Abstract
Background and purpose:
The mechanism(s) of action responsible for the beneficial effects of phosphodiesterase 5 (PDE5) inhibitors including sildenafil on lower urinary tract symptoms suggestive of benign prostate hyperplasia are unclear. In particular, the role of the NO-cGMP signalling pathway in regulating human bladder dome smooth muscle relaxation is questionable. Thus, we assessed the ability of a PDE5 inhibitor, sildenafil, to relax such tissue, and identified the signalling pathways involved in this relaxation.
Experimental approach:
Human bladder samples were obtained from 20 patients with no overactive bladder undergoing cystectomy for bladder cancer. Detrusor strips were mounted isometrically in Krebs–HEPES solution. Concentration–response curves for sildenafil (10 nM–30 µM) were generated in the presence of various inhibitors on carbachol-induced pre-contraction.
Key results:
Sildenafil relaxed carbachol-pre-contracted human detrusor strips, starting at 3 µM. This effect was not modified by NO donors, S-nitroso-N-acetylpenicillamine (10 µM) or sodium nitroprusside (300 nM), but was significantly inhibited by inhibition of guanylate cyclase (with ODQ, 10 µM) or adenylyl cyclase (with MDL-12,330A, 10 µM), by the ATP-sensitive potassium channel inhibitor, glibenclamide (10 µM), or inhibition of the large (with iberiotoxin, 30 nM) or small (with apamin, 100 nM) conductance calcium-activated potassium channels.
Conclusions and implications:
Sildenafil-induced relaxation of human detrusor smooth muscle involved cGMP-, cAMP- and K+ channel-dependent signalling pathways, with a minor contribution from NO. The effect of this sildenafil-induced relaxation on the clinical benefit of PDE5 inhibitors on urinary storage symptoms in men deserves further investigation.
Keywords: PDE5 inhibitor, bladder, lower urinary tract symptom, cAMP, K channels
Introduction
Phosphodiesterase 5 (PDE5) inhibitors, such as sildenafil, vardenafil and tadalafil, are the standard first-line therapy in erectile dysfunction (ED). All relax penile erectile tissue by blocking PDE5-mediated hydrolysis of cGMP, the key second messenger of the nitric oxide (NO) pathway (Corbin et al., 2002). ED is often associated with lower urinary tract symptoms (LUTS), independently of age and cardiovascular co-morbidities (Rosen et al., 2003). There is emerging evidence that PDE5 inhibitors may also have a potential in treating patients with LUTS associated with benign prostatic hyperplasia. Indeed, several randomized placebo-controlled trials have recently demonstrated that the three available PDE5 inhibitors improve both voiding (obstructive) and storage (irritative) urinary symptoms (McVary et al., 2007a,b; Roehrborn et al., 2008; Stief et al., 2008).
Voiding symptoms are associated with obstruction of the bladder outlet, often due to benign prostatic enlargement (Chapple et al., 2008). It is now widely accepted that the mechanisms by which PDE5 inhibitors improve these voiding symptoms involve the NO-cGMP pathway as the expression and/or functional role of the key mediators of this signalling pathway (i.e. neuronal nitric oxide synthase; endothelial nitric oxide synthase; soluble guanylate cyclase, which catalyses the conversion of GTP to cGMP; cGMP-dependent protein kinase type I, which is the principal effector of NO, cGMP and PDE5) have all been described in the outflow region represented by the prostate, bladder neck and urethra (Hedlund, 2005).
Conversely, urinary storage symptoms, currently largely encompassed by the term overactive bladder syndrome, are more specifically related to bladder dome dysfunction (Chapple et al., 2008). Interestingly, a recent double-blinded, placebo-controlled study in male patients with spinal cord injuries and neurogenic detrusor overactivity has also reported a significant improvement of urodynamic parameters after vardenafil, supporting an effect on bladder function (Gacci et al., 2007). Thus, it is tempting to speculate that PDE5 inhibitors could relieve bladder storage symptoms through a direct action on detrusor smooth muscle, all the more because PDE5 isoenzymes are expressed in human detrusor, suggesting a functional role of these enzymes in this tissue (Truss et al., 1996; Filippi et al., 2007). However, the role of the NO-cGMP signalling pathway in regulating bladder smooth muscle (detrusor) relaxation is still unclear. Indeed, various studies have attempted to evaluate the effect of modulators of the NO-cGMP signalling pathway on detrusor relaxation, and the results obtained are quite contradictory depending on the species, type of detrusor contraction and pharmacological agent investigated (Persson and Andersson, 1992; Moon, 2002; Tinel et al., 2006; Yanai et al., 2008; Morelli et al., 2009; Werkstrom et al., 2009). Indeed, the relaxant effect of PDE5 inhibitors in rat or guinea pig bladder has been widely reported (Filippi et al., 2007; Yanai et al., 2008; Morelli et al., 2009; Werkstrom et al., 2009). Conversely, NO donors evoked a complex response (relaxation and contraction) in pre-contacted human detrusor muscle (Moon, 2002), and enhanced spontaneous contractions in guinea pig detrusor. Moreover, chronic treatment with a PDE5 inhibitor increased contractile force in normal bladder in rats (Matsumoto et al., 2009).
Therefore, the aim of this study was to assess the ability of the PDE5 inhibitor, sildenafil, to relax human bladder dome smooth muscle and to investigate the signalling pathways that might be involved in this relaxation.
Methods
Human detrusor strip preparation
All samples were obtained from patients with their informed consent. Bladder dome samples were obtained from 20 patients (mean age: 65 ± 2.1 years; 80% males and 20% females) undergoing cystoprostatectomy for infiltrating bladder cancer with no history of bladder dysfunction according to their medical history.
After the surgical procedures, the bladders were immediately transported to pathologist facilities where a sample with no macroscopic malignant tissue was removed. These tissue samples were stored at 4°C in Krebs–HEPES buffer (118 mM NaCl, 4.7 mM KCl, 1.2 mM MgSO4, 1.2 mM KH2PO4, 2.5 mM CaCl2, 4.2 mM NaHCO3; 11.1 mM glucose and 20.8 mM HEPES; pH 7.4) containing penicillin (100 IU·mL–1) and streptomycin (0.1 mg·mL–1) for optimal conservation until use (within 24 h maximum). The samples were cleared of adherent tissue and blood, and sections were excised from each donor sample for each experiment. The serosal layer was removed, and paired longitudinal detrusor strips were isolated. Depending on the experiment, the urothelium was carefully removed from either one of the paired strips so as to obtain strips with urothelium (8 × 6 × 4 mm) or strips without urothelium (8 × 4 × 4 mm).
Strips were suspended in 5 mL organ chambers filled with Krebs–HEPES buffer maintained at 37°C and continuously bubbled with 95% O2 and 5% CO2 to maintain pH at 7.4. They were connected to force transducers for isometric tension recording (LCM Systems, Newport, UK), and an initial tension was applied (0.5–1 g). Following amplification, the tension changes were computerized with Mac Lab/8 using Chart 5 software (AD Instruments Ltd., Chalgrove, UK). The tissue preparations were allowed to equilibrate for 60 min, while being washed periodically with fresh Krebs–HEPES buffer and they were primed by KCl (100 mM, 10 min).
In vitro contractile experiments with detrusor strips
The strips were exposed to carbachol (10 µM, 10 min) to evaluate their contractile capacity. Then, the strips were incubated for a 20 min period with various inhibitors or their vehicle: the inhibitor of adenylate cyclase, MDL-12,330A (10 µM); the inhibitor of guanylate cyclase, ODQ (10 µM); the KATP channel blocker, glibenclamide (10 µM); the large conductance K+ channel (BKCa) blocker, iberiotoxin (30 nM); the small conductance K+ channel (SKCa) blocker, apamin (100 nM); drug and channel nomenclature follows Alexander et al. (2009). The strips were then pre-contracted with carbachol (1 µM) and allowed to re-equilibrate until a stable response was obtained (20 min). In some experiments, sodium nitroprusside (SNP) 3.10−7 M or S-nitroso-N-acetylpenicillamine (SNAP) 10 µM, two NO donors, or their vehicle were added and the strips allowed to reach a stable pre-contraction level for 20 min. Then, concentration–response curves to sildenafil (10 nM–30 µM) or vehicle were obtained.
Finally, the strips were washed repeatedly, and the ability of the tissue to contract was assessed with KCl (100 mM, 10 min).
Data analysis
Results were expressed as a percentage of inhibition of the contractile response to carbachol. Pre-incubation with various inhibitors followed by concentration–response curves to sildenafil's vehicle had no effect on human detrusor. Thus, in order to gain clarity and better compare the effects of the various inhibitors, for some results, the net relaxant effect of sildenafil was calculated by subtracting the effect of the vehicle.
For concentration–response curves to sildenafil in the absence of treatment, a pD2 value (–log [EC50] where EC50 was the concentration of drug that produced 50% of the maximum effect) and a maximal effect value (Emax, maximum response that can be produced by the highest concentration of the drug used) were determined using the four parameter logistic model.
The data were expressed as mean ± SEM for N bladders. N corresponds to the number of bladder samples used from different patients. Statistical comparisons of the concentration–response curves were performed with a two-way anova and Bonferroni's post-test. In case of interaction between the two factors (concentration and drug) during the two-way anova, a modified Student's t-test with the Bonferroni adjustment for multiple comparisons was performed. P values < 0.05 were considered statistically significant. Statistical analysis was performed with GraphPad Prism 4.03 software.
Materials
All drugs and chemicals were purchased from Sigma (St Louis, MO, USA). Sildenafil was purchased from Alsachim SAS (Strasbourg, France). ODQ, MDL-12330A and glibenclamide were prepared in dimethyl sulphoxide (DMSO), with a final concentration of 0.01% in the organ bath, except for glibenclamide 10 µM, for which the final concentration was 0.1%. Apamin was prepared in acetic acid (5 µM final concentration in the organ bath). At the concentrations used, DMSO and acetic acid had no effect on detrusor activity (data not shown). All other drugs were prepared in distilled water.
Results
Effect of sildenafil on human detrusor smooth muscle
The levels of contraction induced by carbachol were 2868 ± 455 mg and 2816 ± 456 mg before vehicle and sildenafil concentration–response curves, respectively, and were not significantly different.
Sildenafil elicited a significant concentration-dependent relaxation of carbachol-induced contraction of human bladder strips when compared to vehicle (P < 0.001, Figure 1). This relaxant effect was particularly marked at high concentrations, and was significant starting at 3 µM and reached −62.0 ± 2.3% at 30 µM, with a pD2 value of 5.0 ± 0.1.
Figure 1.
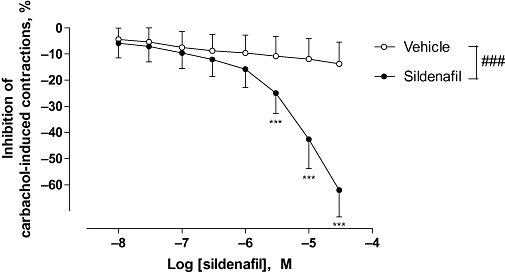
Effect of sildenafil on carbachol-induced human detrusor contraction. Concentration–response curves to sildenafil or vehicle were performed on human detrusor strips, pre-contracted with carbachol (1 µM). The data are mean ± SEM of 20 bladders. ###P < 0.001, two-way anova followed by a modified Student's t-test with the Bonferroni adjustment for multiple comparisons, because of interaction in the two-way anova analysis ***P < 0.001.
Involvement of the NO/cGMP pathway in sildenafil-induced relaxation
The effect of SNAP was investigated on carbachol-induced contractions of human detrusor strips. The levels of contraction induced by carbachol were 1529 ± 341 mg and 1842 ± 447 mg before vehicle and SNAP concentration–response curves, respectively, and were not significantly different.
As shown in Figure 2, SNAP exerted a very slight and non-significant relaxant effect, starting at 3 µM. Thus, a high concentration of SNAP was later chosen in order to evaluate the influence of pre-incubation with an NO donor on the effect of sildenafil. SNAP at 10 µM did not modify the relaxant effect of sildenafil on carbachol-pre-contracted detrusor strips (Figure 3A). This result was also confirmed on pre-incubation with SNP at 300 nM (Figure 3B). Concentration–response curves to sildenafil were performed with detrusor strips with urothelium. In these conditions, the sildenafil-induced relaxation was also unchanged (Figure 3B). The guanylate cyclase inhibitor, ODQ at 10 µM, significantly attenuated relaxation to sildenafil (P < 0.001; Figure 3A). The pre-incubation of the strips with both SNAP and ODQ seemed to abolish the effect of ODQ alone (Figure 3A).
Figure 2.
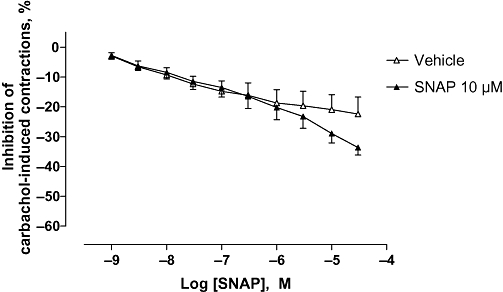
Effect of SNAP on carbachol-induced human detrusor contraction. Concentration–response curves to SNAP or vehicle were performed on human detrusor strips on carbachol (1 µM)-induced detrusor contractions. The data are mean ± SEM of five bladders.
Figure 3.
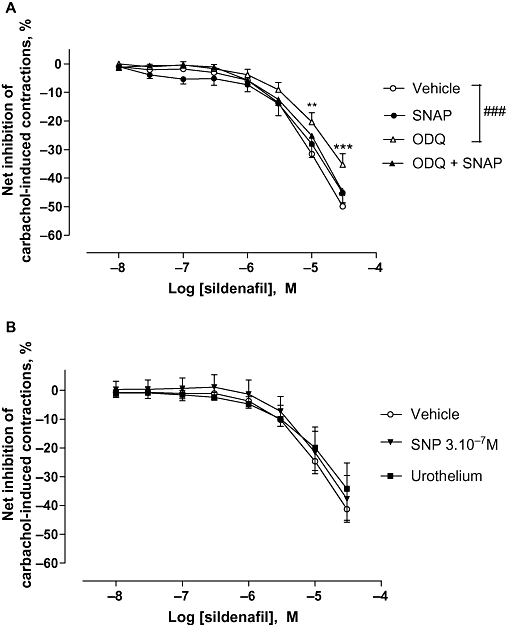
Effect of ODQ and/or NO donors on sildenafil-induced relaxation on carbachol-induced human detrusor contraction. (A) Concentration–response curves to sildenafil or vehicle were performed in the presence of SNAP (10 µM; N = 10) or ODQ (10 µM; N = 17) or their combination (N = 7) or their respective vehicle (N = 20) on carbachol (1 µM)-induced detrusor contraction. (B) Concentration–response curves to sildenafil or vehicle were performed in the presence of SNP (30 mM; N = 5) or in the presence of urothelium (N = 4). Results are expressed as the net effect of sildenafil where the effect of its vehicle has been subtracted. The data are mean ± SEM of N bladders. Vehicle versus ODQ: ###P < 0.001, two-way anova followed by a modified post hoc Student's t-test with the Bonferroni adjustment for multiple comparisons **P < 0.01; ***P < 0.001, at the concentrations shown.
At the concentrations used, ODQ, SNP or SNAP did not modify levels of contraction induced by carbachol and the maximal inhibition caused by each inhibitor in absence of sildenafil, was not different from that caused by the vehicle (Tables 1 and 2). It is worth noting that the contractile response to carbachol of the strips with urothelium seemed slightly less than that of the strips without urothelium (Table 2) as previously described (Chaiyaprasithi et al., 2003), but this effect was not significant.
Table 1.
Effect of ODQ and/or SNAP on carbachol-induced contractions on human detrusor
| ODQ | − | − | − | − | + | + | + | + |
|---|---|---|---|---|---|---|---|---|
| SNAP | − | − | + | + | − | − | + | + |
| CRC sildenafil | − | + | − | + | − | + | − | + |
| Level of contraction induced by carbachol (mg) | 2644 ± 496 | 2644 ± 495 | 2674 ± 446 | 2681 ± 305 | 1976 ± 543 | 2361 ± 519 | 2066 ± 663 | 3058 ± 1323 |
| Maximal inhibition (%) | 12.7 ± 1.8 | 62.1 ± 2.3a | 8.9 ± 2.4b | 51.5 ± 3.5a | 14.0 ± 1.8b | 49.5 ± 3.8a | 10.5 ± 2.7b | 55.3 ± 6.0a |
| Number of experiments | 20 | 20 | 10 | 10 | 17 | 17 | 7 | 7 |
Levels of contraction induced by carbachol were not significantly different between each condition (one-way anova).
P < 0.0001, Student's t-test versus corresponding vehicle condition.
Non-significant versus first column.
Table 2.
Effect of SNP or urothelium on carbachol-induced contractions on human detrusor
| Urothelium | − | − | − | − | + | + |
|---|---|---|---|---|---|---|
| SNP | − | − | + | + | − | − |
| CRC sildenafil | − | + | − | + | − | + |
| Level of contraction induced by carbachol (mg) | 1289 ± 196 | 2092 ± 435 | 2157 ± 1007 | 2655 ± 900 | 1049 ± 204 | 1021 ± 155 |
| Maximal inhibition (%) | 15.6 ± 3.1 | 57.1 ± 4.6a | 19.5 ± 5.6b | 55.8 ± 7.3a | 15.3 ± 2.2b | 53.8 ± 9.1a |
| Number of experiments | 10 | 10 | 5 | 5 | 4 | 4 |
Levels of contraction induced by carbachol were not significantly different between each condition (one-way anova).
P < 0.0001, Student's t-test versus corresponding vehicle condition.
Non-significant versus first column.
Involvement of the cAMP pathway in sildenafil-induced relaxation
The adenylate cyclase inhibitor, MDL-12,330A at 10 µM, significantly inhibited the relaxant effect of sildenafil on carbachol pre-contracted detrusor strips (P < 0.001, Figure 4). Pre-incubation of the strips with SNAP in addition to MDL-12,330A significantly counteracted the effect of MDL-12,330A alone by enhancing the relaxant effect of sildenafil.
Figure 4.
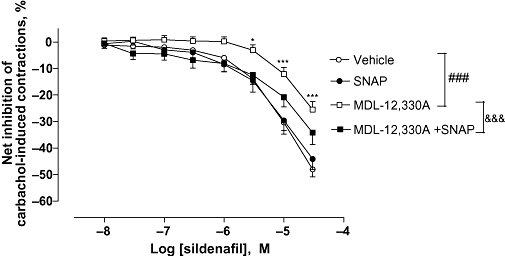
Effect of MDL-12,330A and/or SNAP on sildenafil-induced relaxation on carbachol-induced human detrusor contraction. Concentration–response curves to sildenafil or vehicle were performed in the presence of MDL-12,330A (10 µM; N = 17) or SNAP (10 µM; N = 9) or their combination (N = 6), or their respective vehicle (N = 20) on carbachol (1 µM)-induced detrusor contraction. Results are expressed as the net effect of sildenafil where the effect of its vehicle has been subtracted. The data are mean ± SEM of N bladders. Vehicle versus MDL-12.330A: ###P < 0.001, two-way anova followed by a modified post hoc Student's t-test with the Bonferroni adjustment for multiple comparisons *P < 0.05, ***P < 0.001 at the concentrations shown MDL-12.330A versus MDL-12.330A + SNAP: & & &P < 0.001, two-way anova.
Pretreatment with the combination of ODQ and MDL-12.330A did not further reduce sildenafil-induced relaxation, compared with MDL-12,330A alone, whereas an additive effect might have been expected (Figure 5).
Figure 5.
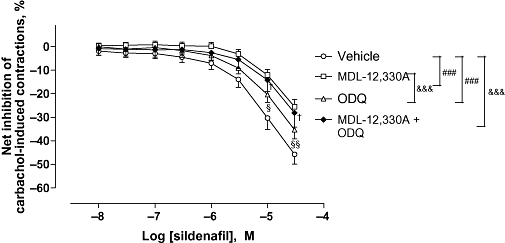
Effect of the combination of MDL-12,330A and ODQ on sildenafil-induced relaxation on carbachol-induced human detrusor contraction. Concentration–response curves to sildenafil or vehicle were performed in the presence of MDL-12,330A (10 µM; N = 17) or ODQ (10 µM; N = 17), or the combination of both (N = 6) or vehicle (N = 20) on carbachol (1 µM)-induced detrusor contraction. Results are expressed as the net effect of sildenafil where the effect of its vehicle has been subtracted. The data are mean ± SEM of N bladders. Vehicle versus MDL-12.330A or vehicle versus ODQ: ###P < 0.001, two-way anova. Vehicle versus MDL-12.330A + ODQ: & & &P < 0.001, two-way anova followed by Bonferroni post-test: †P < 0.05; at the concentrations shown. MDL-12,330A versus ODQ: & & &P < 0.001, two-way anova followed by Bonferroni post-test: §P < 0.05; §§P < 0.001 at the concentrations shown.
At the concentration used, MDL-12,330A did not modify levels of contraction induced by carbachol, and the maximal inhibition caused by MDL-12,330A in the absence of sildenafil was not different from that caused by the vehicle (Table 3). In contrast, the combination of ODQ and MDL-12,330A significantly increased the maximal inhibition of carbachol-induced contraction in the absence of sildenafil when compared to the vehicle (Table 4).
Table 3.
Effect of MDL-12,330A and/or SNAP on carbachol-induced contractions on human detrusor
| MDL-12,330A | − | − | − | − | + | + | + | + |
|---|---|---|---|---|---|---|---|---|
| SNAP | − | − | + | + | − | − | + | + |
| CRC sildenafil | − | + | − | + | − | + | − | + |
| Level of contraction induced by carbachol (mg) | 2868 ± 455 | 2816 ± 456 | 3246 ± 547 | 3122 ± 624 | 2204 ± 435 | 2377 ± 359 | 2272 ± 907 | 3043 ± 820 |
| Maximal inhibition (%) | 13.8 ± 1.9 | 62.1 ± 2.3a | 9.01 ± 2.1b | 53.2 ± 3.5a | 19.4 ± 2.1b | 44.2 ± 2.5a | 15.6 ± 2.7b | 49.5 ± 4.6a |
| Number of experiments | 20 | 20 | 9 | 9 | 17 | 17 | 6 | 6 |
Levels of contraction induced by carbachol were not significantly different between each condition (one-way anova).
P < 0.0001, Student's t-test versus corresponding vehicle condition.
Non-significant versus first column.
Table 4.
Effect of the combination of MDL-12,330A and ODQ on carbachol-induced contractions on human detrusor
| MDL-12,330A | − | − | − | − | + | + | + | + |
|---|---|---|---|---|---|---|---|---|
| ODQ | − | − | + | + | − | − | + | + |
| CRC sildenafil | − | + | − | + | − | + | − | + |
| Level of contraction induced by carbachol (mg) | 2868 ± 455 | 2816 ± 456 | 1976 ± 543 | 2361 ± 519 | 2204 ± 435 | 2377 ± 359 | 2168 ± 709 | 1926 ± 541 |
| Maximal inhibition (%) | 13.8 ± 1.9 | 62.1 ±2.3a | 14.0 ± 1.8b | 49.5 ± 3.8a | 19.4 ± 2.1b | 44.2 ± 2.5a | 21.3 ± 2.8c | 47.4 ± 5.2a |
| Number of experiments | 20 | 20 | 17 | 17 | 17 | 17 | 6 | 6 |
Levels of contraction induced by carbachol were not significantly different between each condition (one-way anova).
P < 0.001, Student's t-test versus corresponding vehicle condition.
Non-significant versus first column.
P < 0.05 versus first column.
Involvement of K+ channels in sildenafil-induced relaxation
The KATP channel blocker, glibenclamide (10 µM), the BKCa channel blocker iberiotoxin (30 nM) and the SKCa channel blocker apamin (100 nM) significantly reduced the relaxation to sildenafil in carbachol-pre-contracted detrusor strips to 23.6 ± 3.8, 35.0 ± 11.3 and 38.2 ± 1.8%, respectively (Figure 6). At the concentrations used, these K+ channel modulators did not modify contractions induced by carbachol, and the maximal inhibition caused by each modulator in the absence of sildenafil was not different from that caused by the vehicle (Table 5).
Figure 6.
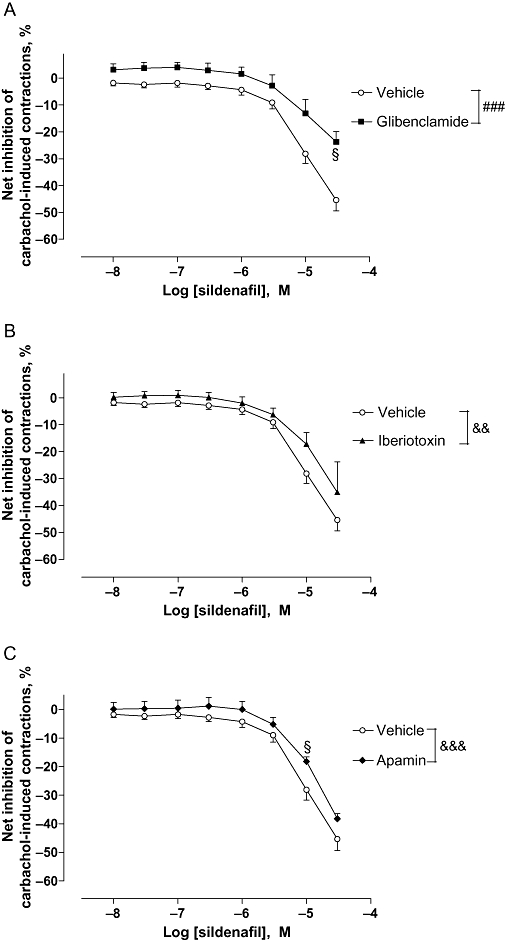
Effect of glibenclamide, iberiotoxin and apamin on sildenafil-induced relaxation on carbachol-induced human detrusor contraction. Concentration–response curves to sildenafil or vehicle were performed in the presence of (A) glibenclamide (10 µM; N = 7), (B) iberiotoxin (30 nM; N = 9) or (C) apamin (100 nM; N = 7) or their respective vehicle (N = 10). Results are expressed as the net effect of sildenafil where the effect of its vehicle has been subtracted. The data are mean ± SEM of N bladders. Vehicle versus glibenclamide: ###P < 0.001, two-way anova followed by a modified post hoc Student's t-test with the Bonferroni adjustment for multiple comparisons §P < 0.05 at the concentration shown. Vehicle versus iberiotoxin: & &P < 0.01. Vehicle versus apamin & & &P < 0.001, two-way anova followed by Bonferroni post-test: §P < 0.05 at the concentration shown.
Table 5.
Effect of K+ channel modulators on carbachol-induced contractions on human detrusor
| K+channel inhibitor | − | − | Glibenclamide | Glibenclamide | Iberiotoxin | Iberiotoxin | Apamin | Apamin |
|---|---|---|---|---|---|---|---|---|
| CRC sildenafil | − | + | − | + | − | + | − | + |
| Level of contraction induced by carbachol (mg) | 3437 ± 654 | 3931 ± 661 | 2852 ± 912 | 3064 ± 879 | 2641 ± 570 | 2970 ± 396 | 3392 ± 1030 | 3756 ± 833 |
| Maximal inhibition (%) | 14.0 ± 2.4 | 59.4 ± 2.3a | 18.9 ± 0.8b | 42.6 ± 3.8a | 11.8 ± 2.5b | 49.9 ± 1.4a | 12.2 ± 2.5b | 50.5 ± 3.4a |
| Number of experiments | 10 | 10 | 7 | 7 | 9 | 9 | 7 | 7 |
Levels of contraction induced by carbachol were not significantly different between each condition (one-way ANOVA).
Vehicle used for drug pre-incubation was a mixture of each drug's vehicle i.e DMSO 0.1%, acetic acid 5 µM and distilled water.
P < 0.0001, Student's t-test versus corresponding vehicle condition.
Non significant versus first column.
Discussion and conclusions
The clinical efficacy of PDE5 inhibitors in the management of LUTS has been recently demonstrated. Increasing evidence now suggests that their effect on voiding symptoms involves the NO-cGMP signalling pathway (Hedlund, 2005). In contrast, their effect on storage symptoms is less well understood. The present study demonstrates that, in vitro, sildenafil exerts a direct relaxant effect on human detrusor smooth muscle. However, high concentrations of sildenafil are needed to attain this effect. Moreover, this relaxant effect also involves the cGMP-, cAMP- and K+ channel-dependent signalling pathways. In contrast, the contribution of NO to sildenafil-induced relaxation appeared to be minor.
First, we showed that sildenafil relaxed carbachol-pre-contracted strips of human detrusor muscle. Its pD2 on human detrusor smooth muscle was high but was identical to its pD2 on human corpus cavernosum tissue (Oger et al., 2009). As in human corpus cavernosum, the relaxant effect of PDE5 inhibitors is strongly increased by the stimulation of the NO pathway (Corbin, 2004), we hypothesized that the presence of urothelium, which is a potential source of NO in the detrusor (Birder et al., 1998), or the presence of NO donors should enhance sildenafil-induced relaxation in detrusor strips. However, all of these conditions failed to increase the relaxation induced by sildenafil. This result highlights the fact that regulation of human bladder smooth muscle by the NO/cGMP pathway differs markedly according to the region of the bladder studied. Indeed, Filippi et al. (2007), who investigated the effect of NO donors/PDE5 inhibitors in the human bladder neck, reported that SNP relaxed human bladder neck, and this effect was enhanced in the presence of vardenafil confirming that the NO/cGMP pathway was a major influence in the regulation of bladder outlet control. This relaxant effect of PDE5 inhibitors at the bladder neck may in fact explain why PDE5 inhibitors improve voiding symptoms. However, the reason why PDE5 inhibitors also improve storage symptoms, which are more specifically related to bladder dome dysfunction (Chapple et al., 2008), is still unknown. In our study, we have used samples taken from the bladder dome. Clearly, in this region of the bladder, NO does not seem to be a major contributor to sildenafil-induced relaxation in human detrusor, as a NO donor, such as SNAP, did not relax human detrusor and did not enhance relaxation to sildenafil. This is in accordance with earlier investigations showing that the nitrergic innervation of the bladder dome was very sparse when compared to the innervation of the outflow region, that is, the bladder neck, urethra and prostate (Mamas et al., 2003). Moreover, the regulation of bladder smooth muscle by the NO/cGMP pathway may be also very dependent on the species investigated. Indeed, in rat bladder strips, SNP enhanced relaxation to a PDE5 inhibitor (Filippi et al., 2007), while in our study of human bladder muscle, it did not. Such discrepancies could be explained by the fact that PDE5 gene expression is twice as high in rats as it is in human tissues (Behr-Roussel et al., 2008). Thus, the effect exerted by sildenafil on the human bladder dome may not completely explain the improvement of urinary storage symptoms observed in sildenafil-treated patients. Indeed, it is likely that an effect of sildenafil on bladder sensory afferent signalling pathways may be more responsible for this improvement (Giuliano, 2008).
This study was pursued by investigating the signalling pathways involved in sildenafil-induced relaxation. These relaxant responses were significantly reduced by the inhibition of guanylate cyclase by ODQ, confirming the involvement of the cGMP pathway, although the pD2 for inhibiting human detrusor smooth muscle was higher than the pD2 of sildenafil for inhibiting the cGMP-dependent PDE5 isoenzyme (Bischoff, 2004). However, relaxation to sildenafil was only slightly inhibited by ODQ, indicating one or several additional mechanism(s) of relaxation, independent of the cGMP pathway. Interestingly, sildenafil-induced relaxation was more extensively reduced by the inhibition of adenylate cyclase, suggesting that the cAMP signalling pathway was also involved in this relaxation. Such an effect could be ascribed to the inhibition of PDEs, specific for cAMP. In human detrusor muscle, the PDEs that specifically hydrolyse cAMP, PDE1, PDE2, PDE3 and PDE4 are expressed, but only PDE1 and PDE4 have been shown to have a functional role in regulating the contractile activity (Truss et al., 2000; Oger et al., 2007). As the selectivity of sildenafil is better for PDE1 than for PDE4 (Bischoff, 2004), sildenafil could elicit relaxation through an increase in endogenous cAMP levels mediated by inhibition of PDE1. An involvement of the cAMP signalling pathway has already been described in other smooth muscles such as human corpus cavernosum or rat duodenum in which sildenafil (at a concentration of 1 µM) was shown to increase cAMP levels (Stief et al., 2000; Prieto et al., 2006b; Clemente et al., 2008). Interestingly, when the cAMP signalling pathway was inhibited with MDL-12,330A, the addition of SNAP counteracted in part the effect of the inhibitor, allowing an increased relaxation to sildenafil, while the addition of SNAP without MDL-12,330A did not influence sildenafil-induced relaxation. Thus, it may be that the inhibition of cAMP production promotes the activation of cGMP pools by NO, which could be involved in sildenafil relaxation, supporting a cross-talk between the cAMP and cGMP signalling pathways in human detrusor. It would be interesting to evaluate if the mechanisms of action of sildenafil differ in a system where cAMP response is impaired, such as in animal models of bladder outlet obstruction (Khan et al., 1999).
As with many other smooth muscle cell types, K+ channels play a critical role in the modulation of detrusor myocyte tone. The central role played by K+ channels in modulating bladder function derives from their functionally antagonistic relationship with transmembrane calcium flux through voltage-dependent calcium channels (Christ and Hodges, 2006). Thus, KCa and KATP channels are physiologically relevant modulators of detrusor myocyte excitability. In the present study, we found that sildenafil-induced relaxation of detrusor involved KATP, BKCa and SKCa channels. Similar mechanisms have been previously described in several other systems and are compatible with the fact that activation of BKCa and KATP channels can be regulated by cGMP or cAMP through PKG or PKA (Lee & Kang, 2001; Geeson et al., 2002; Gragasin et al., 2004; Prieto et al., 2006a; Medeiros et al., 2008).
In conclusion, the present study contributes to the understanding of the mechanisms responsible for the beneficial effect of PDE5 inhibitors on urinary storage symptoms. Indeed, we showed that sildenafil exerted a relaxant effect on human bladder dome smooth muscle. The major components of sildenafil-induced relaxation of human detrusor tissue, pre-contracted with carbachol, also included signalling pathways, independent of the nitrergic component, unlike many other smooth muscles. Sildenafil-induced relaxation involved both the cGMP and the cAMP signalling pathways, and also the activation of KCa and KATP channels. However, the contribution of this relaxation to sildenafil that we have shown in vitro to the clinical benefit of PDE5 inhibitors on storage symptoms is still unknown. Further investigations are needed, more particularly on the effect of PDE5 inhibitors on the micturition reflex. A fuller understanding of the mechanisms of action of PDE5 inhibitors on LUTS is crucial for the further development of these compounds in this clinical setting.
Conflict of interest
None to declare.
Glossary
Abbreviations:
- ATP
activated potassium channel: KATP channel
- BKCa
channel: large conductance calcium-activated potassium channel
- DMSO
dimethyl sulphoxide
- ED
erectile dysfunction
- LUTS
lower urinary tract symptom
- ODQ
[1H-[1,2,4]oxadiazolo-[4,3-a]quinoxalin-1-one]
- PDE5
phosphodiesterase 5
- PKA
protein kinase cAMP dependent
- PKG
protein kinase cGMP dependent
- SKCa
channel: small conductance calcium-activated potassium channel
- SNAP
S-nitroso-N-acetylpenicillamine
- SNP
sodium nitroprusside
References
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC) Br J Pharmacol. (4th edn.) 2009;158(Suppl. 1):S1–S254. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behr-Roussel D, Oger S, Tinel H, Sandner P, Giuliano F. PDE5 gene expression and relaxant effects of vardenafil in male and female human and rat detrusor muscle. J Urol. 2008;176:541. [Google Scholar]
- Birder LA, Apodaca G, De Groat WC, Kanai AJ. Adrenergic- and capsaicin-evoked nitric oxide release from urothelium and afferent nerves in urinary bladder. Am J Physiol. 1998;275:F226–F229. doi: 10.1152/ajprenal.1998.275.2.F226. [DOI] [PubMed] [Google Scholar]
- Bischoff E. Potency, selectivity, and consequences of nonselectivity of PDE inhibition. Int J Impot Res. 2004;16(Suppl. 1):S11–S14. doi: 10.1038/sj.ijir.3901208. [DOI] [PubMed] [Google Scholar]
- Chaiyaprasithi B, Mang CF, Kilbinger H, Hohenfellner M. Inhibition of human detrusor contraction by a urothelium derived factor. J Urol. 2003;170:1897–1900. doi: 10.1097/01.ju.0000091870.51841.ae. [DOI] [PubMed] [Google Scholar]
- Chapple CR, Wein AJ, Abrams P, Dmochowski RR, Giuliano F, Kaplan SA, et al. Lower urinary tract symptoms revisited: a broader clinical perspective. Eur Urol. 2008;54:563–569. doi: 10.1016/j.eururo.2008.03.109. [DOI] [PubMed] [Google Scholar]
- Christ GJ, Hodges S. Molecular mechanisms of detrusor and corporal myocyte contraction: identifying targets for pharmacotherapy of bladder and erectile dysfunction. Br J Pharmacol. 2006;147(Suppl. 2):S41–S55. doi: 10.1038/sj.bjp.0706627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemente CM, Araujo PV, Palheta RC, Jr, Ratts ZM, Fernandes GH, Rola FH, et al. Sildenafil inhibits duodenal contractility via activation of the NO-K+ channel pathway. Fundam Clin Pharmacol. 2008;22:61–67. doi: 10.1111/j.1472-8206.2007.00549.x. [DOI] [PubMed] [Google Scholar]
- Corbin JD. Mechanisms of action of PDE5 inhibition in erectile dysfunction. Int J Impot Res. 2004;16(Suppl. 1):S4–S7. doi: 10.1038/sj.ijir.3901205. [DOI] [PubMed] [Google Scholar]
- Corbin JD, Francis SH, Webb DJ. Phosphodiesterase type 5 as a pharmacologic target in erectile dysfunction. Urology. 2002;60:4–11. doi: 10.1016/s0090-4295(02)01686-2. [DOI] [PubMed] [Google Scholar]
- Filippi S, Morelli A, Sandner P, Fibbi B, Mancina R, Marini M, et al. Characterization and functional role of androgen-dependent PDE5 activity in the bladder. Endocrinology. 2007;148:1019–1029. doi: 10.1210/en.2006-1079. [DOI] [PubMed] [Google Scholar]
- Gacci M, Del Popolo G, Macchiarella A, Celso M, Vittori G, Lapini A, et al. Vardenafil improves urodynamic parameters in men with spinal cord injury: results from a single dose, pilot study. J Urol. 2007;178:2040–2043. doi: 10.1016/j.juro.2007.07.048. [DOI] [PubMed] [Google Scholar]
- Geeson J, Larsson K, Hourani SM, Toms NJ. Sodium nitroprusside-induced rat fundus relaxation is ryanodine-sensitive and involves L-type Ca2+ channel and small conductance Ca(2+)-sensitive K+ channel components. Auton Autacoid Pharmacol. 2002;22:297–301. doi: 10.1046/j.1474-8673.2002.00271.x. [DOI] [PubMed] [Google Scholar]
- Giuliano F. Phosphodiesterase type 5 inhibitors improve male lower urinary tract symptoms. Eur Urol. 2008;53:1121–1123. doi: 10.1016/j.eururo.2008.02.026. [DOI] [PubMed] [Google Scholar]
- Gragasin FS, Michelakis ED, Hogan A, Moudgil R, Hashimoto K, Wu X, et al. The neurovascular mechanism of clitoral erection: nitric oxide and cGMP-stimulated activation of BKCa channels. FASEB J. 2004;18:1382–1391. doi: 10.1096/fj.04-1978com. [DOI] [PubMed] [Google Scholar]
- Hedlund P. Nitric oxide/cGMP-mediated effects in the outflow region of the lower urinary tract – is there a basis for pharmacological targeting of cGMP? World J Urol. 2005;23:362–367. doi: 10.1007/s00345-005-0019-1. [DOI] [PubMed] [Google Scholar]
- Khan MA, Thompson CS, Angelini GD, Morgan RJ, Mikhailidis DP, Jeremy JY. Prostaglandins and cyclic nucleotides in the urinary bladder of a rabbit model of partial bladder outlet obstruction. Prostaglandins Leukot Essent Fatty Acids. 1999;61:307–314. doi: 10.1054/plef.1999.0105. [DOI] [PubMed] [Google Scholar]
- Lee SW, Kang TM. Effects of nitric oxide on the Ca2+-activated potassium channels in smooth muscle cells of the human corpus cavernosum. Urol Res. 2001;29:359–365. doi: 10.1007/s002400100211. [DOI] [PubMed] [Google Scholar]
- McVary KT, Monnig W, Camps JL, Jr, Young JM, Tseng LJ, van den Ende G. Sildenafil citrate improves erectile function and urinary symptoms in men with erectile dysfunction and lower urinary tract symptoms associated with benign prostatic hyperplasia: a randomized, double-blind trial. J Urol. 2007a;177:1071–1077. doi: 10.1016/j.juro.2006.10.055. [DOI] [PubMed] [Google Scholar]
- McVary KT, Roehrborn CG, Kaminetsky JC, Auerbach SM, Wachs B, Young JM, et al. Tadalafil relieves lower urinary tract symptoms secondary to benign prostatic hyperplasia. J Urol. 2007b;177:1401–1407. doi: 10.1016/j.juro.2006.11.037. [DOI] [PubMed] [Google Scholar]
- Mamas MA, Reynard JM, Brading AF. Nitric oxide and the lower urinary tract: current concepts, future prospects. Urology. 2003;61:1079–1085. doi: 10.1016/s0090-4295(03)00131-6. [DOI] [PubMed] [Google Scholar]
- Matsumoto S, Hanai T, Uemura H. Chronic treatment with a PDE5 inhibitor increases contractile force of normal bladder in rats. Int Urol Nephrol. 2009;42:53–56. doi: 10.1007/s11255-009-9564-7. [DOI] [PubMed] [Google Scholar]
- Medeiros JV, Gadelha GG, Lima SJ, Garcia JA, Soares PM, Santos AA, et al. Role of the NO/cGMP/K(ATP) pathway in the protective effects of sildenafil against ethanol-induced gastric damage in rats. Br J Pharmacol. 2008;153:721–727. doi: 10.1038/sj.bjp.0707605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon A. Influence of nitric oxide signalling pathways on pre-contracted human detrusor smooth muscle in vitro. BJU Int. 2002;89:942–949. doi: 10.1046/j.1464-410x.2002.02795.x. [DOI] [PubMed] [Google Scholar]
- Morelli A, Filippi S, Sandner P, Fibbi B, Chavalmane AK, Silvestrini E, et al. Vardenafil modulates bladder contractility through cGMP-mediated inhibition of RhoA/Rho kinase signaling pathway in spontaneously hypertensive rats. J Sex Med. 2009;6:1594–1608. doi: 10.1111/j.1743-6109.2009.01249.x. [DOI] [PubMed] [Google Scholar]
- Oger S, Behr-Roussel D, Gorny D, Denys P, Lebret T, Alexandre L, et al. Relaxation of phasic contractile activity of human detrusor strips by cyclic nucleotide phosphodiesterase type 4 inhibition. Eur Urol. 2007;51:772–780. doi: 10.1016/j.eururo.2006.10.023. [DOI] [PubMed] [Google Scholar]
- Oger S, Behr-Roussel D, Gorny D, Lecoz O, Lebret T, Denoux Y, et al. Combination of doxazosin and sildenafil exerts an additive relaxing effect compared with each compound alone on human cavernosal and prostatic tissue. J Sex Med. 2009;6:836–847. doi: 10.1111/j.1743-6109.2008.01138.x. [DOI] [PubMed] [Google Scholar]
- Persson K, Andersson KE. Nitric oxide and relaxation of pig lower urinary tract. Br J Pharmacol. 1992;106:416–422. doi: 10.1111/j.1476-5381.1992.tb14349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto D, Rivera L, Benedito S, Recio P, Villalba N, Hernandez M, et al. Ca2+-activated K+ (KCa) channels are involved in the relaxations elicited by sildenafil in penile resistance arteries. Eur J Pharmacol. 2006a;531:232–237. doi: 10.1016/j.ejphar.2005.12.033. [DOI] [PubMed] [Google Scholar]
- Prieto D, Rivera L, Recio P, Rubio JL, Hernandez M, Garcia-Sacristan A. Role of nitric oxide in the relaxation elicited by sildenafil in penile resistance arteries. J Urol. 2006b;175:1164–1170. doi: 10.1016/S0022-5347(05)00320-4. [DOI] [PubMed] [Google Scholar]
- Roehrborn CG, McVary KT, Elion-Mboussa A, Viktrup L. Tadalafil administered once daily for lower urinary tract symptoms secondary to benign prostatic hyperplasia: a dose finding study. J Urol. 2008;180:1228–1234. doi: 10.1016/j.juro.2008.06.079. [DOI] [PubMed] [Google Scholar]
- Rosen R, Altwein J, Boyle P, Kirby RS, Lukacs B, Meuleman E, et al. Lower urinary tract symptoms and male sexual dysfunction: the multinational survey of the aging male (MSAM-7) Eur Urol. 2003;44:637–649. doi: 10.1016/j.eururo.2003.08.015. [DOI] [PubMed] [Google Scholar]
- Stief CG, Uckert S, Becker AJ, Harringer W, Truss MC, Forssmann WG, et al. Effects of sildenafil on cAMP and cGMP levels in isolated human cavernous and cardiac tissue. Urology. 2000;55:146–150. doi: 10.1016/s0090-4295(99)00371-4. [DOI] [PubMed] [Google Scholar]
- Stief CG, Porst H, Neuser D, Beneke M, Ulbrich E. A randomised, placebo-controlled study to assess the efficacy of twice-daily vardenafil in the treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia. Eur Urol. 2008;53:1236–1244. doi: 10.1016/j.eururo.2008.01.075. [DOI] [PubMed] [Google Scholar]
- Tinel H, Stelte-Ludwig B, Hutter J, Sandner P. Pre-clinical evidence for the use of phosphodiesterase-5 inhibitors for treating benign prostatic hyperplasia and lower urinary tract symptoms. BJU Int. 2006;98:1259–1263. doi: 10.1111/j.1464-410X.2006.06501.x. [DOI] [PubMed] [Google Scholar]
- Truss MC, Uckert S, Stief CG, Kuczyk M, Jonas U. Cyclic nucleotide phosphodiesterase (PDE) isoenzymes in the human detrusor smooth muscle. I. Identification and characterization. Urol Res. 1996;24:123–128. doi: 10.1007/BF00304074. [DOI] [PubMed] [Google Scholar]
- Truss MC, Stief CG, Uckert S, Becker AJ, Schultheiss D, Machtens S, et al. Initial clinical experience with the selective phosphodiesterase-I isoenzyme inhibitor vinpocetine in the treatment of urge incontinence and low compliance bladder. World J Urol. 2000;18:439–443. doi: 10.1007/pl00007088. [DOI] [PubMed] [Google Scholar]
- Werkstrom V, Hedlund P, Lee T, Andersson KE. Vardenafil-induced relaxation and cyclic nucleotide levels in normal and obstructed rat urinary bladder. BJU Int. 2009;104:1740–1745. doi: 10.1111/j.1464-410X.2009.08651.x. [DOI] [PubMed] [Google Scholar]
- Yanai Y, Hashitani H, Hayase M, Sasaki S, Suzuki H, Kohri K. Role of nitric oxide/cyclic GMP pathway in regulating spontaneous excitations in detrusor smooth muscle of the guinea-pig bladder. Neurourol Urodyn. 2008;27:446–453. doi: 10.1002/nau.20517. [DOI] [PubMed] [Google Scholar]


