Abstract
Background and purpose:
AE9C90CB (N- [(1R, 5S, 6R)-3-azabicyclo [3.1.0] hex-6-ylmethyl]-2-hydroxy-N-methyl-2, 2-diphenylacetamide), a novel muscarinic receptor antagonist, was synthesized for the treatment of overactive bladder. Here we describe the in vitro and in vivo profiles of AE9C90CB for action in bladder over salivary gland and compare it with four agents already in clinical use (tolterodine, oxybutynin, solifenacin and darifenacin).
Experimental approach:
Radioligand binding assay and isolated tissue-based functional assay were used to evaluate affinity, potency, and receptor subtype selectivity of compounds. Inhibition of carbachol-induced increase in intravesicular pressure and salivary secretion were measured in anaesthetized rabbits to assess the functional selectivity.
Key results:
In vitro radioligand binding study using human recombinant muscarinic receptors showed that AE9C90CB had greater affinity for M3 muscarinic receptors with pKi of 9.90 ± 0.11 and was 20-fold more selective for M3 than for M2 muscarinic receptors. AE9C90CB exhibited an unsurmountable antagonism on rat bladder strips (pKB, 9.13 ± 0.12). In anaesthetized rabbits after intravenous administration, AE9C90CB dose dependently inhibited carbachol-induced increase in intravesicular pressure and salivary secretion, and exhibited functional selectivity for urinary bladder over salivary gland which was ninefold better than that of oxybutynin.
Conclusions and implications:
We have identified AE9C90CB, a compound exhibiting moderate selectivity for M3 over M2 receptors but greater selectivity for urinary bladder over salivary gland than oxybutynin, tolterodine, solifenacin and darifenacin. Therefore, AE9C90CB may be a promising compound for the treatment of overactive bladder with reduced potential to cause dry mouth than currently available antimuscarinic drugs.
Keywords: muscarinic receptors, salivary gland, urinary bladder, overactive bladder, oxybutynin, tolterodine, solifenacin, darifenacin
Introduction
Overactive bladder (OAB) is one of the most common causes of bladder control problems. It derives from the uncontrolled activity of the detrusor muscle during bladder filling, leading to the symptoms of urinary urgency and increased frequency of micturition with or without incontinence (Abrams et al., 2003). Symptoms of OAB are highly prevalent in men and women of all ages, and the risk of developing this condition increases with age (Wein and Rovner, 1999), with a sharp increase among patients 40 years of age or older (Stewart et al., 2003). OAB is a disruptive condition that not only can cause embarrassment but also can have a significant impact on the patient's quality of life (Abrams et al., 2000; Bogner et al., 2002). Despite the disturbing nature of OAB, only 20% of patients are on medical treatments, and in addition, OAB is an under-diagnosed condition. Although not life-threatening, the quality of life of patients with OAB is poorer than that of diabetics (Liberman et al., 2001).
Urinary bladder contraction is predominantly under the control of the parasympathetic system (de Groat et al., 1981), where the primary input is via the muscarinic cholinoceptors (Hegde and Eglen, 1999). It is well established that the normal and involuntary bladder contraction involves acetylcholine-induced stimulation of post-ganglionic muscarinic receptors on the detrusor smooth muscle (Andersson, 1997). The smooth muscle of human urinary bladder contains a mixed population of M2 and M3 muscarinic receptors (Hegde and Eglen, 1999; Yamanishi et al., 2002; nomenclature follows Alexander et al., 2009). Although the M2 receptors are the predominant cholinoreceptors present in the bladder, the smaller population of M3 receptors appears to be the most functionally important, and they mediate direct contraction of the detrusor muscle (Chess-Williams et al., 2001; Fetscher et al., 2002). The contribution of M2 receptors to bladder contraction is unclear, and it has been suggested that M2 receptors oppose detrusor relaxation mediated by β-adrenoceptors (Hegde et al., 1997; Yamanishi et al., 2002). The cholinergic nerve terminals of the human bladder are also enriched with presynaptic facilitatory and inhibitory muscarinic receptors, M1 and M4 receptors, respectively, which are involved in the regulation of acetylcholine release.
Although the exact etiology of OAB remains largely unknown, the symptoms of OAB are often associated with uncontrolled spontaneous activity of the detrusor muscle during filling phase that leads to the symptoms of urinary urgency and increased frequency of micturition (Kumar et al., 2003). As bladder contraction is largely regulated by the stimulation of muscarinic receptors, pharmacotherapy with muscarinic receptor antagonists forms the mainstay of treatment for the symptoms of OAB. Despite their proven efficacy, the utility of currently available muscarinic receptor antagonists has been limited by poor tolerability because of the high incidence of treatment-related adverse events such as dry mouth, constipation, blurred vision, headache, somnolence and tachycardia. Of these, dry mouth has been the most unacceptable and is the leading cause of treatment discontinuation and failure. In order to achieve better tolerability, there is an unmet need to target the bladder selectively with this class of drugs. Given the major involvement of M3 receptors in bladder contraction, compounds selective for the M3 muscarinic receptor may prove effective in the treatment of OAB without the systemic adverse effects that would arise from action at multiple muscarinic receptors. The present study was therefore undertaken to evaluate the in vitro pharmacological profile of AE9C90CB by radioligand binding studies and functional studies. Further, we evaluated the effects of AE9C90CB in vivo on carbachol-induced urinary bladder contraction and salivary secretion in anaesthetized rabbits. Four other muscarinic antagonists already in clinical use for OAB (tolterodine, oxybutynin, solifenacin and darifenacin) were evaluated in parallel in all the assays.
Methods
In vitro studies
Binding affinity for human recombinant muscarinic receptor subtypes
The affinity of AE9C90CB and the standard compounds, namely tolterodine, oxybutynin, solifenacin and darifenacin, for M1–M5 receptor subtypes were evaluated by studying their ability to displace specific [3H] N-methyl-scopolamine (NMS) binding from the membranes of Chinese hamster ovary cells overexpressing human M1–M5 receptors. Binding assays were performed according to Moriya et al. (1999) with minor modifications. Briefly, the membrane homogenates (5–20 µg protein), along with the compounds, were incubated in 96 well microtiter plates in 250 µL of assay buffer (HEPES 20 mM, pH 7.4) at 23–27°C for 3 h using [3H] NMS as ligand. Non-specific binding was determined in the presence of 1 µM of atropine. The incubation was terminated by vacuum filtration through 0.1% polyethyleneimine pretreated GF/B fiber filters using Skatron cell harvester (Skatron Instruments, Lier, Norway). Filters were then washed with ice-cold 50 mM Tris HCl buffer (pH 7.4). Filter mats were then dried and transferred to 24 well plates. Finally, radioactivity retained on filters was counted in 500 µL of Supermix in a Microbeta counter (Wallac, Turku, Finland).
Specific binding was used for the analysis. IC50 of the compounds was determined in competitive binding assays. Saturation binding assays were used to determine Kd (apparent dissociation constant) for [3H] NMS. The IC50 value was determined by using the non-linear curve-fitting programme using GraphPad Prism software (GraphPad Software, Inc., La Jolla, CA, USA). In built equation ‘One site competition’ was used for the estimation of IC50. The value of the inhibition constant Ki was calculated from competitive binding studies by using the equation of Cheng and Prusoff (1973):
where [L] was the concentration of ligand used in the particular experiment and Kd was the dissociation constant of ligand [3H] NMS in each receptor subtype. Subtype selectivity was expressed as antilogarithm of difference of mean pKi at one receptor to mean pKi at another receptor. Results were expressed as mean ± SEM for each group and statistical significance for pKi values was assessed by one-way analysis anova by GraphPad Prism software.
Functional studies
All animal care and experimental protocols complied with the guidelines of the Committee for the Purpose of Control and Supervision on Experiments on Animals and were approved by the Institutional Animal Ethics Committee at Ranbaxy Laboratories Ltd.
Male Wistar rats weighing between 250 and 400 g, obtained from the experimental animal facility of Ranbaxy Laboratories Ltd. (Gurgaon, Haryana, India), were used for the experiments. Rats were killed with an overdose of thiopentone sodium (∼300 mg·kg−1). The urinary bladder was isolated and placed in oxygenated Tyrode buffer of the following composition (mM): NaCl 137, KCl 2.7, CaCl2 1.8, MgCl2 0.5, NaHCO3 11.9, NaH2PO4 0.4 and glucose 5.55. The bladder was freed of connective tissue and then cut into four longitudinal sections. These bladder strips were then mounted in the buffer maintained at 30°C and aerated with carbogen (95% oxygen and 5% carbon dioxide) during the entire length of experiment. A resting tension of 1 g was applied. Tissues were equilibrated for 90 min with frequent washes at 15-min intervals. After equilibration, tissues were stimulated by 1 µM carbachol until two reproducible responses were obtained and the contractile responses were recorded on POWERLAB data acquisition system (AD Instruments, Bella Vista, NSW, Australia) or Grass Polygraph Model 7 (Grass Instrument Co., Quincy, MA, USA) and expressed as tension in g.
Cumulative concentration response curves to carbachol in rat bladder were obtained following, which the tissues were allowed to relax to the baseline while they were given a wash every 15 min. Tissues were incubated with different concentrations of AE9C90CB, tolterodine, oxybutynin, solifenacin and darifenacin or vehicle for 20 min, after which a second cumulative concentration response curve to carbachol was obtained.
For rat bladder, Emax was expressed as the percentage of maximum response obtained in control tissue. Percent data were analysed using a non-linear curve-fitting programme (GraphPad Prism). EC50 values were calculated with the help of GraphPad software. Agonist potencies were expressed as pD2, value [the negative log10 (EC50)]. pKB value, an index of antagonist potency, was calculated from EC50 data using the following relationship:
In tissues where Emax attained was less than 50%, pKB was calculated by Kenakin's double reciprocal plot (Kenakin, 1993). The pKB of AE9C90CB, tolterodine, oxybutynin, solifenacin and darifenacin were compared between the different tissues using one-way anova followed by Dunnett's t-test. A P-value of <0.05 was considered to be statistically significant.
Ex vivo brain receptor occupancy study
Brain muscarinic receptor occupancy was determined by measuring the effect of drugs on extracts of mouse whole brain (except cerebellum) with [3H] NMS, using the modified method of Petitet et al. (1999) and Taylor et al. (1992). Mice (Swiss Albino female mice, 25–30 g supplied by the animal facility at Ranbaxy Laboratories Ltd.) were given a single i.v. injection of compound, in a final volume of 1 mL·kg−1. Thirty minutes after drug administration, animals were anaesthetized with sodium pentobarbital (40 mg·kg−1, i.p). Blood was collected in sodium citrate (20%), from the retro-orbital fossa using a capillary. After death, the upper body region and head were perfused transcardially with ice-cold HEPES (20 mM, pH 7.4) buffer to remove free drug from the vasculature. The brain was then removed, the cerebellum discarded and the remainder placed on ice. The brain samples were minced, homogenized using chilled HEPES (20 mM, pH 7.4) buffer. The homogenate was centrifuged at 1000×g, 4°C for 10 min and supernatant obtained was used for receptor binding assay. 200–250 µg protein was used for receptor binding assay in a total volume of 50 µL using 0.5 nM of [3H] NMS as ligand. Incubations were carried out for 3 h at room temperature and reaction was terminated by filtration on Whatman GF/B filtermats (Whatman, Manchester, UK) using 50 mM TRIS buffer (pH 7.4). The filtermats were dried and radioactivity retained on filters was counted in 500 µL of Supermix in a Microbeta counter.
Estimation of muscarinic activity in plasma
Plasma was obtained from the blood sample by centrifuging at 1000×g for 5 min. Ex vivo receptor binding assay was performed to estimate the muscarinic activity in plasma. Aliquots (25 µL) of plasma were incubated with 1 nM of [3H] NMS at room temperature for 3 h using 80–100 µg of protein. The samples were immediately filtered under vacuum through Whatman GF/B filters, which were rinsed twice using 50 mL TRIS buffer. The filtermats were dried and transferred to 24 well plates (PET A No cross talk). Radioactivity retained on filters was counted in 500 µL of Supermix in a Microbeta counter.
In vivo studies
The study was conducted on male New Zealand White rabbits weighing between 1.5 and 3.0 kg. Rabbits were obtained from the Experimental Animal Facility, Ranbaxy Laboratories Ltd. The animals were housed in standard cages and maintained at a temperature of 24 ± 2°C with controlled illumination to provide a light–dark cycle of 12 h. The treatment groups comprised five to six animals each.
Anaesthesia and surgery
The rabbits were anaesthetized with a slow intravenous infusion of urethane (1.5 g·kg−1). Both femoral arteries were catheterized with polyethylene tubing. One of these was connected to a blood pressure transducer (Statham P 10 EZ, Spectramed, Oxnard, CA, USA) and the other was used for i.a. injection of carbachol. Both femoral veins were catheterized with polyethylene tubing for i.v administration of test compounds and infusion of normal saline (to maintain homeostasis), respectively. Through a midline incision, the bladder was exposed and the ureters were bilaterally identified, ligated, and catheterized to drain the urine to the exterior. A polyethylene catheter was inserted into the urinary bladder through an incision in the proximal urethra. The bladder was drained and subsequently filled with 12–17 mL of saline to attain a basal intravesical pressure of 7–10 cm of H2O at 37°C. The catheter inserted into the bladder was later connected to a pressure transducer (Statham P 10 EZ) for recording of intravesicular pressure. Intravesicular pressure and blood pressure were recorded on a polygraph (Grass model 7H). The animal was stabilized for 30 min subsequent to surgery.
Experimental procedure
Rabbits were divided into six groups receiving vehicle (n = 5), AE9C90CB (n = 6), oxybutynin (n = 6), tolterodine (n = 6), darifenacin (n = 6) and solifenacin (n = 5). Urinary bladder contraction and salivary secretion were evoked by i.a. injection of a submaximal dose of carbachol (1.5 µg·kg−1) as determined by a dose-response curve to carbachol (0.1, 0.3, 1, 3 and 10 µg·kg−1) that was generated in a separate set of animals prior to the start of the study. For the measurement of saliva, the buccal cavity was thoroughly wiped and a pre-weighed cotton pad was placed in it. Two minutes after carbachol administration, the cotton pad was removed and weighed. At least two stable responses were obtained before administration of the test compounds. AE9C90CB, tolterodine, oxybutynin, solifenacin and darifenacin were administered as an i.v. bolus in increasing doses (five to six doses). Fifteen minutes after each dose of the antagonists, a dose-response curve to carbachol was generated. Animals were left for 30 min between the doses. All the drugs were dissolved in milli Q water. In the vehicle-treated group, vehicle was administered six times and responses to carbachol were obtained over 3 h, which was the duration of the experiment in all groups.
Effects of vehicle and drugs on carbachol-induced urinary bladder contractions and salivary secretion are expressed as percentages of the basal responses. ED50 values (dose of antagonist producing 50% of the basal responses) for the above-mentioned parameters were computed from non-linear regression analysis of percent basal response using GraphPad Prism software, version 2.01; bladder versus salivary gland selectivity was calculated from the formula
 |
Additionally, bladder versus salivary gland selectivity was also expressed relative to that for oxybutynin.
Data are expressed as mean ± SEM of five to six animals. Differences between the ED50 values for the effect on carbachol-induced increase in intravesical pressure for AE9C90CB, oxybutynin, tolterodine, darifenacin and solifenacin were analysed using one-way anova followed by Tukey's multiple comparison test on GraphPad Prism. Differences between bladder and salivary selectivity were also analysed. P < 0.05 was considered significant.
Materials
AE9C90CB; tolterodine, darifenacin and solifenacin were synthesized at Ranbaxy Laboratories Ltd. Oxybutynin was obtained from Orgamol (Evionnaz, Switzerland). All of the chemicals used were of AR grade and were purchased from Sigma (Ronkonkoma, NY, USA). Stock solutions of AE9C90CB, tolterodine, oxybutynin, solifenacin and darifenacin were prepared fresh every day. Subsequent dilutions were prepared from the stock in MilliQ/assay buffer.
Results
In vitro studies
Binding affinity of AE9C90CB, tolterodine, oxybutynin, solifenacin and darifenacin for human recombinant muscarinic receptor subtypes
AE9C90CB and all the standard compounds inhibited [3H] NMS binding to human muscarinic M1, M2, M3, M4, and M5 receptors. In competitive binding assays, AE9C90CB exhibited significantly higher affinity for muscarinic M3, M1, M4 and M5 receptor subtypes compared with the muscarinic M2 subtype (Table 1). Oxybutynin, solifenacin and darifenacin exhibited lower affinity for M2 receptors than for the M3 receptors. In contrast, tolterodine displayed similar affinity for all the subtypes of the muscarinic receptors. The observed affinities for M1, M3, M4 and M5 muscarinic receptors were significantly greater for AE9C90CB compared with those found for tolterodine, oxybutynin, solifenacin and darifenacin.
Table 1.
Binding affinities of AE9C90CB, tolterodine, oxybutynin, solifenacin and darifenacin at human recombinant muscarinic receptor subtypes
| Compound | pKi | ||||
|---|---|---|---|---|---|
| M1 | M2 | M3 | M4 | M5 | |
| AE9C90CB | 9.7 ± 0.11 | 8.6 ± 0.02# | 9.9 ± 0.11 | 9.5 ± 0.05 | 9.5 ± 0.08 |
| Tolterodine | 8.7 ± 0.17* | 8.4 ± 0.13 | 8.4 ± 0.17* | 8.4 ± 0.07* | 8.8 ± 0.15* |
| Oxybutynin | 8.6 ± 0.01* | 8.1 ± 0.09# | 8.8 ± 0.15* | 8.7 ± 0.04* | 7.9 ± 0.04* |
| Solifenacin | 7.6 ± 0.02* | 7.1 ± 0.1# | 7.7 ± 0.04* | 6.8 ± 0.08* | 7.2 ± 0.06* |
| Darifenacin | 7.5 ± 0.04* | 7.2 ± 0.03# | 8.6 ± 0.08* | 7.3 ± 0.07* | 7.9 ± 0.03* |
Data are expressed as mean ± SEM of five experiments.
P < 0.01, significantly different from corresponding values for pKi of AE9C90CB at each receptor subtype versus pKi of tolterodine, oxybutynin, darifenacin and solifenacin.
P < 0.01. significantly different from pKi at M3 receptor for each compound.
Subtype selectivity
AE9C90CB exhibited greater selectivity for M3 over M2 receptors when compared with tolterodine, oxybutynin and solifenacin, but its selectivity was comparable to that shown by darifenacin. The selectivity of these five antagonists for the M3 receptor was derived from the pKi values in Table 1 and is presented in Table 2, as fold selectivity, that is, ratio of pKi at the pair of receptors compared (M1/M3, M2/M3, etc.).
Table 2.
Selectivity of AE9C90CB, tolterodine, oxybutynin, solifenacin and darifenacin at human recombinant muscarinic receptor subtypes
| Compound | Fold selectivity | |||
|---|---|---|---|---|
| M1/M3 | M2/M3 | M4/M3 | M5/M3 | |
| AE9C90CB | 2 | 20 | 3 | 3 |
| Tolterodine | 1 | 1 | 1 | 0.4 |
| Oxybutynin | 2 | 5 | 1 | 8 |
| Solifenacin | 1 | 4 | 8 | 3 |
| Darifenacin | 13 | 25 | 20 | 5 |
Fold selectivity is antilog of the difference between pKi at muscarinic M3 receptors and those at M1, M2, M4, M5 subtypes.
In vitro functional studies
In rat bladder strips, carbachol exerted a concentration-dependent increase in the force of contraction with a pD2 of 5.89 ± 0.06 and an Emax of 5.97 ± 0.25 g, and AE9C90CB exhibited a concentration-dependent, unsurmountable antagonism to this effect (Figure 1A). Solifenacin. tolterodine, oxybutynin and darifenacin also exhibited a similar trend and shifted the concentration–contraction response curve of carbachol to the right in a concentration-dependent manner (Figure 1B–E). The corresponding pKB values (index of functional antagonism) are summarized in Table 3 and show that AE9C90CB was the most potent (up to 15-fold more potent than solifenacin) antagonist of carbachol-induced responses in rat bladder strips, of all the other compounds tested.
Figure 1.
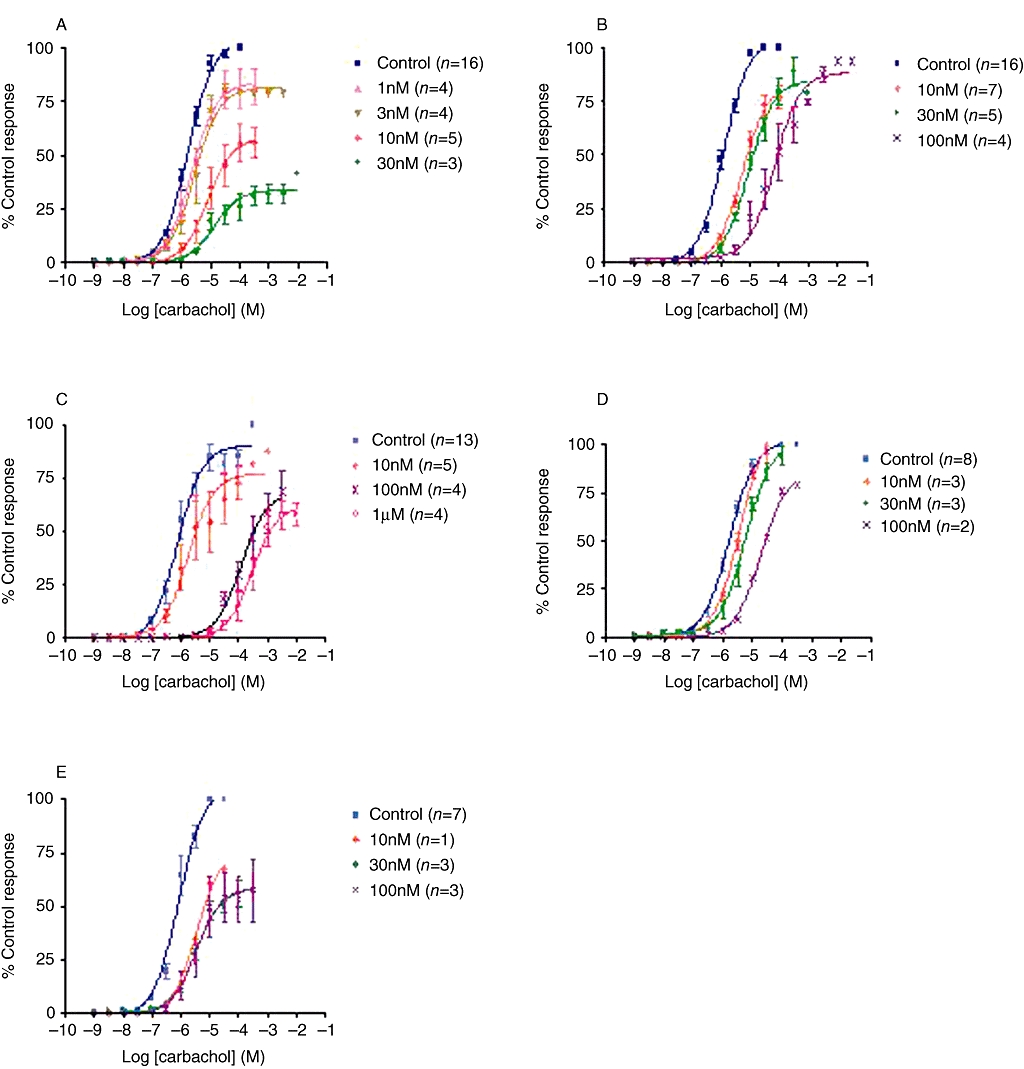
Double cumulative concentration response curves to carbachol-evoked contractions in strips of rat bladder. Data are expressed as % maximum response in control tissue. Each data point represents mean ± SEM of the number of experiments shown in parentheses. (A) AE9C90CB, (B) tolterodine, (C) oxybutynin, (D) solifenacin and (E) darifenacin all produced concentration-dependent rightward shifts in the concentration response curve to carbachol in rat bladder strips.
Table 3.
Functional antagonistic potencies of AE9C90CB, tolterodine, oxybutynin, solifenacin and darifenacin in rat bladder strips
| Compound | pKB |
|---|---|
| Bladder (M3) | |
| AE9C90CB (n = 16) | 9.13 ± 0.12 |
| Tolterodine (n = 16) | 8.67 ± 0.08* |
| Oxybutynin (n = 13) | 9.05 ± 0.19 |
| Solifenacin (n = 8) | 7.94 ± 0.09* |
| Darifenacin (n = 7) | 8.55 ± 0.15* |
Functional antagonism is expressed as pKB (mean ± SEM); n, number of experiments.
(P < 0.05) significantly different from value for AE9C90CB tolterodine; comparative potency ratio of AE9C90CB versus tolterodine: 3; comparative potency ratio of AE9C90CB versus oxybutynin: 1; comparative potency ratio of AE9C90CB versus solifenacin: 15; comparative potency ratio of AE9C90CB versus darifenacin: 4.
Ex vivo brain receptor occupancy study
In brain samples taken from mice, 30 min after i.v. injection, AE9C90CB exhibited 0.9% central muscarinic receptor occupancy (Figure 2). This value was well below those found for tolterodine, oxybutynin, solifenacin and darifenacin. These results suggest that AE9C90CB showed the least tendency to penetrate the blood brain barrier, indicating a low potential for CNS-related side effects.
Figure 2.
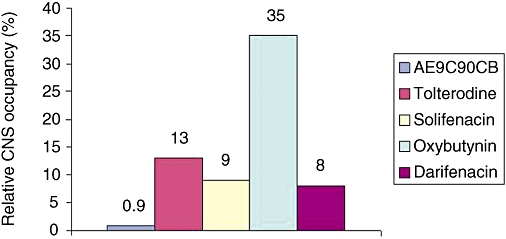
Relative CNS occupancy of muscarinic receptors was calculated as the ratio of binding of [3H] NMS in brain homogenate and plasma samples taken 30 min after in vivo injection of drug. Each group had eight animals.
In vivo studies
In anaesthetized rabbits, the basal mean changes in intravesical pressure subsequent to injection of the standard dose of carbachol (1.5 µg·kg−1) were in the range of 9.1–10.2 cm of H2O. The mean basal salivation evoked by this dose of carbachol was in the range of 0.5–0.7 g. No statistically significant differences in the basal carbachol-induced responses were evident among the different treatment groups (Table 4). Repeated administration of vehicle did not elicit any appreciable change in the intravesical pressure and salivary response to carbachol administration from the basal values over the 3 h experimental period.
Table 4.
Basal values of carbachol-induced changes in anaesthetized rabbits
| Treatment group | Number of animals | Intravesicular pressure (cm H2O) | Salivation (g) |
|---|---|---|---|
| Vehicle | 5 | 9.1 ± 0.8 | 0.6 ± 0.1 |
| Oxybutynin | 6 | 9.5 ± 0.8 | 0.5 ± 0.1 |
| Tolterodine | 6 | 10.1 ± 1.0 | 0.7 ± 0.1 |
| Solifenacin | 5 | 10.2 ± 0.4 | 0.5 ± 0.1 |
| Darifenacin | 6 | 9.8 ± 1.2 | 0.5 ± 0.1 |
| AE9C90CB | 6 | 9.3 ± 1.4 | 0.5 ± 0.1 |
All the muscarinic antagonists tested produced a dose-dependent inhibition of carbachol-induced increase in intravesical pressure and salivary secretion (Figure 3), and the corresponding ED50 values (in µg·kg−1) are shown in Tables 4 and 5. AE9C90CB, darifenacin and solifenacin were more potent in inhibiting the carbachol-induced increase in intravesical pressure than the carbachol-evoked salivation (Table 5, Figure 3A,D,E). However, oxybutynin was more potent in inhibiting the effect on salivation (Table 5, Figure 3B), whereas tolterodine showed equal potency in the inhibition of either effect (Table 5, Figure 3C).
Figure 3.
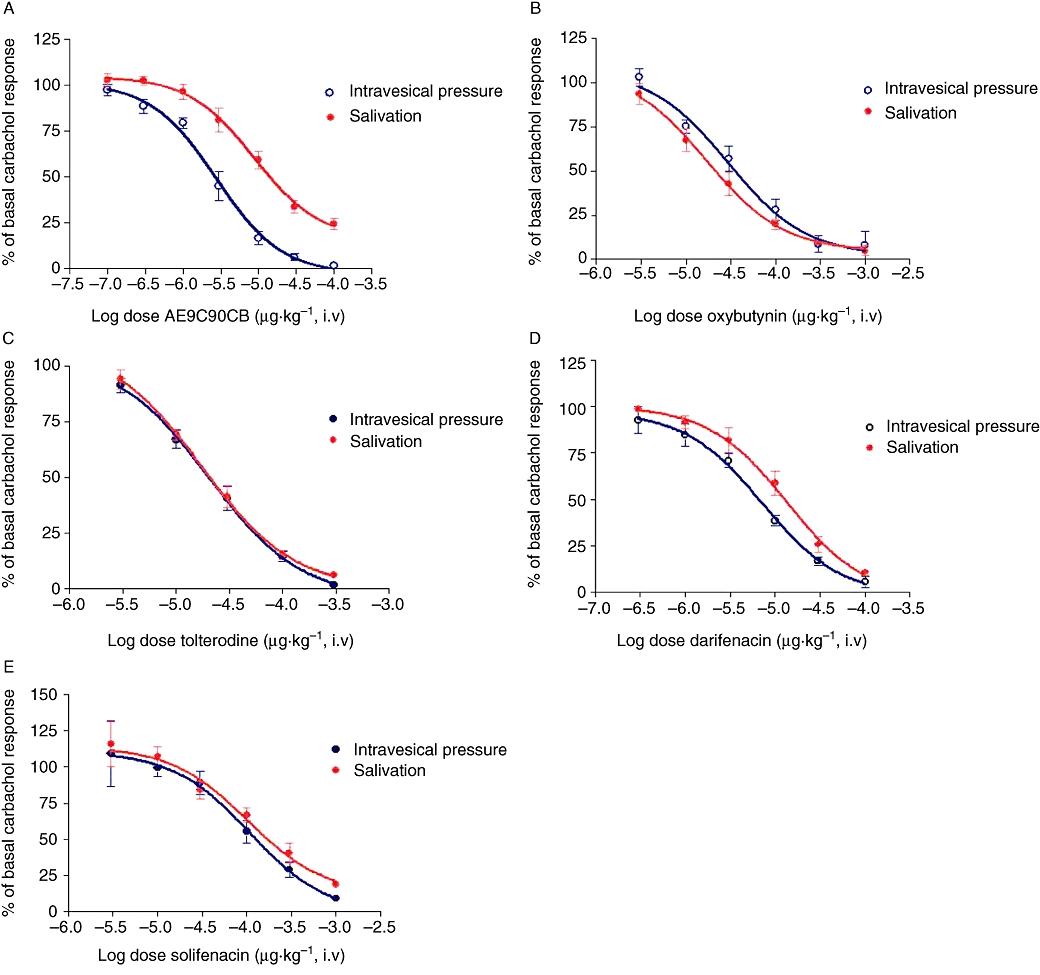
Dose-response curves of muscarinic antagonists drugs on carbachol-induced urinary bladder contractions and salivary secretion in rabbits were determined and are expressed as percentage of the basal responses. Data are expressed as mean ± SEM of five to six animals. Compound was administered as an intravenous bolus in increasing doses. Fifteen minutes after each dose of the antagonist, a dose-response curve of carbachol was generated. (A) AE9C90CB (seven doses). (B) Oxybutynin (six doses). (C) Tolterodine (five doses). (D) Darifenacin (six doses). (E) Solifenacin (six doses).
Table 5.
Antagonism of carbachol-induced changes by AE9C90CB, tolterodine, oxybutynin, solifenacin or darifenacin in anaesthetized rabbits
| Compound | ED50 (µg·kg−1); i.v. (mean ± SEM) | Bladder versus salivary gland selectivity (mean ± SEM) | Bladder versus salivary gland selectivity (fold of oxybutynin) | |
|---|---|---|---|---|
| Intravesicular pressure | Salivation | |||
| Oxybutynin | 49 ± 16 | 25 ± 5 | 0.6 ± 0.2*** | 1 |
| Tolterodine | 22 ± 4 | 23 ± 3 | 1.1 ± 0.2*** | 2 |
| Solifenacin | 148 ± 35*** | 194 ± 36 | 1.7 ± 0.5*** | 3 |
| Darifenacin | 6 ± 0.8 | 14 ± 0.3 | 2.3 ± 0.5*** | 4 |
| AE9C90CB | 3 ± 0.4 | 16 ± 3.4 | 5.4 ± 0.7 | 9 |
ED50 is defined as the dose inhibiting responses to 50% of control responses to carbachol and was calculated from non-linear regression analysis of percent control response using GraphPad Prism. Bladder versus salivary gland selectivity is defined as ED50 salivation/ED50 intravesicular pressure from the individual animals.
P < 0.001 significantly different from corresponding values for AE9C90CB; one-way anova; Tukey's multiple comparison test.
From these data, the bladder versus salivary gland selectivity (essentially the ratio of the corresponding ED50 values) was highest for AE9C90CB. As oxybutynin is commonly used in patients, this organ selectivity can also be expressed relative to that of oxybutynin, and such comparison (Table 5, last column) showed that although all the newer agents were more organ selective than oxybutynin, AE9C90CB was still the most selective for bladder versus salivary gland.
Discussion
Several species have been used to investigate urinary bladder function. Among them, rats and rabbits are the most commonly used species. There are numerous studies that provide detailed information regarding urinary bladder morphology, cellular biochemistry, contractile function and normal micturition in these species (Levin et al., 1983, 1990, 1994; Longhurst et al., 1990; Steers, 1994; Damaser et al., 2000). In mammals, acetylcholine is the principal neurotransmitter that acts at muscarinic receptors of the detrusor muscle leading to contraction of the smooth muscle; therefore, compounds that act as antagonists at these receptors would reduce bladder overactivity. Detrusor smooth muscle cells are densely populated with two of the five muscarinic receptor subtypes (M2 and M3 receptors), and it is the M2 receptors which are predominantly expressed and account for >80% of the muscarinic receptors in the bladder (Wang et al., 1995). However, the M3 receptors, although well outnumbered by the M2 receptors, are mainly responsible for eliciting detrusor contraction (Fetscher et al., 2002). These findings gave rise to the initial development of M3 receptor-selective compounds as treatment for OAB.
However, M3 receptors are also present in many different tissues such as the salivary glands, eyes, brain and gastrointestinal tract. Indeed, M3 receptors mediate salivary secretion, and ∼90% of the receptors in salivary glands are of this receptor subtype (Wang et al., 1995). Therefore, the specific antagonists of M3 receptors would also be expected to cause dry mouth, which is the most common side effect observed with this class of drugs and the main reason for discontinuation of treatment (Abrams et al., 2000). A further complication is that the detrusor contractions are not exclusively mediated by M3 receptors (Fetscher et al., 2002). The M3 receptor subtypes mediate normal contraction, whereas M2 receptors are involved in the detrusor contraction after selective M3 receptor inactivation. The finding was corroborated by knockout (KO) studies in mice that demonstrated the persistence of a small, direct contractile response in the urinary bladder of M3 receptor-KO mice. The residual response was completely lost in M2 receptor-KO mice, demonstrating the role of M2 receptors in the direct contraction of the urinary bladder (Matsui et al., 2002). However, the magnitude of the contractile response elicited by M2 receptors was very small (∼5%) compared with the 95% response mediated by M3 receptors. Furthermore, M2 receptors have been shown to mediate urinary bladder contractions indirectly by enhancing M3 receptor-mediated contractions and inhibiting relaxation mediated by β-adrenoceptors (Ehlert et al., 2005).
Overall, the question of M3 versus M2 receptor selectivity for the treatment of OAB has been widely debated. In OPERA (OAB: Performance of Extended Release Agents) and STAR (solifenacin succinate OD flexible dosing with 5 and 10 mg doses and tolterodine ER 4 mg OD as an active comparator in randomized) trials (Diokno et al., 2003; Chapple et al., 2005), where an M3 receptor selective drug (oxybutynin or solifenacin) was compared with a M3-M2 non-selective drug (tolterodine), the M3 selective drugs were considerably better in terms of efficacy over the M3-M2 non-selective compound, thereby emphasizing the importance of moderate selectivity for M3 over M2 receptors. Subsequent development focused on organ selectivity (uroselectivity) rather than receptor selectivity, seeking compounds which were selective for bladder over salivary gland, along with high selectivity for M3 receptors (Tiwari and Naruganahalli, 2006). The organ selectivity of a drug does not necessarily derive from its receptor selectivity (Kenakin, 1982), suggesting that other properties such as binding characteristics may also be important in addition to selectivity at receptor subtypes. Our work with AE9C90CB was based on these considerations of organ selectivity.
We found AE9C90CB to have 20-fold selectivity as an antagonist at M3 over M2 muscarinic receptors. It also had a functional selectivity of ninefold for bladder smooth muscle, using the pKb of AE9C90CB generated in rat bladder strips, in comparison with the pKi obtained in the PARC-5 cell line, measuring changes in intracellular calcium assays (data not shown). Our results also provide the first evaluation of organ selectivity for AE9C90CB in vivo using a rabbit model to demonstrate functional antagonism of M3 muscarinic receptors in urinary bladder and in the salivary gland, simultaneously in the same animal (Naruganahalli et al., 2007). Thus, we assessed organ selectivity subsequent to establishing receptor selectivity and were able to gain a better estimate of the potential for side effects at the same time as measuring its potency on bladder functions. The physiology of rabbit bladder is similar to that of the human bladder (Tobin, 1995; Choppin et al., 1998; Fetscher et al., 2002), and it would seem that the responsiveness of rabbit bladder to muscarinic receptor antagonists might closely reflect that of human bladder.
We examined the bladder versus salivary selectivity of AE9C90CB and four other currently available muscarinic receptor antagonists in our rabbit model, using a carbachol-induced increase in intravesical pressure and salivary secretion. We selected the dose of carbachol (1.5 µg·kg−1) in order to induce a close to physiological increase in intravesicular pressure, as reported in an in vitro whole-bladder study where the maximum pressure generated by the rabbit bladder was ∼12 cm H2O (Levin et al., 1983), close to the intravesicular pressure (9–10 cm H2O) elicited by carbachol in our study. Although all the drugs at the highest doses almost completely inhibited the carbachol-induced increase in intravesicular pressure and salivary response, the separation between the dose-response curves (see Figure 3) which represents the differing sensitivity of the two effects (bladder contractions or saliva secretion) to blockade was greatest for AE9C90CB. Quantitative estimates of this organ selectivity by comparing ED50 values showed AE9C90CB to be twice as selective as darifenacin and ninefold better than oxybutynin.
Another potential side effect of these M3 antagonists reflects their potency at M1 receptors, at which they were nearly effective as they were at M3 receptors (Table 2). The M1 receptors are typically found in the CNS, and our assessment of the occupancy of brain receptors showed that here AE9C90CB had the lowest values, 10-fold less than solifenacin and almost 40-fold less than oxybutynin. These data would suggest that AE9C90CB has a correspondingly lower liability to induce CNS-related side effects. Thus, overall, AE9C90CB exhibited greater receptor (M2 vs. M3) and organ (bladder vs. salivary gland) selectivity combined with a lower penetration into the CNS than the four clinically used muscarinic antagonists, tolterodine, oxybutynin, solifenacin, and darifenacin.
The prevalence of side effects in the present available muscarinic antagonists for treatment of OAB has led to the development of different formulations in order to reduce these side effects.
Extended-release (ER) formulations of oxybutynin, tolterodine, trospium chloride and other agents do reduce adverse events, especially the rate of dry mouth, compared with immediate-release formulations. Another variation is to use transdermal drug delivery systems such as skin patches and topical gel, which offer improved pharmacokinetics, convenient dosing schedules, and substantially lower incidence of adverse events. Many patients may find the option of transdermal treatment appealing because it avoids the addition of yet another pill to their personal polypharmacy. But even though these drug delivery strategies might ultimately improve the clinical efficacy of existing dugs for OAB, compounds with inherently decreased side-effect potential, that is, increased organ selectivity will still be needed.
In conclusion, our data have clearly demonstrated that AE9C90CB is a potent and selective M3 muscarinic antagonist, with higher organ selectivity for the urinary bladder and improved efficacy, compared with the currently available antimuscarinic drugs. Hence, if the favourable pharmacological profile observed in the rabbit model also translates into humans, AE9C90CB may be a promising drug for the treatment of urinary urgency and frequency in patients with OAB syndrome.
Acknowledgments
We would like to acknowledge the Department of Biotechnology at Ranbaxy for providing us with the cell lines, over expressing the muscarinic receptors.
Glossary
Abbreviations:
- NMS
[3H] N-methyl-scopalamine
- OAB
overactive bladder
Conflict of interest
There is no conflict of interest.
References
- Abrams P, Kelleher CJ, Kerr LA, Rogers RG. Overactive bladder significantly affects quality of life. Am J Manag Care. 2000;6:S580–S590. [PubMed] [Google Scholar]
- Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, et al. The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Urology. 2003;61:37–49. doi: 10.1016/s0090-4295(02)02243-4. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to receptors and channels (GRAC), 4th edn. Br J Pharmacol. 2009;158(Suppl. 1):S1–S254. doi: 10.1111/j.1476-5381.2009.00499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson KE. The overactive bladder: pharmacologic basis of drug treatment. Urology. 1997;50:74–84. doi: 10.1016/s0090-4295(97)00595-5. [DOI] [PubMed] [Google Scholar]
- Bogner HR, Gallo JJ, Sammel MD, Ford DE, Armenian HK, Eaton WW. Urinary incontinence and psychological distress in community-dwelling older adults. J Am Geriatr Soc. 2002;50:489–495. doi: 10.1046/j.1532-5415.2002.50115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapple CR, Martinez-Garcia R, Selvaggi L, Toozs-Hobson P, Warnack W, Drogendijk T, et al. A comparison of the efficacy and tolerability of solifenacin succinate and extended release tolterodine at treating overactive bladder syndrome: results of the STAR trial. Eur Urol. 2005;48:464–470. doi: 10.1016/j.eururo.2005.05.015. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Prusoff WH. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Chess-Williams R, Chapple CR, Yamanishi T, Yasuda K, Sellers DJ. The minor population of M3-receptors mediate contraction of human detrusor muscle in vitro. J Auton Pharmacol. 2001;21:243–248. doi: 10.1046/j.1365-2680.2001.00231.x. [DOI] [PubMed] [Google Scholar]
- Choppin A, Eglen RM, Hegde SS. Pharmacological characterization of muscarinic receptors in rabbit isolated iris sphincter muscle and urinary bladder smooth muscle. Br J Pharmacol. 1998;124:883–888. doi: 10.1038/sj.bjp.0701920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damaser MS, Whitbeck C, Barreto M, Horan P, Benno H, O’Connor LJ, et al. Comparative physiology and biochemistry of rat and rabbit urinary bladder. BJU Int. 2000;85:519–525. doi: 10.1046/j.1464-410x.2000.00444.x. [DOI] [PubMed] [Google Scholar]
- Diokno AC, Appell RA, Sand PK, Dmochowski RR, Gburek BM, Klimberg IW, et al. Prospective, randomized, double-blind study of the efficacy and tolerability of the extended-release formulations of oxybutynin and tolterodine for overactive bladder: results of the OPERA trial. Mayo Clin Proc. 2003;78:687–695. doi: 10.4065/78.6.687. [DOI] [PubMed] [Google Scholar]
- Ehlert FJ, Griffin MT, Abe DM, Vo TH, Taketo MM, Manabe T, et al. The M2 muscarinic receptor mediates contraction through indirect mechanisms in mouse urinarybladder. J Pharmacol Exp Ther. 2005;313:368–378. doi: 10.1124/jpet.104.077909. [DOI] [PubMed] [Google Scholar]
- Fetscher C, Fleichman M, Schmidt M, Krege S, Michel MC. M(3) muscarinic receptors mediate contraction of human urinary bladder. Br J Pharmacol. 2002;136:641–643. doi: 10.1038/sj.bjp.0704781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groat WC, Nadelhaft I, Milne RJ, Booth AM, Morgan C, Thor K. Organization of the sacral parasympathetic reflex pathways to the urinary bladder and large intestine. J Auton Nerv Syst. 1981;3:135–160. doi: 10.1016/0165-1838(81)90059-x. [DOI] [PubMed] [Google Scholar]
- Hegde SS, Eglen RM. Muscarinic receptor subtypes modulating smooth muscle contractility in the urinary bladder. Life Sci. 1999;64:419–428. doi: 10.1016/s0024-3205(98)00581-5. [DOI] [PubMed] [Google Scholar]
- Hegde SS, Choppin A, Bonhaus D, Briaud S, Loeb M, Moy TM, et al. Functional role of M2 and M3 muscarinic receptors in the urinary bladder of rats in vitro and in vivo. Br J Pharmacol. 1997;120:1409–1418. doi: 10.1038/sj.bjp.0701048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenakin T. Pharmacologic Analysis of Drug Receptor Interaction. 2nd edn. New York: Raven Press Ltd; 1993. [Google Scholar]
- Kenakin TP. Organ selectivity of drugs, alternative to receptor selectivity. Trends Pharmacol Sci. 1982;3:153–156. [Google Scholar]
- Kumar V, Templeman L, Chapple CR, Chess-Williams R. Recent developments in the management of detrusor overactivity. Curr Opin Urol. 2003;13:285–291. doi: 10.1097/00042307-200307000-00004. [DOI] [PubMed] [Google Scholar]
- Levin RM, Brendler K, Wein AJ. Comparative pharmacological response of an in vitro whole bladder preparation (rabbit) with response of isolated smooth muscle strips. J Urol. 1983;130:377–381. doi: 10.1016/s0022-5347(17)51172-6. [DOI] [PubMed] [Google Scholar]
- Levin RM, Longhurst PA, Kato K, McGuire EJ, Elbadawi A, Wein AJ. Comparative physiology and pharmacology of the cat and rabbit urinary bladder. J Urol. 1990;143:848–852. doi: 10.1016/s0022-5347(17)40115-7. [DOI] [PubMed] [Google Scholar]
- Levin RM, Haugaard N, Eika B, Packard D, McGuire EJ, Elbadawi A, et al. Comparative biochemical characteristics of the cat and rabbit urinary bladder. Neurourol Urodyn. 1994;13:307–314. doi: 10.1002/1520-6777(1994)13:3<307::aid-nau1930130312>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Liberman JN, Hunt TL, Stewart WF, Wein A, Zhou Z, Herzog AR, et al. Health-related quality of life among adults with symptoms of overactive bladder: results from a U.S. community-based survey. Urology. 2001;57:1044–1050. doi: 10.1016/s0090-4295(01)00986-4. [DOI] [PubMed] [Google Scholar]
- Longhurst PA, Kang J, Wein AJ, Levin RM. The influence of intravesical volume upon contractile responses of the whole bladder preparation from streptozotocin-diabetic rats. Gen Pharmacol. 1990;21:687–692. doi: 10.1016/0306-3623(90)91018-m. [DOI] [PubMed] [Google Scholar]
- Matsui M, Motomura D, Fujikawa T, Jiang J, Takahashi S, Manabe T, et al. Mice lacking M2 and M3 muscarinic acetylcholine receptors are devoid of cholinergic smooth muscle contractions but still viable. J Neurosci. 2002;22:10627–10632. doi: 10.1523/JNEUROSCI.22-24-10627.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriya H, Takagi Y, Nakanishi T, Hayashi M, Tani T, Hirotsu I. Affinity profiles of various muscarinic antagonists for cloned human muscarinic acetylcholine receptor (mAChR) subtypes and mAChRs in rat heart and submandibular gland. Life Sci. 1999;64:2351–2358. doi: 10.1016/s0024-3205(99)00188-5. [DOI] [PubMed] [Google Scholar]
- Naruganahalli KS, Sinha S, Hegde LG, Meru AV, Chugh A, Kumar N, et al. Comparative in vivo uroselectivity profiles of anticholinergics, tested in a novel anesthetized rabbit model. Eur J Pharmacol. 2007;572:207–212. doi: 10.1016/j.ejphar.2007.06.009. [DOI] [PubMed] [Google Scholar]
- Petitet F, Jeantaud B, Bertrand P, Imperato A. Cannabinoid penetration into mouse brain as determined by ex vivo binding. Eur J Pharmacol. 1999;374:417–421. doi: 10.1016/s0014-2999(99)00189-2. [DOI] [PubMed] [Google Scholar]
- Steers WD. Rat: overview and innervation. Neurourol Urodyn. 1994;13:97–118. doi: 10.1002/nau.1930130204. [DOI] [PubMed] [Google Scholar]
- Stewart WF, Van Rooyen JB, Cundiff GW, Abrams P, Herzog AR, Corey R, et al. Prevalence and burden of overactive bladder in the United States. World J Urol. 2003;20:327–336. doi: 10.1007/s00345-002-0301-4. [DOI] [PubMed] [Google Scholar]
- Taylor SJ, Michel AD, Kilpatrick GJ. In vivo occupancy of histamine H3 receptors by thioperamide and R-alpha-methylhistamine measured using histamine turnover and an ex vivo labeling technique. Biochem Pharmacol. 1992;44:1261–1267. doi: 10.1016/0006-2952(92)90524-m. [DOI] [PubMed] [Google Scholar]
- Tiwari A, Naruganahalli KS. Current and emerging investigational medical therapies for the treatment of overactive bladder. Expert Opin Investig Drugs. 2006;15:1017–1037. doi: 10.1517/13543784.15.9.1017. [DOI] [PubMed] [Google Scholar]
- Tobin G. Muscarinic receptor subtypes in the submandibular gland and the urinary bladde of the rabbit: in vivo and in vitro functional comparisons of receptor antagonists. J Auton Pharmacol. 1995;15:451–463. doi: 10.1111/j.1474-8673.1995.tb00410.x. [DOI] [PubMed] [Google Scholar]
- Wang P, Luthin GR, Ruggieri MR. Muscarinic acetylcholine receptor subtypes mediating urinary bladder contractility and coupling to GTP binding proteins. J Pharmacol Exp Ther. 1995;273:959–966. [PMC free article] [PubMed] [Google Scholar]
- Wein AJ, Rovner ES. The overactive bladder: an overview for primary care health providers. Int J Fertil Womens Med. 1999;44:56–66. [PubMed] [Google Scholar]
- Yamanishi T, Chapple CR, Yasuda K, Yoshida K, Chess-Williams R. The role of M2 muscarinic receptor subtypes mediating contraction of the circular and longitudinal smooth muscle of the pig proximal urethra. J Urol. 2002;168:308–314. [PubMed] [Google Scholar]


