Abstract
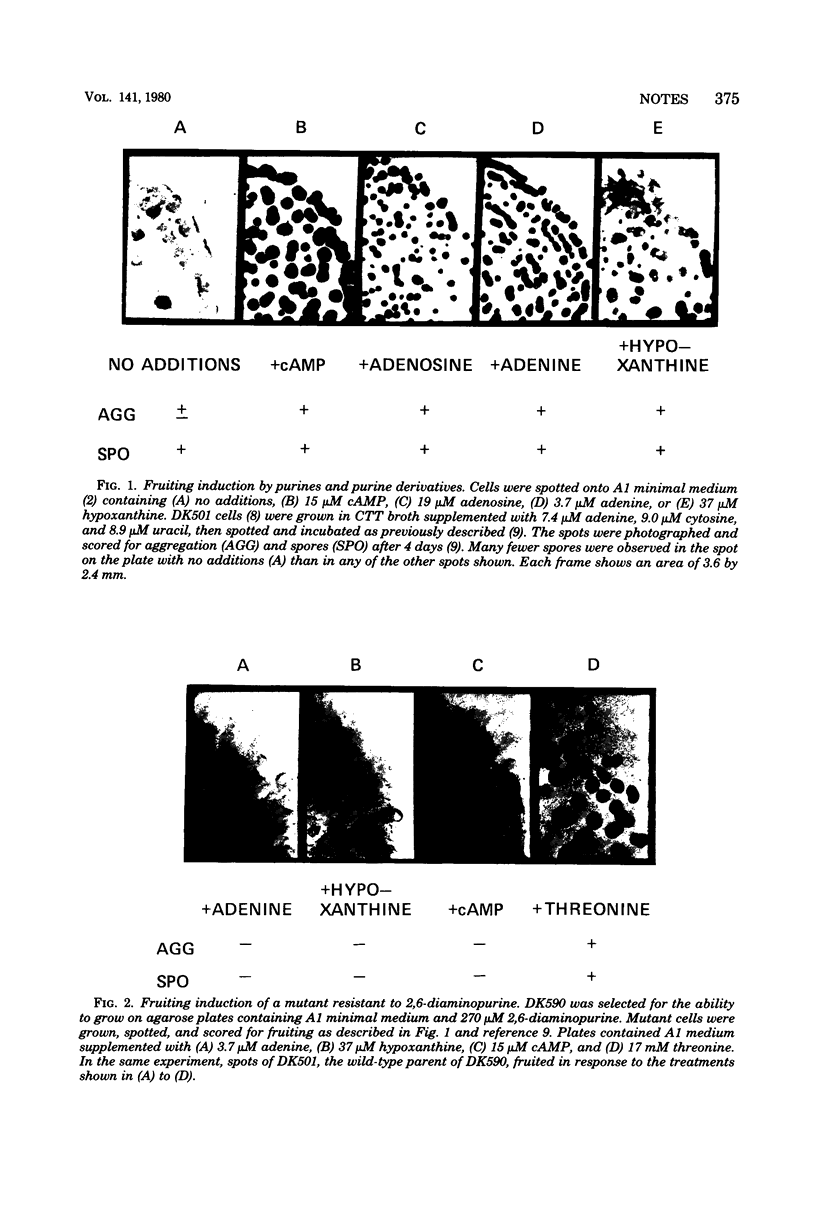
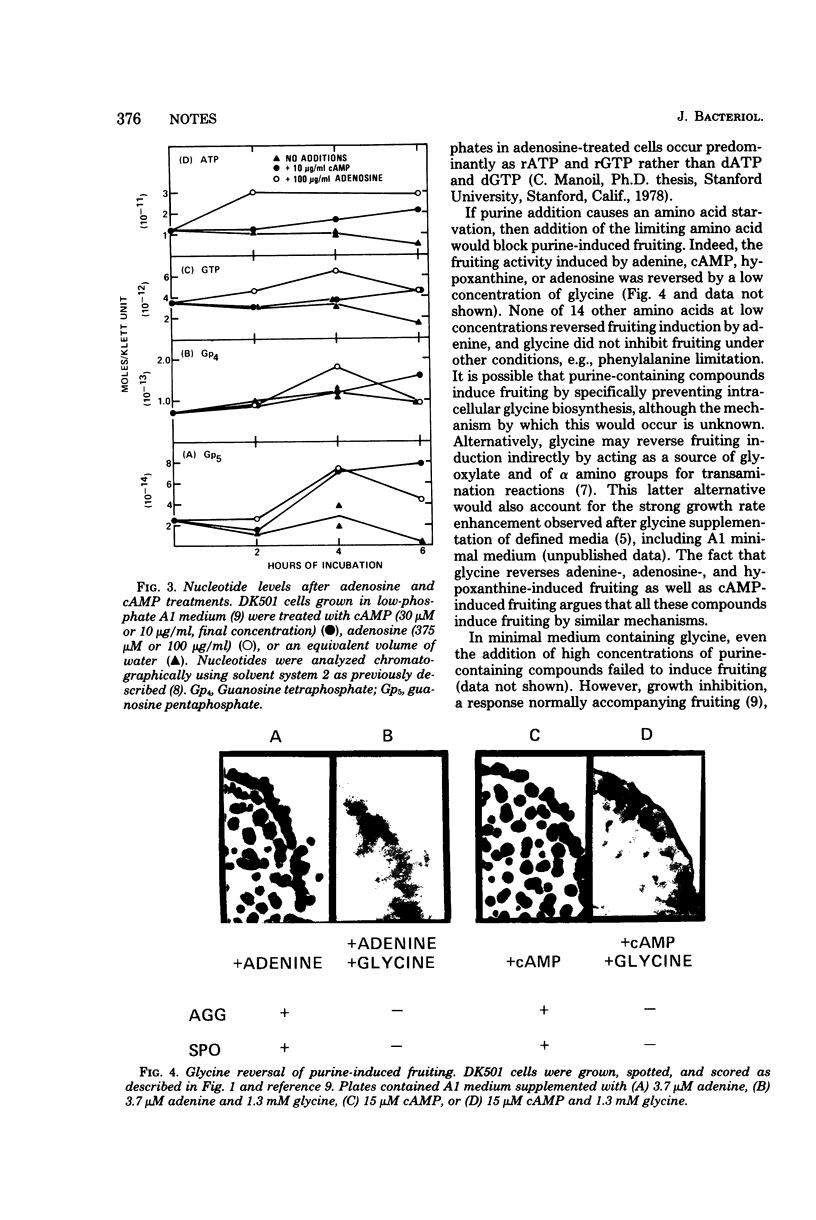
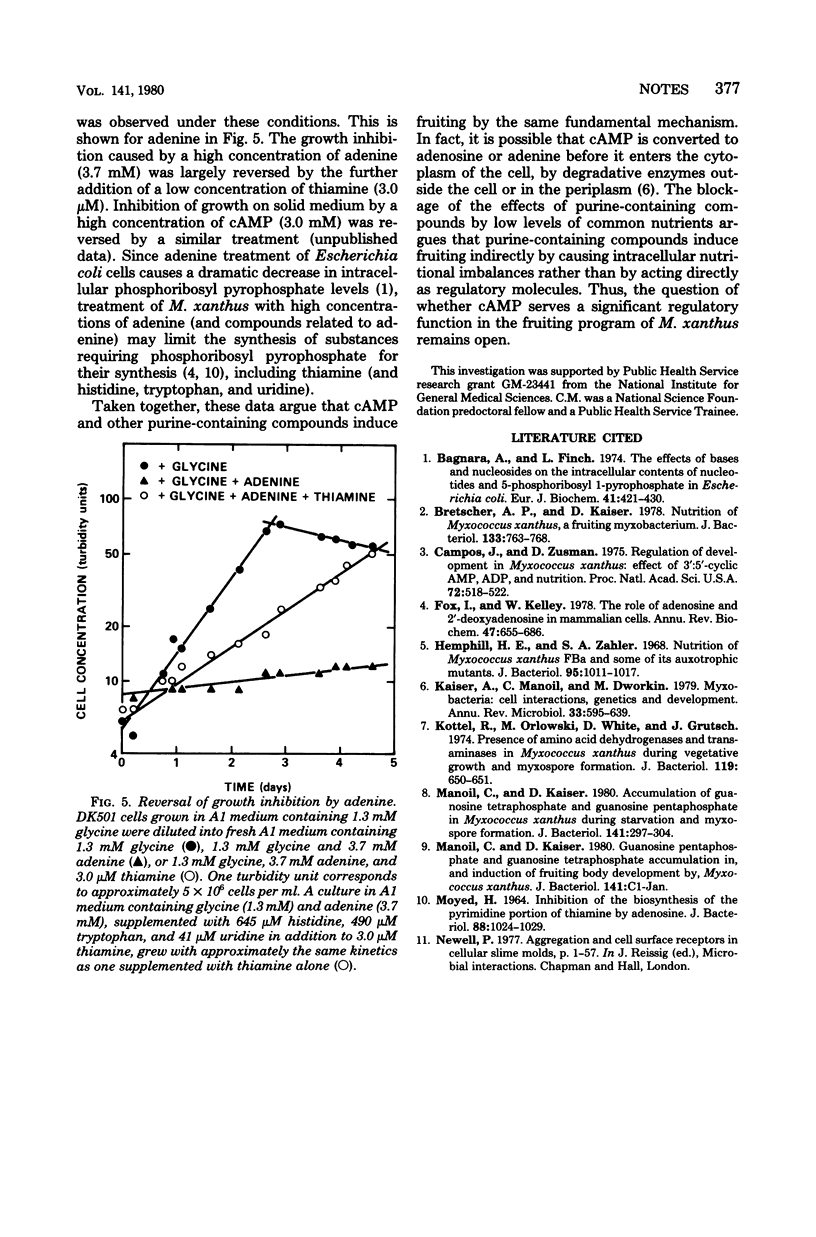
Induction of Myxococcus xanthus fruiting by a number of different purine-containing compounds, including cyclic adenosine 3',5'-monophosphate, is defective in a mutant resistant to 2,6-diaminopurine. Furthermore, the purine-induced fruiting of wild-type cultures is uniquely blocked by a low concentration of added glycine. These results imply that different purine-containing compounds induce fruiting through a single mechanism involving nutritional imbalance.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagnara A. S., Finch L. R. The effects of bases and nucleosides on the intracellular contents of nucleotides and 5-phosphoribosyl 1-pyrophosphate in Escherichia coli. Eur J Biochem. 1974 Feb 1;41(3):421–430. doi: 10.1111/j.1432-1033.1974.tb03283.x. [DOI] [PubMed] [Google Scholar]
- Bretscher A. P., Kaiser D. Nutrition of Myxococcus xanthus, a fruiting myxobacterium. J Bacteriol. 1978 Feb;133(2):763–768. doi: 10.1128/jb.133.2.763-768.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos J. M., Zusman D. R. Regulation of development in Myxococcus xanthus: effect of 3':5'-cyclic AMP, ADP, and nutrition. Proc Natl Acad Sci U S A. 1975 Feb;72(2):518–522. doi: 10.1073/pnas.72.2.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox I. H., Kelley W. N. The role of adenosine and 2'-deoxyadenosine in mammalian cells. Annu Rev Biochem. 1978;47:655–686. doi: 10.1146/annurev.bi.47.070178.003255. [DOI] [PubMed] [Google Scholar]
- Hemphill H. E., Zahler S. A. Nutrition of Myxococcus xanthus FBa and some of its auxotrophic mutants. J Bacteriol. 1968 Mar;95(3):1011–1017. doi: 10.1128/jb.95.3.1011-1017.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser D., Manoil C., Dworkin M. Myxobacteria: cell interactions, genetics, and development. Annu Rev Microbiol. 1979;33:595–639. doi: 10.1146/annurev.mi.33.100179.003115. [DOI] [PubMed] [Google Scholar]
- Kottel R. H., Orlowski M., White D., Grutsch J. Presence of amino acid dehydrogenases and transaminases in Myxococcus xanthus during vegetative growth and myxospore formation. J Bacteriol. 1974 Aug;119(2):650–651. doi: 10.1128/jb.119.2.650-651.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOYED H. S. INHIBITION OF THE BIOSYNTHESIS OF THE PYRIMIDINE PORTION OF THIAMINE BY ADENOSINE. J Bacteriol. 1964 Oct;88:1024–1029. doi: 10.1128/jb.88.4.1024-1029.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoil C., Kaiser D. Accumulation of guanosine tetraphosphate and guanosine pentaphosphate in Myxococcus xanthus during starvation and myxospore formation. J Bacteriol. 1980 Jan;141(1):297–304. doi: 10.1128/jb.141.1.297-304.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]





