Abstract
Background and purpose:
Sildenafil, a specific inhibitor of phosphodiesterase 5A (PDE5A), is currently tested as a treatment for severe Raynaud's phenomenon. Here, we tested whether sildenafil, alone or combined with local sodium nitroprusside (SNP) delivered through skin iontophoresis, increased forearm cutaneous blood conductance in healthy volunteers, and to assess how well this combination was tolerated.
Experimental approach:
Ten healthy volunteers were enrolled. Variations in cutaneous vascular conductance (CVC) following oral administration of 50 or 100 mg of sildenafil with or without SNP iontophoresis were expressed as a percentage of maximal CVC, and were monitored using laser Doppler imaging. SNP iontophoresis was performed on the ventral surface of the forearm, 1 h after application of lidocaine/prilocaine cream.
Key results:
Sildenafil at 100 mg, but not 50 mg, increased overall responses (area under the curve) (44%) and peak responses (29%) to SNP iontophoresis. Sildenafil at 100 mg, but not 50 mg, increased baseline CVC (75%). Incidence of headache was not changed when SNP iontophoresis was combined with sildenafil. One episode of symptomatic arterial hypotension occurred in a volunteer given 50 mg sildenafil, 30 min after the beginning of SNP iontophoresis.
Conclusions and implications:
Oral sildenafil at 100 mg potentiated local skin hyperaemia induced by SNP iontophoresis, with no increased incidence of headaches. The combination of oral specific PDE5A inhibitor and nitrates administered through skin iontophoresis deserves further investigation in diseases such as severe Raynaud's phenomenon, with particular attention to the incidence of arterial hypotension.
Keywords: sildenafil, iontophoresis, sodium nitroprusside, microcirculation, ClinicalTrials.gov (NCT00710099)
Introduction
Sildenafil is a specific phosphodiesterase 5A (PDE5A) inhibitor used as a first- or second-line treatment in scleroderma patients presenting with pulmonary arterial hypertension. Most of these patients suffer from severe Raynaud's phenomenon, and 30–50% of them exhibit cutaneous finger ulcerations, despite long-term treatment with calcium channel blockers. Specific PDE5A inhibitors have been tested as a second-line treatment of severe Raynaud's phenomenon (Levien, 2006). There are a few case reports in which the authors tested sildenafil in the treatment of fingertip ulceration in patients with secondary Raynaud's phenomenon (Colglazier et al., 2005), and a recent double-blinded, randomized clinical trial showed a functional benefit in such patients (Fries et al., 2005). In addition, this study showed that sildenafil increased nail-fold capillary blood flow.
Nitrates have been used for decades for the treatment of Raynaud's phenomenon (Franks, 1982). However, systemic nitrates exhibit frequent side effects related to their potency as vasodilators. Recent investigations have shown that a new topical nitroglycerin formulation may improve the severity of Raynaud's phenomenon (Chung et al., 2009), strengthening the potential interest in local administration of nitrates. Iontophoresis, a non-invasive transdermal drug delivery method using a low-intensity electric current, could be an interesting alternative to ointments. Indeed, we recently showed that iontophoresis of sodium nitroprusside (SNP) leads to a non-axon-reflex-dependent, increased cutaneous vascular conductance (CVC) on the forearm of patients with secondary Raynaud's phenomenon (Roustit et al., 2009). Moreover, some authors suggest there is potential interest in vasodilator iontophoresis as a treatment for digital ulcerations (Murray et al., 2005; 2008;). Indeed, long iontophoretic stimulation may be of clinical and therapeutic value with less systemic side effects.
Sildenafil is a specific PDE5A inhibitor which decreases the metabolism of cGMP. The effect of endothelial nitric oxide (NO) via cGMP is increased and leads to enhanced vasodilatation. SNP is a donor of NO, which leads to an activation of guanylate cyclase and an increased production of cGMP in smooth muscle cells. The resulting vasodilation is therefore not dependent on the endothelial production of NO. Therefore, specific PDE5A inhibitors and nitrates have a synergistic pharmacodynamic action, and their co-administration significantly reduces blood pressure compared to nitrates alone (Webb et al., 2000). As a consequence, the combination of oral sildenafil and nitrates is contra-indicated due to sustained arterial hypotension (Cheitlin et al., 1999). Combining orally administered-specific PDE5A inhibitors with the local diffusion of a short half-life nitrate, using a controlled delivery system such as iontophoresis, could potentially reduce systemic side effects while increasing local microvascular blood flow. However, such a concept has not been demonstrated to date.
The main objective of the present study was to test whether 50 or 100 mg oral sildenafil potentiated the increase in CVC induced by local SNP iontophoresis, in the forearm of healthy volunteers. In addition, we tested whether such a combination increased the side effects related to sildenafil, with a specific focus on arterial hypotension and headaches. Cutaneous microvascular effects of the systemic administration of sildenafil, with or without SNP iontophoresis, were quantified using non-invasive laser Doppler imaging, which can routinely be used to assess skin blood flux (Cracowski et al., 2006; Turner et al., 2008).
Methods
Study population
This was a proof of concept study, enrolling healthy subjects recruited through local newspaper advertisements. Inclusion criteria were age of 18 years or older, no significant medical history and the absence of any severe disease (diabetes mellitus, cancer, cardiac and/or pulmonary failure, pulmonary arterial hypertension, myocardial infarction, angina pectoris). Pregnancy was excluded by urinary tests at the beginning of each visit. For all subjects, non-inclusion criteria included any allergies to local anaesthetics, cigarette smoking or dermatological disease on the arm or forearm. Grenoble Institutional Review Board (IRB n°6705) approval was obtained in May 2008, and each subject gave written informed consent before participation. The study was registered in July 2008 at ClinicalTrials.gov (NCT00710099).
Study design
This was an open-label pharmacological study. Upon arrival at the laboratory, the subjects were placed in a temperature-controlled room (23 ± 1°C). All subjects were sober. They were supine for the duration of the whole experiment. Heart rate and blood pressure were monitored automatically. Blood pressure was recorded every 5 min.
Each volunteer attended seven consecutive visits (V1–V7) at the Clinical Research Center (Figure 1). Each visit was separated by 7 ± 2 days. V1 was the initial enrollment visit. For the subsequent visits, the volunteers were fasted for at least 6 h before the visits. During V2, SNP iontophoresis alone was performed in one location and a control site was chosen in a different location. During V3, 50 mg sildenafil alone was given orally; at V4, 50 mg sildenafil was given orally, together with SNP iontophoresis 1 h later; at V5, 100 mg sildenafil alone was given orally; and at V6, 100 mg sildenafil was given orally together with SNP iontophoresis 1 h later. V7 was the last safety clinical visit. We intentionally chose not to randomize the different sequences for safety reasons, with a ‘go/no go’ decision at the end of each visit, based on clinical side effect criteria: drop in humeral systolic or diastolic blood pressure of more than 30 mm Hg, heart rate higher than 120 min−1, any ophthalmic symptoms or the physician's decision.
Figure 1.
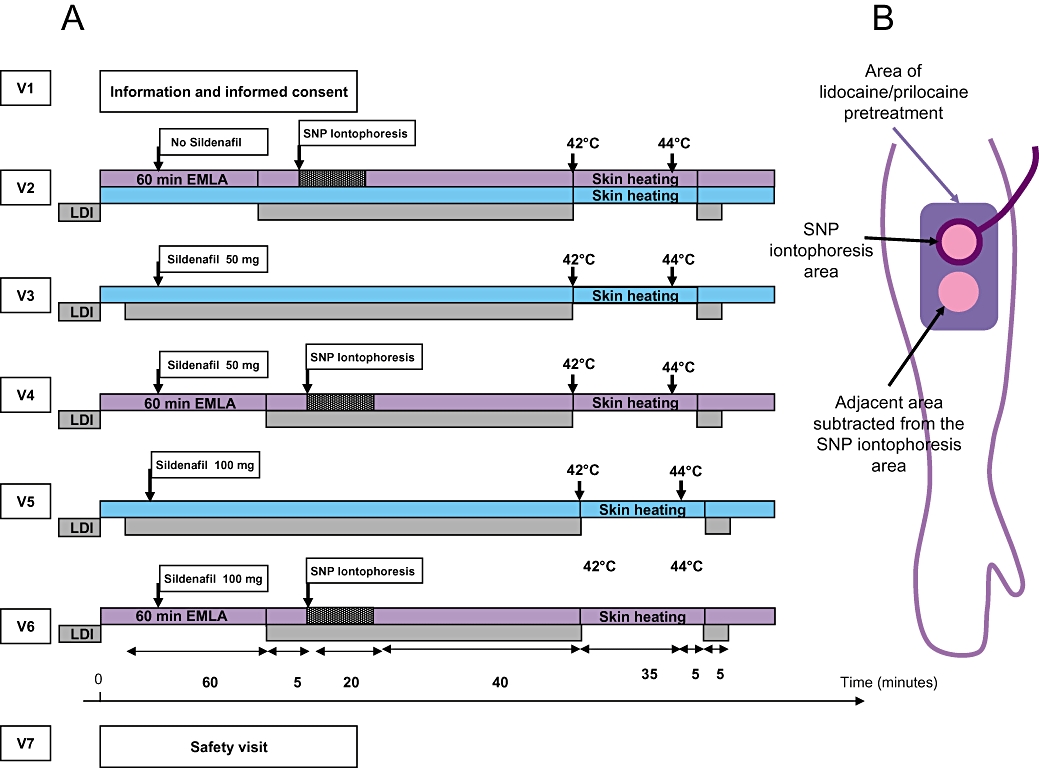
(A) Study protocol. Each volunteer (n = 10) underwent seven consecutive visits separated by 7 ± 2 days. For safety reasons, the visit sequence was not randomized, with a go/no go decision at the end of each visit based on side effects. In order to study the effect of 50 or 100 mg oral sildenafil on forearm CVC compared with no sildenafil, blue bars are used to show the sites compared. In order to study the effect of 50 mg, 100 mg oral sildenafil associated to forearm SNP iontophoresis, compared to SNP iontophoresis alone, purple bars are used to show the sites compared. In the latter case, SNP iontophoresis data were expressed as response in iontophoresis minus response in adjacent area (both subjected to surface anaesthesia with lidocaine/prilocaine) to correct for the effects of sildenafil alone, as shown (B). LDI, laser Doppler imaging; V, visit.
Iontophoresis protocol and laser Doppler imaging
In order to avoid axon reflex-induced hyperaemia during iontophoresis, 1 g of lidocaine/prilocaine cream (5 g tubes containing 125 mg lidocaine and 125 mg prilocaine) was applied on the iontophoresis site 1 h before starting iontophoresis, as previously described (Cracowski et al., 2007; Roustit et al., 2008). The initial application of lidocaine/prilocaine cream covered 1 cm2 of skin surface. An occlusive transparent dressing covering a larger skin area was placed over the cream to enhance cutaneous diffusion. The anaesthetized area of skin was larger than the size of the local iontophoresis probe. In order to avoid interference, the sites on the forearm were at least 3 cm apart. One hour later, the lidocaine/prilocaine cream was removed with a cotton swab. Using this procedure, we were unable to detect any change in cutaneous blood flux during NaCl 0.9% iontophoresis at 20 µA during 20 min, 60 min after lidocaine/prilocaine application, in four subjects, showing that under our experimental protocol, lidocaine/prilocaine was able to inhibit any potential axon reflex response.
Before recording, the arm was immobilized with a vacuum cushion as previously described (Roustit et al., 2009). The use of this cushion decreases the artefacts associated with arm movement. After the 1 h acclimatization period at room temperature (23 ± 1°C), baseline laser Doppler imaging data were recorded for 5 min. Iontophoresis was then performed on the ventral face of the non-dominant forearm, followed by local heating to reach maximal blood flow.
Iontophoresis probes were used together with laser Doppler imaging (Perilont System and PeriScan PIM 3 System, Perimed, Järfälla, Sweden). The laser head was placed 20 cm above the skin. The resolution was set as ‘high’ (1 mm step length). Laser Doppler imaging scans were taken every minute. SNP iontophoresis was performed for 20 min at 20 µA with 180 µL of a 57.8 mM SNP solution (Nitriate, powder for IV injection; one vial containing 50 mg of SNP diluted in 4 mL of 0.9% NaCl solution). SNP was purchased from Serb, Paris, France. A negative charge was used for SNP iontophoresis. Our iontophoresis pads did not enable us to apply a thermoneutral temperature; however, all experiments were performed in a specific temperature-controlled room (23 ± 1°C) where room temperature is constantly monitored. Size of the region of interest was 1.5 cm2. The coefficient of variation of the peak SNP iontophoresis, calculated in eight volunteers in two measurements performed 7 days apart ± 1, was 22%. The intraclass coefficient (ICC) of correlation was 72%. ICC values of <0.40, 0.40–0.75 and >0.75 represent poor, fair to good and excellent agreements, respectively (Landis and Koch, 1977). One single vial of SNP was used for each visit and discarded thereafter. The length of the data acquisition was 1 h after the start of the iontophoresis, or 2 h after administration of sildenafil alone.
At the two visits when the patient received sildenafil alone (once at 50 mg and the other at 100 mg), and at the control site at visit V2, lidocaine/prilocaine cream was not applied; probes were located on the forearm as described above. We used the iontophoresis probes, but did not connect them to the current generator. Skin blood flux was recorded using the laser Doppler imager. Heart rate and blood pressure were monitored for 2 h after the oral intake of sildenafil.
At the end of the experiment, the skin was heated locally at all locations, for 30 min at 42°C followed by 5 min at 44°C to reach maximal skin vasodilation, using a Perimed integrated heating probe (P457, Perimed). The reason of heating first to 42°C for 30 min, and then to 44°C to obtain maximal vasodilatation was based on our practical experience in patients who exhibit a decreased capillary density like scleroderma patients who do not tolerate thermal heating to 44°C. As a consequence, we used a 42°C heating during 30 min, with only 5 min at 44°C, given the fact that 42°C does not lead to a maximal hyperaemia. Five minutes of laser Doppler imaging was recorded after the end of the skin heating sequence.
Data analysis
Data were initially expressed as CVC, which is the flux in perfusion units (PU) divided by the mean arterial pressure (MAP) in mm Hg. Subsequently, all CVC data were expressed as a percentage of maximal vasodilatation (44°C thermal plateau) (Cracowski et al., 2006; Salvat-Melis et al., 2006; Roustit et al., 2008) and area under the curve (AUC) for SNP iontophoresis, the latter being calculated over 60 min. Following data recording, a region of interest was chosen underneath the probes on the laser Doppler scans, and flux data were used for data analysis. Data from an adjacent area (control) were subtracted from those of the iontophoresis region of interest to take into account any potential basal variation in CVC induced by sildenafil alone, and data were therefore expressed as values from the iontophoresis region of interest minus values from the control area (Figure 1B).
Quantitative data are expressed as mean and standard deviation. Qualitative data are expressed as numerical values and percentage in parentheses. Quantitative data were analysed by anova for repeated measures, and with 2 × 2 t-tests for paired analyses, corrected by Bonferroni's correction, with each subject serving as his/her own control. P values less than 0.05 were considered statistically significant. The number of subjects, 10, was based on the hypothesis that sildenafil would induce a 25% increase in the peak hyperaemic response to SNP iontophoresis, with previous unpublished data showing that the mean SNP induced peak was 50 ± 25%, with α= 0.05, a power of 80% and a unilateral test (JD Elashoff; nQuery Advisor for Windows v 6.01, Statistical Solutions, Saugus, MA, USA).
Materials
Sildenafil, a specific PDE5A inhibitor, was purchased from Pfizer France, Paris, France. SNP, a nitrate leading to soluble guanylyl cyclase activation through NO release, was purchased from Serb, Paris, France.
Results
Baseline characteristics
The demographic and clinical characteristics of the 10 subjects included in this study were: six females and four males, mean age 22.9 years (SD 5.3), body mass index 21.4 kg·m−2 (2.8), mean systolic arterial pressure at the beginning of the study = 117.8 mm Hg (SD 13.9), mean diastolic arterial pressure = 70.6 mm Hg (SD 10.3).
Effect of oral 50 and 100 mg sildenafil on thermal hyperaemia
Oral sildenafil did not modify the amplitude of heat (44°C)-induced hyperaemia 2 h after intake (3.15 ± 1.2 PU·mm Hg−1 without sildenafil, 3.27 ± 1 PU·mm Hg−1 after 50 mg sildenafil and 3.3 ± 1.2 PU·mm Hg−1 after 100 mg sildenafil, NS).
Main objective: effect of oral sildenafil at 50 and 100 mg on SNP iontophoresis-induced hyperaemia
The higher dose of sildenafil (100 mg), but not the lower dose (50 mg), increased the overall response to SNP iontophoresis. The corresponding AUC values were 101 100 ± 70 415% CVCmax·s without sildenafil, 95 212 ± 62 119% CVCmax·s after 50 mg sildenafil and 146 285 ± 63 315% CVCmax·s after 100 mg sildenafil (P = 0.03 for 100 mg vs. control; Figure 2). Similarly, 100 mg, but not 50 mg, sildenafil increased the peak response to SNP iontophoresis (peak CVC 49.5 ± 18.9% CVCmax without sildenafil, 46.6 ± 20.3% CVCmax after 50 mg sildenafil and 63.9 ± 24.4% CVCmax after 100 mg sildenafil; P = 0.05 for 100 mg vs. without control). Time to peak of SNP iontophoresis was unchanged after sildenafil (17.2 ± 8.1 min without sildenafil, 20.3 ± 9.1 min after 50 mg sildenafil and 17.3 ± 7.4 min after 100 mg sildenafil). Finally, residual flux at 60 min remained higher under sildenafil at 100 mg (13.2 ± 12.7% CVCmax without sildenafil, 7.6 ± 20.2% CVCmax after 50 mg sildenafil and 25.5 ± 10.9% CVCmax after 100 mg sildenafil; P = 0.05 vs. without sildenafil).
Figure 2.
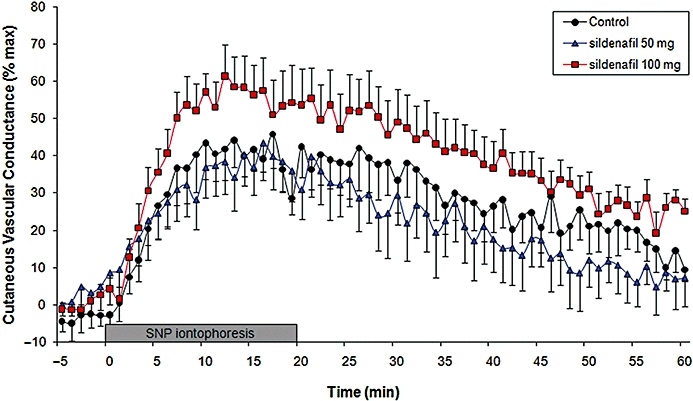
Effect of 50 or 100 mg oral sildenafil combined with forearm SNP iontophoresis (20 µA during 20 min) on forearm CVC (mV·mm Hg−1). Oral sildenafil was taken 1 h before the iontophoresis onset. CVC was expressed as a percentage of maximal CVC at 44°C. Data from an adjacent control region of interest were subtracted from the iontophoresis region of interest to take into account any potential basal CVC variation induced by sildenafil alone. The increased CVC following SNP iontophoresis was significantly higher after 100 mg oral sildenafil than control (P = 0.03), as measured by the AUC over 60 min.
Effect of oral 50 and 100 mg sildenafil given alone on baseline CVC
Sildenafil at 100 mg, but not at 50 mg, increased baseline CVC (peak CVC 8.5 ± 1% CVCmax without sildenafil, 10.5 ± 3.5% CVCmax after 50 mg sildenafil and 14.9 ± 10.2% CVCmax after 100 mg sildenafil; P = 0.03 for 100 mg vs. without sildenafil; Figure 3). Note that the peak increase in CVC with sildenafil alone occurred at 60 ± 0.8 min.
Figure 3.
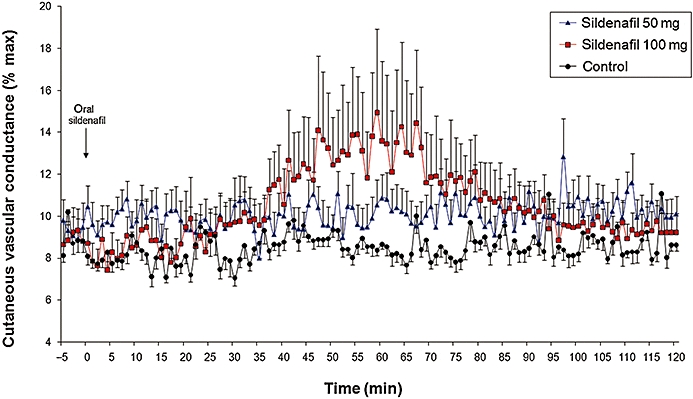
Effect of 50 or 100 mg oral sildenafil on forearm CVC (mV·mm Hg−1). CVC was expressed as a percentage of maximal CVC at 44°C.
Side effects
No serious side effects occurred. We were able to give 100 mg sildenafil to all subjects. While sildenafil decreased MAP 1 h after oral intake, no individual variation in MAP was observed during the 20 min of SNP cutaneous iontophoresis, with or without sildenafil (Figure 4). At V4 (sildenafil 50 mg plus SNP iontophoresis), 10 min after the end of SNP iontophoresis, one woman exhibited pallor and dizziness associated with a 10 mm Hg drop in MAP from baseline (systolic/diastolic arterial pressure at 100/52 mm Hg at baseline, at 85/48 mm Hg during the symptoms). Her glycaemia was normal. She recovered after infusion of 100 mL NaCl 0.9%, exhibited no further side effect during the next 2 h and was able to leave the Clinical Research Center. The safety committee considered this event as not serious, but possibly related to the combination of sildenafil plus SNP iontophoresis. With her approval, she returned for the V5 and V6 (sildenafil 100 mg without and with SNP iontophoresis, respectively), where she exhibited no side effects.
Figure 4.
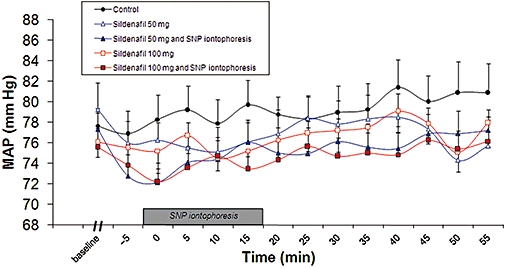
Effect of 50 or 100 mg oral sildenafil with or without forearm SNP iontophoresis on MAP. Oral sildenafil was taken 1 h before the iontophoresis onset. Baseline refers to MAP before sildenafil was taken. Data from baseline to SNP iontophoresis are not shown for clarity.
No headaches occurred during V2 (SNP iontophoresis alone). In contrast, four, three, three and three volunteers reported headaches at V3–V6, respectively (NS between sildenafil groups). Paracetamol (1 g orally) was taken by one, three, two and two volunteers, respectively (NS). No ophthalmic symptoms were reported.
Discussion and conclusions
We have shown that 100 mg oral sildenafil potentiated the increase in cutaneous blood conductance induced by local SNP iontophoresis, in the forearm of healthy volunteers. This pharmacodynamic potentiation occurred without an increased frequency of headaches compared with sildenafil alone. Symptomatic arterial hypotension occurred in one subject receiving 50 mg sildenafil 30 min after skin SNP iontophoresis.
Microvascular function can routinely be studied in human skin using non-invasive laser Doppler imaging (Turner et al., 2008), and thermal hyperaemia is commonly used as a reference of maximal vasodilatation (Cracowski et al., 2006), such as in the present study. In our study, 50 and 100 mg of sildenafil did not alter maximal CVC 2 h after drug intake. Indeed, such results compare with those of Gooding et al. (2006) who showed that maximal thermal hyperaemia was unchanged following 100 mg sildenafil taken orally. However, this treatment did increase cutaneous flux 30 min after the end of the local heating, that is, sildenafil increased the duration of the hyperaemia due to local heating, but not its amplitude. We chose to provide data as CVC rather than flux to allow for potential variations in arterial blood pressure, and we further normalized conductance over the maximal hyperaemia, as this is less variable than expressing data as a percentage of baseline (Cracowski et al., 2006).
Topical delivery of SNP via iontophoresis, in combination with oral sildenafil, could be considered as an alternative method of administration to increase SNP concentration locally, thus avoiding the major systemic adverse effects of both drugs. In this study, SNP iontophoresis was performed 1 h after oral intake of sildenafil, in order to match the maximal sildenafil plasma concentration, which is close to 1 h (Wright, 2006). Sildenafil absorption decreases during meals, and for this reason we chose to study fasting volunteers, in order to decrease the variability. Iontophoresis of SNP is a commonly used technique to assess non-endothelium-dependent skin microvascular function. SNP is a pro-drug, releasing NO through both enzymatic and non-enzymatic pathways, which leads to an activation of guanylate cyclase and an increased production of cGMP in smooth muscle cells. When infused intravenously, SNP has an onset of action within 30 s, with a peak effect occurring within 2 min. In our study, the effect of SNP, using iontophoresis, was delayed compared to that observed during intravenous infusion, with a peak close to 10 min. The more plausible reason for the observed potentiation of SNP increase in CVC by sildenafil is their synergistic pharmacodynamic action. However, we cannot rule out the possibility that sildenafil decreased skin resistance. Such a phenomenon would enable an increased iontophoretic SNP flux through a clearly different mechanism. As shown by Ramsay et al. (2002), variability in skin resistance was shown to increase the variability of iontophoresis, with high skin resistance being associated with lower vasodilation. However, in terms of therapy, the result would be similar, as it would tend to increase the vasodilator response to SNP, although through another mechanism.
Iontophoresis can cause non-specific axon reflex vasodilation. Lidocaine/prilocaine cream, applied for 1 h prior to iontophoresis, was therefore used in order to avoid such an axon reflex response during SNP iontophoresis (Cracowski et al., 2007). Indeed, using lidocaine/prilocaine cream as an anaesthetic inhibits the trigger for axon reflex (C fibre action potentials). As a consequence, the effect observed is not associated with a non-specific axon reflex, but with the effect of the vasodilating substances themselves. In addition, SNP iontophoresis data were expressed as values from the iontophoresis region of interest minus the value from the adjacent region of interest to correct for any effect of oral sildenafil. The adjacent region of interest was within the anaesthetized zone. Local thermal equilibrium might have been slightly different from that within the chamber, and thus this ‘adjacent area’ was not perfect. However, the effects of sildenafil alone, as shown in Figure 3, were minimal in comparison with those of SNP iontophoresis. This means that the potentiation observed after 100 mg sildenafil was not related to the baseline effect of sildenafil.
Oral sildenafil at 100 mg, but not at 50 mg, increased baseline CVC in the forearm. However, with a sample size of 10 volunteers, the study may have been under-powered to detect any effect with 50 mg. Indeed, such a short-term microvascular effect on skin vasculature is well known, as 50 mg oral sildenafil increased resting and post-ischaemic cutaneous capillary circulation in patients with coronary artery disease 1 h later (by 47%) (Park et al., 2004). In addition, mean nail-fold capillary flow velocity was increased by more than 400% in patients treated with 50 mg sildenafil twice daily for 4 weeks (Fries et al., 2005). Sildenafil improved the clinical condition and the anemometric flow velocity in nail-fold capillaries of patients with non-scleroderma Raynaud's phenomenon (Fries et al., 2005), and was associated with thermographic and symptomatic improvement in patients with systemic sclerosis and Raynaud's phenomenon (Kumar et al., 2006) or in severe digital ischaemia (Kumana et al., 2004). In some series, the effects of sildenafil on Raynaud's phenomenon or digital ulcers in systemic sclerosis were spectacular and appeared within a few weeks (Gore and Silver, 2005). Regarding the side effects, headache, facial flushing and nasal congestion are the most common adverse events observed following oral administration. Headaches after sildenafil occurred as expected, but were not increased by SNP iontophoresis. Decreased MAP was observed, as expected, 1 h after oral administration of sildenafil (Figure 4), without any significant additional effect of SNP iontophoresis. However, the MAP drop associated with dizziness and pallor that occurred 10 min after the end of SNP iontophoresis (i.e. 30 min after the start of SNP iontophoresis), in a young fasting woman, was possibly related to a pharmacodynamic interaction, but the cause was uncertain given the discrepancy between the 30 min delay and the short half-life of SNP when given intravenously (a few minutes). Re-challenge (100 mg sildenafil plus SNP iontophoresis) on a different day was negative. However, such a side effect should be considered as being potentially related to the association, and merits further safety studies.
In conclusion, 100 mg oral sildenafil potentiated local skin hyperaemia induced by iontophoresis of SNP, with no increased incidence of headaches. The combination of oral specific PDE5A inhibitors and nitrates administered through skin iontophoresis should be further investigated in diseases such as severe Raynaud's phenomenon. However, further studies are required to determine the incidence of arterial hypotension induced by this combination.
Acknowledgments
We thank the patient's association, Association des Sclérodermiques de France, and the Groupe Français de Recherche sur la Sclérodermie for their financial support, and Alison Foote for correcting the manuscript.
Glossary
Abbreviations:
- AUC
area under the curve
- CVC
cutaneous vascular conductance
- MAP
mean arterial pressure
- SNP
sodium nitroprusside
Conflicts of interest
Dr Jean-Luc Cracowski has received grant support from Pfizer for another study. The other authors report no conflict of interest.
References
- Cheitlin MD, Hutter AM, Jr, Brindis RG, Ganz P, Kaul S, Russell RO, Jr, et al. ACC/AHA expert consensus document. Use of sildenafil (Viagra) in patients with cardiovascular disease. American College of Cardiology/American Heart Association. J Am Coll Cardiol. 1999;33:273–282. doi: 10.1016/s0735-1097(98)00656-1. [DOI] [PubMed] [Google Scholar]
- Chung L, Shapiro L, Fiorentino D, Baron M, Shanahan J, Sule S, et al. MQX-503, a novel formulation of nitroglycerin, improves the severity of Raynaud's phenomenon: a randomized, controlled trial. Arthritis Rheum. 2009;60:870–877. doi: 10.1002/art.24351. [DOI] [PubMed] [Google Scholar]
- Colglazier CL, Sutej PG, O'Rourke KS. Severe refractory fingertip ulcerations in a patient with scleroderma: successful treatment with sildenafil. J Rheumatol. 2005;32:2440–2442. [PubMed] [Google Scholar]
- Cracowski JL, Minson CT, Salvat-Melis M, Halliwill JR. Methodological issues in the assessment of skin microvascular endothelial function in humans. Trends Pharmacol Sci. 2006;27:503–508. doi: 10.1016/j.tips.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Cracowski JL, Lorenzo S, Minson CT. Effects of local anaesthesia on subdermal needle insertion pain and subsequent tests of microvascular function in human. Eur J Pharmacol. 2007;559:150–154. doi: 10.1016/j.ejphar.2006.11.069. [DOI] [PubMed] [Google Scholar]
- Franks AG., Jr Topical glyceryl trinitrate as adjunctive treatment in Raynaud's disease. Lancet. 1982;1:76–77. doi: 10.1016/s0140-6736(82)90215-x. [DOI] [PubMed] [Google Scholar]
- Fries R, Shariat K, von Wilmowsky H, Bohm M. Sildenafil in the treatment of Raynaud's phenomenon resistant to vasodilatory therapy. Circulation. 2005;112:2980–2985. doi: 10.1161/CIRCULATIONAHA.104.523324. [DOI] [PubMed] [Google Scholar]
- Gooding KM, Hannemann MM, Tooke JE, Clough GF, Shore AC. Maximum skin hyperaemia induced by local heating: possible mechanisms. J Vasc Res. 2006;43:270–277. doi: 10.1159/000091736. [DOI] [PubMed] [Google Scholar]
- Gore J, Silver R. Oral sildenafil for the treatment of Raynaud's phenomenon and digital ulcers secondary to systemic sclerosis. Ann Rheum Dis. 2005;64:1387. doi: 10.1136/ard.2004.034488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumana CR, Cheung GT, Lau CS. Severe digital ischaemia treated with phosphodiesterase inhibitors. Ann Rheum Dis. 2004;63:1522–1524. doi: 10.1136/ard.2003.015677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar N, Griffiths B, Allen J. Thermographic and symptomatic effect of a single dose of sildenafil citrate on Raynaud's phenomenon in patients with systemic sclerosis: a potential treatment. J Rheumatol. 2006;33:1918–1919. [PubMed] [Google Scholar]
- Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- Levien TL. Phosphodiesterase inhibitors in Raynaud's phenomenon. Ann Pharmacother. 2006;40:1388–1393. doi: 10.1345/aph.1H005. [DOI] [PubMed] [Google Scholar]
- Murray AK, Herrick AL, Gorodkin RE, Moore TL, King TA. Possible therapeutic use of vasodilator iontophoresis. Microvasc Res. 2005;69:89–94. doi: 10.1016/j.mvr.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Murray AK, Moore TL, King TA, Herrick AL. Vasodilator iontophoresis a possible new therapy for digital ischaemia in systemic sclerosis? Rheumatology (Oxford) 2008;47:76–79. doi: 10.1093/rheumatology/kem314. [DOI] [PubMed] [Google Scholar]
- Park JW, Mrowietz C, Chung N, Jung F. Sildenafil improves cutaneous microcirculation in patients with coronary artery disease: a monocentric, prospective, double-blind, placebo-controlled, randomized cross-over study. Clin Hemorheol Microcirc. 2004;31:173–183. [PubMed] [Google Scholar]
- Ramsay JE, Ferrell WR, Greer IA, Sattar N. Factors critical to iontophoretic assessment of vascular reactivity: implications for clinical studies of endothelial dysfunction. J Cardiovasc Pharmacol. 2002;39:9–17. doi: 10.1097/00005344-200201000-00002. [DOI] [PubMed] [Google Scholar]
- Roustit M, Simmons GH, Carpentier P, Cracowski JL. Abnormal digital neurovascular response to local heating in systemic sclerosis. Rheumatology (Oxford) 2008;47:860–864. doi: 10.1093/rheumatology/ken065. [DOI] [PubMed] [Google Scholar]
- Roustit M, Blaise S, Cracowski JL. Sodium nitroprusside iontophoresis on the finger pad does not consistently increase skin blood flow in healthy controls and patients with systemic sclerosis. Microvasc Res. 2009;77:260–264. doi: 10.1016/j.mvr.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Salvat-Melis M, Carpentier PH, Minson CT, Boignard A, McCord GR, Paris A, et al. Digital thermal hyperaemia impairment does not relate to skin fibrosis or macrovascular disease in systemic sclerosis. Rheumatology (Oxford) 2006;45:1490–1496. doi: 10.1093/rheumatology/kel116. [DOI] [PubMed] [Google Scholar]
- Turner J, Belch JJ, Khan F. Current concepts in assessment of microvascular endothelial function using laser Doppler imaging and iontophoresis. Trends Cardiovasc Med. 2008;18:109–116. doi: 10.1016/j.tcm.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Webb DJ, Muirhead GJ, Wulff M, Sutton JA, Levi R, Dinsmore WW. Sildenafil citrate potentiates the hypotensive effects of nitric oxide donor drugs in male patients with stable angina. J Am Coll Cardiol. 2000;36:25–31. doi: 10.1016/s0735-1097(00)00705-1. [DOI] [PubMed] [Google Scholar]
- Wright PJ. Comparison of phosphodiesterase type 5 (PDE5) inhibitors. Int J Clin Pract. 2006;60:967–975. doi: 10.1111/j.1742-1241.2006.01049.x. [DOI] [PubMed] [Google Scholar]


