, Bäumer T, Rosenkranz M, Buhmann C, Thomasius R. Plasma concentrations of 5-HT, 5-HIAA, norepinephrine, epinephrine and dopamine in ecstasy users. Neuro Endocrinol Letts. 2002;23:259–261. [PubMed] [Google Scholar]
- Taffe MA, Lay CC, Von Huben SN, Davis SA, Crean RD, Katner SN. Hyperthermia induced by 3,4-methylenedioxymethamphetamine in unrestrained rhesus monkeys. Drug Alcohol Depend. 2006;82:276–281. doi: 10.1016/j.drugalcdep.2005.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tehan B. Ecstasy and dantrolene. Br Med J. 1993;306:146. doi: 10.1136/bmj.306.6870.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topp L, Hando J, Dillon P, Roche A, Solowij N. Ecstasy use in Australia: patterns of use and associated harm. Drug Alcohol Depend. 1999;55:105–115. doi: 10.1016/s0376-8716(99)00002-2. [DOI] [PubMed] [Google Scholar]
- de la Torre R, Farré M, Ortuño J, Mas M, Brenneisen R, Roset PN, et al. Non-linear pharmacokinetics of MDMA (‘ecstasy’) in humans. Br J Clin Pharmacol. 2000;49:104–109. doi: 10.1046/j.1365-2125.2000.00121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Torre R, Puerta E, Aguirre N. Comment on the letter by Green, Gabrielsson, Marsden, Fone, MDMA: on the translation from rodent to human dosing. Psychopharmacology. 2009;204:379–380. doi: 10.1007/s00213-008-1455-6. [DOI] [PubMed] [Google Scholar]
- Tucker GT, Lennard MS, Ellis SW, Woods HF, Cho AK, Lin LY, et al. The demethylenation of methylenedioxymethamphetamine (‘ecstasy’) by debrisoquine hydroxylase (CYP2D6) Biochem Pharmacol. 1994;47:1151–1156. doi: 10.1016/0006-2952(94)90386-7. [DOI] [PubMed] [Google Scholar]
- Verma A, Kulkarni K. Differential role of dopamine receptor subtypes in thermoregulation and stereotypic behavior in naïve and reserpinized rats. Arch Int Pharmacodyn Ther. 1993;324:17–32. [PubMed] [Google Scholar]
- Vollenweider FX, Gamma A, Liechti M, Huber T. Psychological and cardiovascular effects and short-term sequelae of MDMA (‘ecstasy’) in MDMA-naïve healthy volunteers. Neuropsychopharmacology. 1998;19:241–251. doi: 10.1016/S0893-133X(98)00013-X. [DOI] [PubMed] [Google Scholar]
- Von Huben SN, Lay CC, Crean RD, Davis SA, Katner SN, Taffe MA. Impact of ambient temperature on hyperthermia induced by (+/−)3,4-methylenedioxymethamphetamine in rhesus macaques. Neuropsychopharmacology. 2007;32:673–681. doi: 10.1038/sj.npp.1301078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrydag W, Michel MC. Tools to study beta3-adrenoceptors. Naunyn Schmiedebergs Arch Pharmacol. 2007;374:385–398. doi: 10.1007/s00210-006-0127-5. [DOI] [PubMed] [Google Scholar]
- Winstock AR, Griffiths P, Stewart D. Drugs and the dance music scene: a survey of current drug use patterns among a sample of dance music enthusiasts in the UK. Drug Alcohol Depend. 2001;64:9–17. doi: 10.1016/s0376-8716(00)00215-5. [DOI] [PubMed] [Google Scholar]
- Yang JS, Jamei M, Heydari A, Yeo KR, de la Torre R, Farre M, et al. Implications of mechanism-based inhibition of CYP2D6 for the pharmacokinetics and toxicity of MDMA. J Psychopharmacol. 2006;20:842–849. doi: 10.1177/0269881106065907. [DOI] [PubMed] [Google Scholar]
- Yehuda S, Wurtman RJ. Effects of d-amphetamine and related drugs on colonic temperatures of rats kept at various ambient temperatures. Life Sci. 1972a;11:851–859. doi: 10.1016/0024-3205(72)90101-4. [DOI] [PubMed] [Google Scholar]
- Yehuda S, Wurtman RJ. Release of brain dopamine as probable mechanism for hypothermic effect of d-amphetamine. Nature. 1972b;240:477–478. doi: 10.1038/240477a0. [DOI] [PubMed] [Google Scholar]
- Zarrindast MR, Sadeghi S, Sahebgharani M. Influence of α-adrenoceptor agonists and antagonists on imipramine-induced hypothermia in mice. Pharmacol Toxicol. 2003;93:48–53. doi: 10.1034/j.1600-0773.2003.930107.x. [DOI] [PubMed] [Google Scholar]


