Abstract
Background and purpose:
9,10-Dihydro-2,5-dimethoxyphenanthrene-1,7-diol (RSCL-0520) is a phenanthrene isolated from Eulophia ochreata, one of the Orchidaceae family, known by local tradition to exhibit medicinal properties. However, no anti-inflammatory activity or any molecular mechanisms involved have been reported or elucidated. Here, for the first time, we evaluate the anti-inflammatory properties of RSCL-0520 on responses induced by lipopolysaccharide (LPS) and mediated via Toll-like receptors (TLRs).
Experimental approach:
The in vitro anti-inflammatory activities of RSCL-0520 were investigated in LPS-stimulated monocytic cells, measuring activation of cytokine and inflammatory genes regulated by nuclear factor-κB (NF-κB). Tumour necrosis factor (TNF)-α levels in serum following LPS stimulation in mice and carrageenan-induced paw oedema in rats were used as in vivo models.
Key results:
Pretreatment with RSCL-0520 effectively inhibited LPS-induced, TLR4-mediated, NF-κB-activated inflammatory genes in vitro, and reduced both LPS-induced TNF-α release and carrageenan-induced paw oedema in rats. Treatment with RSCL-0520 reduced LPS-stimulated mRNA expression of TNF-α, COX-2, intercellular adhesion molecule-1, interleukin (IL)-8 and IL-1β, all regulated through NF-κB activation. RSCL-0520, however, did not interfere with any cellular processes in the absence of LPS.
Conclusions and implications:
RSCL-0520 blocked signals generated by TLR4 activation, as shown by down-regulation of NF-κB-regulated inflammatory cytokines. The inhibitory effect involved both MyD88-dependent and -independent signalling cascades. Our data elucidated the molecular mechanisms involved, and support the search for plant-derived TLR antagonists, as potential anti inflammatory agents.
Keywords: Eulophia ochreata, monocytes, inflammation, lipopolysaccharide, TLR
Introduction
Inflammation is a response of a tissue to injury, frequently caused by invading organisms such as bacteria. They initiate inflammation through several interconnecting mechanisms mainly through specific surface molecules called pathogen-associated molecular patterns which bind to Toll-like receptors (TLRs; nomenclature follows Alexander and Rietschel, 2001) leading to the release of a wide variety of cytokines. These secreted cytokines provide signals between immune cells to coordinate the inflammatory response. Some cytokines like interleukin-1β (IL-1β) and IL-6 act to broadly provoke the inflammatory response, while others act on specific types of immune cells. Tumour necrosis factor (TNF)-α, the key signalling cytokine, mediates inflammatory process both in a direct way and an indirect way by inducing signalling of other inflammatory cytokines and molecules (Aggarwal, 2003).
Of the 11 TLRs identified in humans (Kawai and Akira, 2006), TLR4, the most studied receptor, recognizes lipopolysaccharide (LPS), the major component of the outer membrane of Gram-negative bacteria. Its three structural elements: a core oligosaccharide, an O-specific chain of repeating sequences of polysaccharides and a lipid A component, are responsible for its pro-inflammatory properties (Alexander and Rietschel, 2001). LPS activates monocytes and macrophages triggering the production of pro-inflammatory mediators, which in turn modulate endothelial functions. These mediators collectively initiate a cascade of events leading to inflammation and various other clinical manifestations. LPS signals mainly via TLR4 receptors (Dauphinee and Karsan, 2006), although TLR2 involvement has also been documented in some Gram-negative bacteria (Darveau et al., 2004). The LPS-initiated signalling cascade leads to stimulation of both a myeloid differentiation primary response gene (88) (MyD88)-dependent and an MyD88-independent pathway (Takeda and Akira, 2004) involving nuclear factor-κB (NF-κB), mitogen-activated protein kinase and phosphatidylinositol 3-kinase/Akt pathways.
MyD88, an adaptor molecule, is recruited to the TLR4 receptor through its interaction with the Toll/IL-1 receptor (TIR) domain of TLR4 (O'Neill et al., 2003), which in turn facilitates the recruitment of subsequent molecules involved in the cascade: IL-1R-associated kinase-1 (IRAK1), IRAK4 (Wesche et al., 1997; Li et al., 2002), Toll receptor IL-1R domain-containing adapter protein (TIRAP) (Fitzgerald et al., 2001) and TNF-α receptor-associated factor-6 (TRAF6). Subsequently, this IRAK1–TRAF6 complex dissociates from the receptor to form a complex with transforming growth factor-β (TGF-β)-activated kinase 1 (TAK1) and its adaptor molecules, TAK1-binding protein 1 and 2 (TAB1 and TAB2) (Jiang et al., 2002). TAK1 activation subsequently leads to signalling through NF-κB essential modulator (NEMO) (Wang et al., 2001), leading to the oligomerization of TRAF6, and enhances the auto-ubiquitinating activity of TRAF6, which facilitates the downstream activation of members of NF-κB (IκB) family. These are released, and translocate to the nucleus and activate synthesis of inflammatory mediators (Guha and Mackman, 2001).
This study reports that a plant-derived molecule, 9,10-dihydro-2,5-dimethoxyphenanthrene-1,7-diol (referred to as RSCL-0520) exhibited novel anti-inflammatory activity. It blocked TLR4-induced signalling via the NF-κB pathway by inhibiting MyD88-mediated signalling mechanisms. RSCL-0520, a naturally occurring phenanthrene, was isolated from a plant species, Eulophia ochreata, belonging to the Orchidaceae. It has been classified botanically by Dr Rajendra Kshirsagar, with its herbarium deposited at Reliance Life Sciences, Dhirubhai Ambani Life Sciences Center, Navi Mumbai, India (herbarium voucher specimen accession no. 157). It is a terrestrial herb, with its rhizome tubers, up to 20 in number, growing in horizontal chains. Very little is known scientifically about its pharmacological properties, although the tubers of this plant have been used in local traditional medicine for a variety of diseases. Tuber juice is also applied externally for curing rheumatism. In the current study, we sought to assess the anti-inflammatory properties of this phenanthrene, isolated from E. ochreata, and have attempted to establish the possible mechanisms involved in its actions.
Methods
Isolation of RSCL-0520
RSCL-0520 (Figure 1A) was isolated from E. ochreata collected from Satpuda mountain range, Maharashtra, India. Powdered E. ochreata tubers (1 kg) were subjected to sequential cold extraction process. The solvents used for the extraction process were in increasing order of polarity. Petroleum ether (PE) (60–80) treatment was followed by dichloromethane, followed by ethyl acetate (EA), methanol and finally water. Throughout the extraction process, the material-to-solvent ratio was maintained at 1:6. The final aqueous extract was prepared using reverse osmosis-purified (removes most of the toxins, bacteria, viruses, suspended solids and dissolved chemicals) water in 1:10 ratio. The extraction processes were carried out by constant stirring for 6–7 h in stainless steel vessels at room temperature. The extracts obtained from each process were filtered and lyophilized. This systematic extraction process was repeated three times, and the extracts (3 × 6 L) from each process were concentrated to dryness under controlled temperature (42–45°C) and reduced pressure using a rotary evaporator. An aliquot from each extraction process was checked for antioxidant activity using 1,1-diphenyl-2-picrylhydrazyl assay. Dichloromethane and EA extracts (∼30 g), which showed significant antioxidant activity, were dissolved in methanol, adsorbed on 30 g silica gel (60–120 mesh) and chromatographed over 300 g silica gel (60–120 mesh) in glass columns (90 × 5 cm). The flow rate of the eluent was 50 mL·min−1, and fraction size was 100 mL. The elution was carried out in the following order, using 1.5 L of each eluent, shown as % EA in PE: 5, 10, 11, 12, 13, 15, 16, 18, 20, 25, 50 and finally 100% EA. All fractions were monitored on TLC plates (silica gel 60 F254; solvent system-PE : EA, 45:55, v/v). Similar fractions were pooled together and concentrated under reduced pressure. The fraction eluted with 11% EA in PE yielded ∼200 mg RSCL-0520, which was checked for purity (>95%) by HPLC (Figure 2). Its structure was confirmed with 2D NMR spectroscopy and MS.
Figure 1.
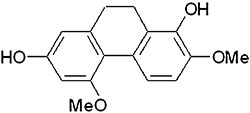
Structure of RSCL-0520.
Figure 2.
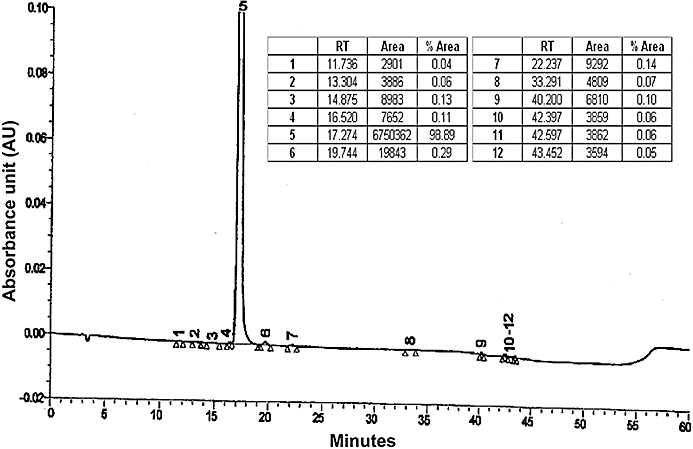
HPLC profile of isolated RCSL 0520. Extracts of Eulophia ochreata were purified, using activity-guided chromatographic fractionation on silica gel. On subsequent HPLC, the most active fraction showed a single peak (fraction 5) with a retention time of 17.3 min, exhibiting >98% of the absorbance eluted and was RSCL-0520 (∼99% pure).
Cells
The THP-1 pro-monocytic cell line and RAW264.7 cells, obtained from ATCC (Manassas, VA, USA), were cultured in Roswell Park Memorial Institute (RPMI) 1640 containing 10% heat-inactivated fetal calf serum (FCS) supplemented with 1% penicillin/streptomycin under standard conditions. Human peripheral blood mononuclear cells (PBMCs) were isolated from peripheral blood obtained from healthy human volunteers with informed consent and after review by the local ethical committee. The PBMCs were prepared by density gradient centrifugation using HistoPaque-1077 (Sigma-Aldrich, St Louis, MO, USA) and suspended in RPMI 1640 medium containing 10% heat-inactivated FCS, 100 U·mL−1 penicillin G and 100 µg·mL−1 streptomycin.
TLR ligand-induced production of TNF-α
THP-1 cells (2 × 105 cells per well) in 96-well culture plates were stimulated with various selective TLR ligands, used according to the manufacturer's instruction: TLR1–2 with Pam3CSK4, a synthetic tripalmitoylated lipopeptide (75 ng·mL−1); TLR3 with poly{I:C} potassium salt, a synthetic analog of double-stranded RNA (75 µg·mL−1); TLR4 with LPS from S. minnesota R595 (250 ng·mL−1); TLR5 with flagellin, the major component of the bacterial flagellar filament (75 ng·mL−1); TLR6 with the macrophage stimulatory lipopeptide MALP-2 (75 ng·mL−1); TLR7–8 with poly{U} potassium salt, a simple motif single-stranded RNA (7.5 µg·mL−1); and TLR9 with CpG ODN 2395, synthetic oligonucleotides containing unmethylated CpG dinucleotides (7.5 µg·mL−1), for 24 h in the presence or absence of RSCL-0520. Culture supernatants were assayed for secreted TNF-α using specific Duo-Set elisa development systems (R&D Systems, Minneapolis, MN, USA) according to the manufacturer's instructions. For assays involving PBMCs and RAW264.7 cells, the 96-well plates were seeded similarly (2 × 105 cells per well). A similar protocol was followed for assaying TNF-α in these cells. For all the experiments, RSCL-0520 was dissolved in DMSO, diluted with appropriate medium and added to the cells 1 h prior to any stimulation.
Cell viability assay
Cell viability was assessed by morphology and by reduction of the tetrazolium salt 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) by mitochondrial dehydrogenases, according to the manufacturer's instruction (Sigma). THP-1 cells (2 × 105 cells) were treated with RSCL-0520 (1–100 µM), and the plates were incubated for 24 h. The cells were then washed once before adding 50 µL of FCS-free medium containing MTT (5 mg·mL−1). After 4 h of incubation at 37°C, the medium was discarded and the formazan blue that formed in the cells was dissolved in DMSO. The optical density was measured at 570 nm.
Western blot analysis
THP-1 cells (1 × 106cells·mL−1) in serum-free RPMI 1640 medium were incubated with RSCL-0520 for 60 min before LPS treatment. As control, the cells were treated with LPS alone. Following incubation at indicated times, the medium was aspirated, and cells lysed in RIPA buffer [1 × PBS, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulphate (SDS), 1 mM sodium orthovanadate, 10 µg·mL−1 PMSF and 1 µL·mL−1 protease inhibitor cocktail]. The concentration of protein in each sample was quantified by the Bradford method (Bio-Rad, Hercules, CA, USA). Then, 25 µg of cytoplasmic proteins was size fractionated in 10% SDS–PAGE gel and transferred to nitrocellulose membrane BioTraceNT (Pall Corporation, Port Washington, NY, USA). To determine the levels of MyD88, TIRAP, NEMO and total IκBα, the blots were probed with rabbit polyclonal antibodies against respective proteins (Stressgen, Assay Designs Inc, Ann Arbor, MI, USA). Horseradish peroxidase-conjugated secondary antibodies were used to develop the membrane, and visualization of bands was performed using Chemiluminescent substrate (ECL, Amersham, Arlington Heights, IL, USA). Blots were stripped and reprobed using a 1/1000 dilution of antibody against ERK-1/2 to normalize the protein loading. The bands obtained were quantitated using ImageJ software version 1.42.
Preparation of nuclear extracts
Nuclear extracts were prepared from THP-1 cells according to the modified procedure of Dignam et al. (1983). Briefly, 3 × 106 cells were pretreated with RSCL-0520 for 1 h followed by LPS treatment for 30, 60 and 120 min. Cells were lysed in 400 µL of lysis buffer [10 mM HEPES (pH 7.9), 100 mM KCl, 1.5 mM MgCl2, 0.1 mM EGTA, 0.5 mM DTT, 0.5 mM PMSF, 0.5% Nonidet P-40 and 1 µL·mL−1 protease inhibitor cocktail (Calbiochem, La Jolla, CA, USA)] on ice for 30 min, followed by vigorous vortex mixing for 5–10 s. The homogenate was centrifuged in a microfuge at 10 000×g for 30 s. The supernatant was discarded, and the nuclear pellet was resuspended in 50 µL of nuclear extraction buffer [10 mM HEPES (pH 7.9), 1.5 mM MgCl2, 420 mM NaCl, 0.1 mM EGTA, 0.5 mM DTT, 5% glycerol, 0.5 mM PMSF and 1 µL·mL−1 protease inhibitor cocktail]. The tube was mixed intermittently for 60 min. The nuclear extract was obtained by centrifuging at 10 000×g for 10 min at 4°C. The concentration of protein in each sample was then quantified by the Bradford method. Nuclear extracts were resolved on 10% SDS–polyacrylamide gels. After electrophoresis, the proteins were electrotransferred to nitrocellulose filters, probed with anti-p65 antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and detected by chemiluminescence. The bands obtained were quantitated using ImageJ software version 1.42.
Real-time quantitative PCR analysis of TNF-α expression
RSCL-0520 (50 µM)-treated THP-1 cells (3 × 106 cells per well) in six-well plates were stimulated with 250 ng·mL−1 LPS for 1 h. Total RNA was isolated from these cells and cDNA was synthesized. LPS-treated cells acted as positive control. All quantitative real-time PCR (TaqMan) primers and probes were obtained from Applied Biosystems (Foster City, CA, USA). For detection of TNF-α, pre-developed assay reagents were used. The PCR was performed utilizing 1 µL cDNA per reaction in triplicates of 25 µL volume on an ABI 7500 Realtime PCR machine using a two-step PCR protocol after the initial denaturing of the cDNA (10 min at 95°C) with 40 cycles of 95°C for 15 s and 60°C for 1 min. Universal master mix obtained from Applied Biosystems included Taq-polymerase and all other reaction reagents excluding specific primers and probes. All amplification batches included no template controls (NTC). Quantitation of mRNA was performed using the comparative threshold cycle method. The highest control level attained by the stimulation (without RSCL-0520) was regarded as 100%, and the levels of control group at other time-points and RSCL-0520-added group were expressed as the percentage of the highest control level. Data were analysed using standard software.
RT-PCR analysis
THP-1 cells (3 × 106 cells) were treated with RSCL-0520 (50 µM) for 1 h followed by incubation with or without 250 ng·mL−1 of LPS. After two washes with ice-cold PBS, the cells were harvested and total cellular RNA was isolated using TRIZOL Reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. cDNA was synthesized using high-capacity cDNA reverse transcription kits (ABI systems, Foster City, CA, USA). Amplification of ICAM-1, COX-2, IL-1β and IL-8 genes from the cDNA was carried out using the respective gene specific primers:
ICAM-1 5′-CTGATGGGCAGTCAACAGCTAAAA-3′ (S)
5′-TCCAGTTCAGTGCGGCACGAGAA-3′ (AS)
COX-2, 5′-ATGAGATTGTGGGAAAATTGCT-3′ (S)
5′-GGTAGATCATCTCTGCCTGAGTATC-3′ (AS)
•IL-1β 5′-GACACATGGGATAACGAGGCT-3′ (S)
5′-TTAGGAAGACACAAATTGCAT-3′ (AS)
•IL- 8,
5′-GCCAAGGAGTGCTAAAGAACTTAG-3′ (S)
5′-GAATTCTCAGCCCTCTTCAAAAAC-3′ (AS)
β-Actin, an internal control, was also amplified using the following primers: 5′-TCCTCCCTGGAGAAGAGC TA-3′ (sense) and 5′-AGTACTTGCGCTCAGGAGGAC-3′ (antisense).
Further, TLR-related genes (TIRAP, MyD88, TRIF, IRAK-1, IRAK-4, IRAK-6 and TRAF6) were amplified from the cDNA extracted from a similar experimental set using the respective gene-specific primers (Nishimura and Naito, 2005).
The amplified PCR products were then analysed on a 2% agarose gel electrophoresis. The bands obtained were quantitated using ImageJ software version 1.42.
Fluorescence-activated cell sorting (FACS)
For the detection of intracellular location of NF-κB phospho-p65 subunits, RSCL-0520-treated THP-1 (5 × 105 cells) were stimulated with LPS for 1 h. Post-LPS treatment, the cells were fixed with 4% paraformaldehyde in PBS for 30 min and washed with FACS buffer (2% in 1× PBS). The cells were then permeabilized with 90% methanol for 30 min at 4°C, followed by washes with FACS buffer. The permeabilized cells were then treated with phospho-p65 monoclonal antibody tagged with Alexa Fluor 488 (Cell Signaling Technology, Inc, Danvers, MA, USA) for 1 h at 37°C, followed by washing with FACS buffer. The cells were then resuspended in PBS and acquired in BD FACS Calibur (BD Biosciences, San Jose, CA, USA).
In vivo experiments
LPS-induced TNF-α release in Balb/c mice
All animal care and experimental protocols were approved by the local animal research board committee: Committee for the Purpose of Control and Supervision of Experiments on Animals and the Institutional Animal Ethics Committee. Specific pathogen-free female Balb/c mice (6–8 weeks old) were treated with two doses of RSCL-520 (10 and 20 mg·kg−1) given i.p., 30 min before LPS (225 µg per mouse). Mice receiving RSCL-0520 without LPS served as negative control. Blood collection was done retro-orbitally under anaesthesia (ether) 1 h after LPS injection. The serum collected was analysed for TNF-α using a Duo-Set elisa (R&D Systems, Minneapolis, MN, USA) according to the manufacturers' instructions.
Carrageenan-induced paw oedema in rats
The carrageenan-induced rat paw oedema model was set up according to the general procedure of Winter et al. (1962). The anti-inflammatory activity was determined as the percent inhibition of oedema formation in the hind paw of male Wistar rats (weighing 180–200 g, n = 5) in response to a sub-plantar injection of carrageenan. The carrageenan was injected as a freshly prepared 1% solution in PBS (0.1 mL), 30 min after injection (i.p.) of 10 and 20 mg·kg−1 of RSCL-0520. Oedema formation was then assessed after the carrageenan injection by measuring the volume (in mL) of the injected paw at 0, 2, 4 and 6 h time intervals using a plethysmometer (Ugo Basile model 7140, Cornerio, Varese, Italy). The increase in volume in the subsequent hours after carrageenan injection constitutes the individual response. The non-treated hind paw of the same rat was used as the control. Swelling (in mL) was then calculated in untreated, vehicle- and drug-treated animals. Inhibition was then derived through comparison with the vehicle control group.
Statistical analysis
Statistical analysis of the responses obtained from control and LPS-treated THP-1 cells, and from the in vivo experiments was conducted by one-way anova using Instat 2 software program (GraphPad, La Jolla, CA, USA). The Newman–Keuls test was used for multiple comparisons. Values of P < 0.05 were considered as significant.
Materials
LPS (from Escherichia coli serotype O55:B5) was from Sigma-Aldrich; penicillin, streptomycin, RPMI 1640 medium, sodium pyruvate and FBS were obtained from Gibco (Invitrogen). Tris, glycine, β-mercaptoethanol, glucose, sodium bicarbonate, sodium chloride (NaCl), SDS, BSA and MTT were obtained from Sigma-Aldrich. Polyclonal antibodies: anti-p65, ERK-1/2 and IκB-α were obtained from Cell Signaling Technology, Inc (Beverly, MA, USA). Antibodies for MyD88 and NEMO were obtained from Stressgen. COX-2 antibody was from BD Biosciences (San Jose, CA, USA). Anti-rabbit secondary HRP was obtained from Jackson Immuno research (West Grove, PA, USA). Trizol was obtained from Invitrogen. Chemiluminescence ECL was purchased from Amersham. TNF-α, IL-1β and IL-8 Duoset elisa detection Kits (R&D Systems), TLR ligands (1–9) were purchased from Apotech, Geneva, Switzerland. RT-PCR kits were from Abgene (Epsom, Surrey, UK). cDNA synthesis kit was from ABI systems. Carrageenan was procured from Sigma. All other reagents and chemicals were purchased from Sigma unless stated otherwise.
Results
RSCL-0520 inhibits the production of inflammatory cytokine TNF-α from LPS-stimulated THP-1, RAW264.7 cells and PBMCs.
THP-1 cells were pre-incubated with various concentrations of RSCL-0520 (1–100 µM), and then exposed to LPS (250 ng·mL−1). TNF-α was measured in the culture supernatants after 24 h incubation with LPS (Figure 3A). RSCL-0520 inhibited TNF-α production in a concentration-dependent manner, with its IC50 value being ∼46 µM (calculated using BioDataFit software). Similar results were seen in both RAW264.7 cells and PBMCs following LPS stimulation (Figure 3C,B). The results clearly indicate that RSCL-0520 inhibits TNF-α production from both mouse and human monocytes/macrophages stimulated with LPS.
Figure 3.
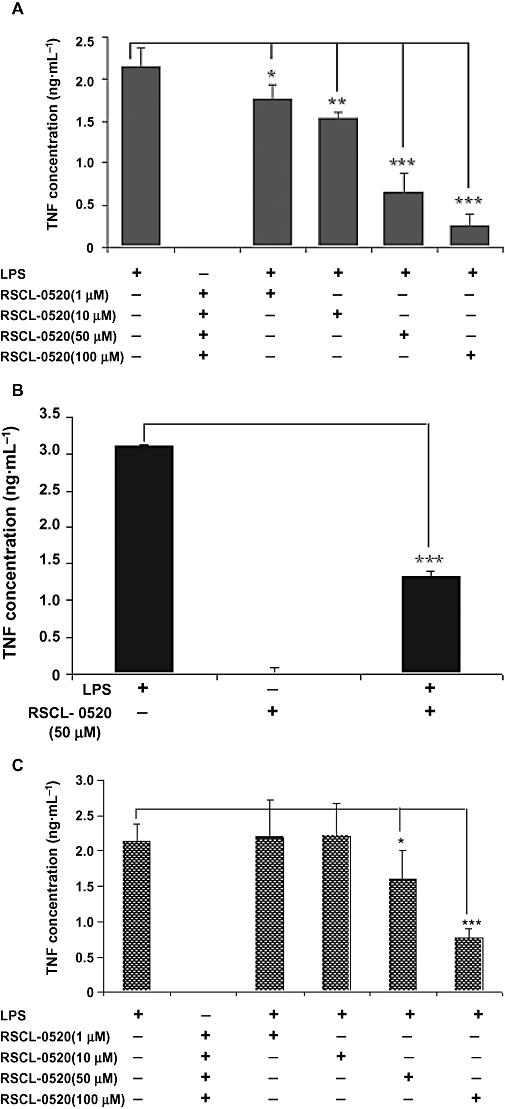
RSCL-0520 suppressed LPS-induced TNF-α output, in a concentration-dependent manner, in different cell lines. Human monocytic cells (THP-1) (A), human PBMCs (B) and mouse macrophage cells (RAW264.7) (C) were pretreated with the indicated concentrations of RSCL-0520 followed by LPS stimulation for 24 h. Culture supernatants were collected and assayed for TNF-α by elisa. Data are expressed as means ± SEM of three independent experiments (***P < 0.001, **P < 0.001, *P < 0.05; LPS treated vs. RSCL-0520 treated).
Selective inhibitory effect on TLR4 ligand-induced signalling
Activation of TLRs by specific ligands leads to the release of many inflammatory cytokines. As the THP-1 cells are known to express all TLRs, we checked TNF-α release in these cells. RSCL-0520 (50 µM)-treated cells were stimulated with various TLR ligands (TLR1-2, Sandor et al., 2003; TLR3, Alexopoulou et al., 2001; TLR4, Yamamoto et al., 2003; TLR5, Sierro et al., 2001; TLR6, Galanos et al., 2000; TLR7–8, Diebold et al., 2004; and TLR9, Vollmer et al., 2004). Cells not treated with RSCL-0520 served as respective controls. As shown in Figure 4A, detectable TNF-α secretions were seen in cells stimulated with TLR1/2, TLR4 and TLR6 ligands. No detectable TNF-α was observed with other ligands. In cells pretreated with RSCL-0520, we observed inhibition of TNF-α secretion in cells stimulated with the TLR4 ligand. We further investigated the inhibitory effect of RSCL-0520 in PBMCs (Figure 3B) and RAW264.7 cells (data not shown). Similar results were seen in both types of cells. These results suggest that RSCL-0520 inhibited TNF-α production mediated mainly by TLR4 in THP-1 cells and TLR1/2, along with TLR4 in PBMCs.
Figure 4.
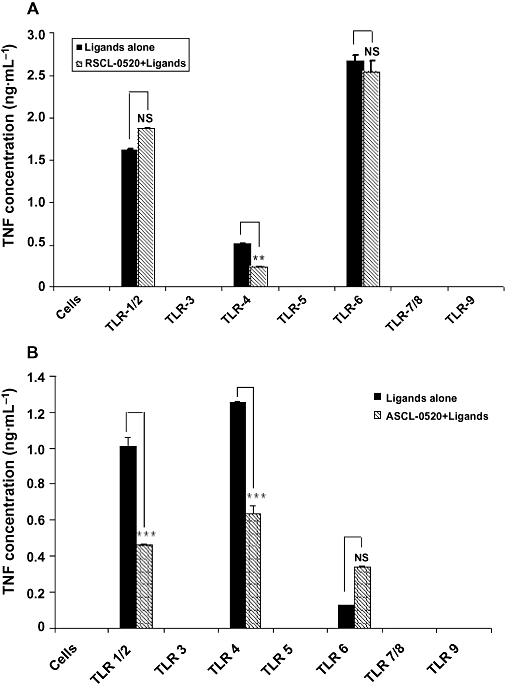
RSCL-0520 inhibits TLR4-induced TNF-α secretion in THP-1 monocytes and PBMCs. THP-1 cells (A) and PBMC (B), 2 × 105 cells per well, were pretreated with RSCL-0520 (50 µM) 1 h prior to TLR ligand treatment. The pretreated cells were stimulated with various TLR ligands at different concentrations (TLR1/2, 75 ng·mL−1; TLR3, 75 µg·mL−1; TLR4, 250 ng·mL−1; TLR5, 75 ng·mL−1; TLR6, 75 ng·mL−1; TLR7/8, 7.5 µg·mL−1; and TLR9, 7.5 µg·mL−1) for 24 h according to the manufacturer's instructions. The culture supernatant was then assayed for TNF-α. Cells untreated with RSCL-0520 served as controls. Data are expressed as mean ± SEM of two independent experiments. ***P < 0.001; ligand-treated cells versus RSCL-0520 treated.
Inhibitory effects of RSCL-0520 on mRNA and protein expression in THP-1 cells
To determine whether the suppressive effect of RSCL-0520 on cytokine production was exerted at mRNA level, we examined TNF-α mRNA expression in THP-1 cells stimulated with LPS using quantitative real-time PCR. As shown in Figure 5, TNF-α mRNA expression significantly increased 1 h after LPS stimulation. The expression levels fell following 1 h pretreatment with RSCL-0520 (50 µM). Further, we checked whether this inhibitory effect was seen on other pro-inflammatory genes producing mRNA for ICAM-1, COX-2, IL-1β and IL-8. The RT-PCR (Figure 6) results clearly show the suppression of these genes at mRNA level. These findings indicate its effectiveness as a good anti-inflammatory agent. It is also noteworthy that cells treated with RSCL-0520 in the absence of the stimulant LPS did not show any effect in any of the genes at mRNA level and protein level (Figure 6, lane 2). The bands obtained were quantitated using ImageJ software.
Figure 5.
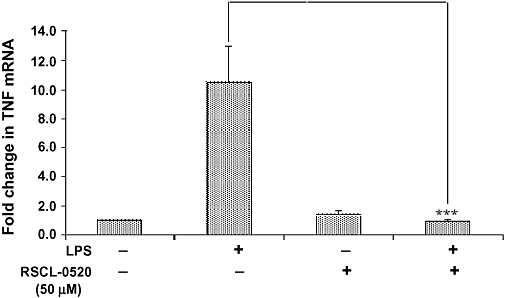
Inhibitory effect of RSCL-0520 on TNF-α mRNA expression in THP-1 cells. Total RNA was isolated from THP-1 cells 1 h after exposure to LPS (250 ng·mL−1) with or without 50 µM RSCL-0520. The cDNA was used for real-time PCR with primers specific for human TNF-α and for the housekeeping gene β-actin. The fold change of TNF-α mRNA in treated cells over control was obtained after correction for the amount of β-actin. Error bars represent the SEM from two separate experiments. ***P < 0.001; LPS-treated cells versus RSCL-0520 + LPS treated.
Figure 6.
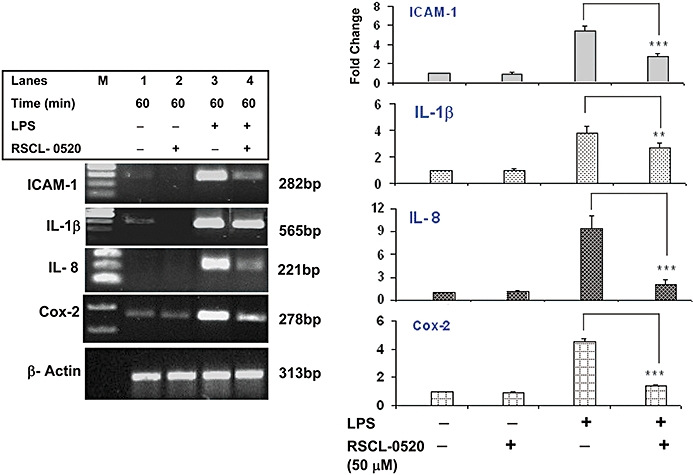
Inhibitory effect of RSCL-0520 on mRNA expression of pro-inflammatory genes in THP-1 cells. cDNA from an experiment similar to Figure 5 was used for amplifying the specific mRNA for pro-inflammatory proteins (ICAM-1, IL-1β, IL-8 and COX-2), using the corresponding specific primers; β-actin served as internal control. The data shown are representative of three independent experiments. Quantification of bands for the specific genes was normalized by β-actin. ***P < 0.001(ICAM-1, IL-8 and COX-2), **P < 0.01 (IL-1β); LPS-treated cells versus RSCL-0520 + LPS treated.
In addition, we have also checked for the effect of RSCL-0520 on IL-8 and IL-1β protein levels. THP-1 cells were pre-incubated with various concentrations of RSCL-0520 (1–50 µM), and then stimulated with LPS. IL-8 and IL-1β were measured in the culture supernatants 24 h later. Treatment with RSCL-0520 inhibited these proteins in a dose-dependent manner (Figure 7A,B). COX-2 protein detected by Western blotting was decreased in RSCL-0520 (50 µM)-treated cells (Figure 7C), corroborating our earlier observation of mRNA levels.
Figure 7.
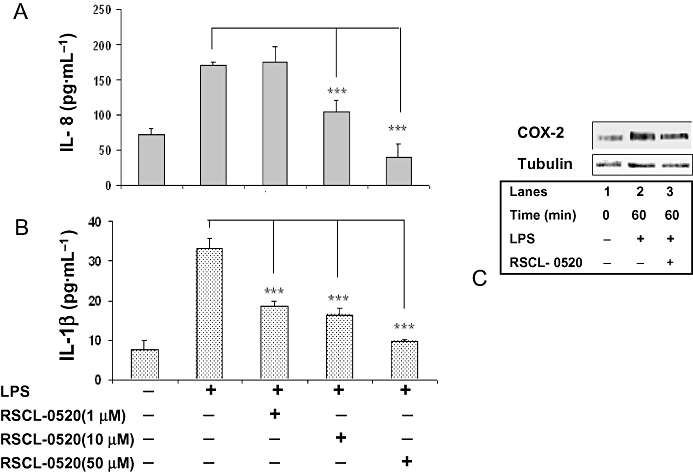
RSCL-0520 inhibits IL-8, IL-1β and COX-2 protein expression in THP-1 cells. THP-1 cells were pretreated with the indicated concentrations of RSCL-0520, and then stimulated with LPS for 24 h. Culture supernatants were collected and assayed for IL-8 (A) and IL-1β (B) by elisa. Data are expressed as means ± SEM of three independent experiments (***P < 0.001; LPS treated vs. RSCL-0520 treated). In Figure 7C, cell lysates were prepared from serum-starved THP-1 cells pretreated with 50 µM RSCL-0520 followed by LPS stimulation for the indicated time-points. Immunoblotting was carried out to determine the COX-2 protein levels. The same blot was probed with tubulin antibody to ensure equal loading.
RSCL-0520 blocks IκBα degradation, activation of NF-κB and nuclear translocation of NF-κB
Stimulation of monocytes with LPS triggers phosphorylation and degradation of the IκB complex allowing the free NF-κB to translocate into the nucleus to activate genes with NF-κB-binding regions. As our mRNA data showed that RSCL-0520 inhibited a number of pro-inflammatory genes which are known to be activated through NF-κB, we decided to check whether it could block any signals leading to nuclear translocation of NF-κB. Figure 8 shows that following LPS stimulation, there were increased levels of NEMO with time, and treatment with RSCL-0520 blocked this stimulation of NEMO (top row, lanes 5–7). Next, to determine whether inhibition of LPS-induced NF-κB activation was due to inhibition of IκBα degradation, we looked at the effects of RSCL-0520 on IκB-α degradation. IκB-α following LPS stimulation, undergoes phosphorylation and subsequently degrades. RSCL-0520 prevented IκB-α degradation (Figure 8, second row, lanes 5–7). Blots were stripped and reprobed with ERK-1/2 to normalize the protein loading. Summary data are shown in the bar graphs.
Figure 8.
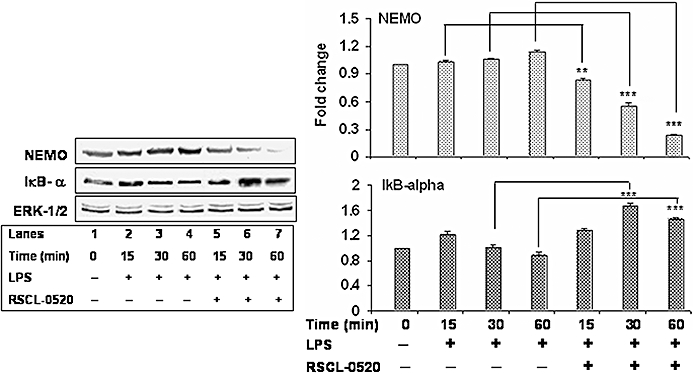
RSCL-0520 blocks activation of NEMO and IκBα degradation. Serum- starved THP-1 cells were pre-incubated with 50 µM RSCL-0520 for 1 h, and then stimulated with LPS for the indicated time. Total protein was isolated from the treated cells, and an equal amount of protein from each sample was used for immunoblots to determine protein levels of NEMO and IκB-α. The blot was stripped and reprobed with an anti-ERK-1/2 antibody to ensure equal loading. Quantification of NEMO and IκB-α bands was normalized by ERK-1/2, and data presented are from three independent experiments. ***P < 0.001; **P < 0.01; LPS treated-15, 30, 60 min versus RSCL-0520 + LPS treated-15, 30 and 60, respectively.
LPS has been shown to induce the phosphorylation of the p65 subunit of NF-κB, which is required for its translocation to the nucleus. Therefore, we checked whether RSCL-0520 blocked LPS-induced phosphorylation of p65. As shown in Figure 9A, LPS induced the phosphorylation of p65, and RSCL-0520 treatment suppressed p65 phosphorylation almost completely.
Figure 9.
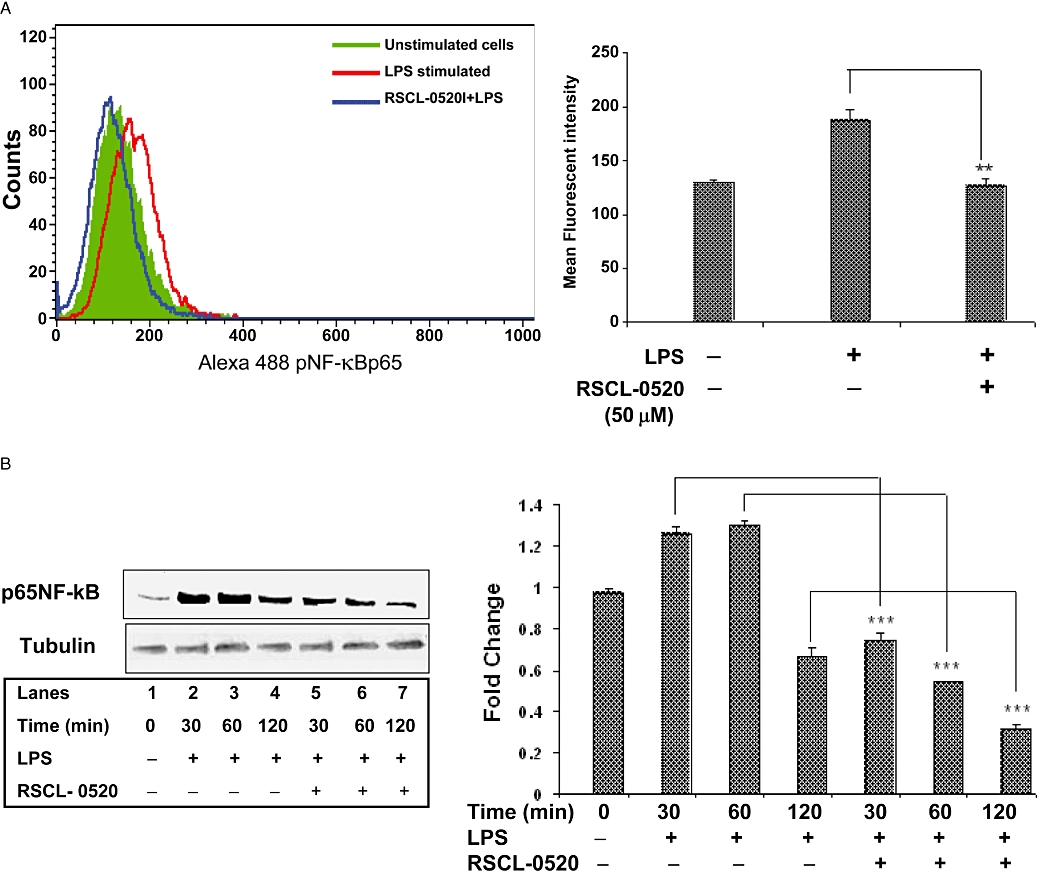
RSCL-0520 blocks phosphorylation and nuclear translocation of NF-κB. Cells stimulated with LPS for 1 h were processed for FACS using an NF-κB-specific antibody tagged with Alexa-Fluor-488. The results shown (A) are representative of three separate experiments. Adjacent graph shows MFI data from three experiments (**P < 0.01; LPS treated vs. RSCL-0520 + LPS treated). Treatment of cells with RSCL-0520 alone did not show any alteration in protein levels (data not shown). In B, the nuclear fractions were obtained from LPS-stimulated THP-1 cells at the indicated times, and processed for immunoblots using an NF-κB-specific antibody, as in A. Quantification of p65NF-κB bands was normalized by tubulin. ***P < 0.001, **P < 0.01; LPS-treated vs. RSCL-0520 + LPS at 30, 60 and 120 min. All the immuno blot results shown are representative of three separate experiments.
Further, following phosphorylation of p65 subunit, LPS has been shown to induce the nuclear translocation of the p65 subunit. So, we tested the effect of RSCL-0520 on LPS-induced nuclear translocation. As shown in Figure 9B, RSCL-0520 treatment prevented p65 translocation into the nucleus.
RSCL-0520 inhibits LPS-induced MyD88-dependent and -independent signalling cascades
Various adapters and signalling molecules are involved in TLR4 signalling. mRNA expression levels of TIRAP, MyD88 and IRAK1 were up-regulated following stimulation with LPS (Figure 10, lane 3). Treatment with RSCL-0520 (50 µM) down-regulated the mRNA expression levels of TIRAP, MyD88 and IRAK-1 as seen in Figure 7A (lane 4). Nonetheless, the mRNA levels of all these genes remained unaffected when treated with RSCL-0520 in the absence of LPS treatment (Figure 7A, lane 2). To investigate the possibility of an MyD88-independent, TRIF-dependent pathway, we checked TRIF expression levels in the LPS-treated cells and found that treatment with RSCL-0520 down-regulated TRIF mRNA levels. Summary data are shown in the bar graphs in Figure 10.
Figure 10.
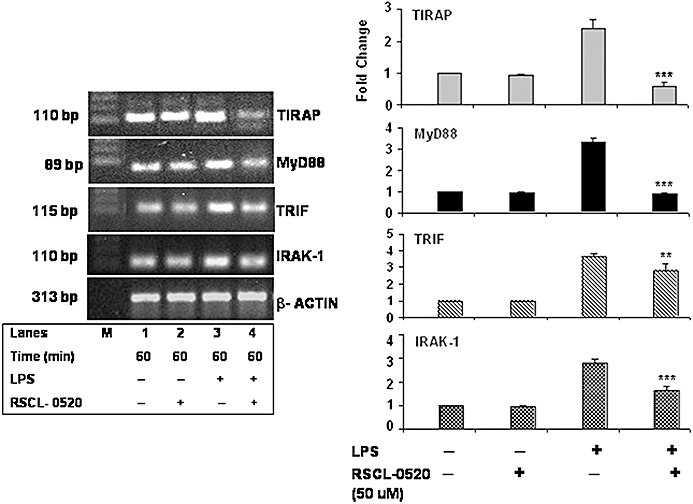
RSCL-0520 inhibited the mRNA expression of the LPS-induced MyD88-dependent and independent-signalling cascades. Serum-starved THP-1 cells were incubated with RSCL-0520 before stimulation with LPS for 1 h. Total RNA was isolated from treated cells after LPS exposure. The cDNA synthesized was used for PCR against specific primers for the TLR-related genes. Quantification of bands for the specific genes was normalized by β-actin. ***P < 0.001(TIRAP, MyD88 and IRAK-1), **P < 0.01 (TRIF); LPS-treated cells versus RSCL-0520 + LPS treated.
The protein levels (Figure 11) of two of these signalling molecules, TIRAP and MyD88, were also increased by LPS, and this increase was inhibited by RSCL-0520. Overall, these results indicate that RSCL-0520 inhibits both MyD88-dependent and -independent signalling triggered by TLR4.
Figure 11.
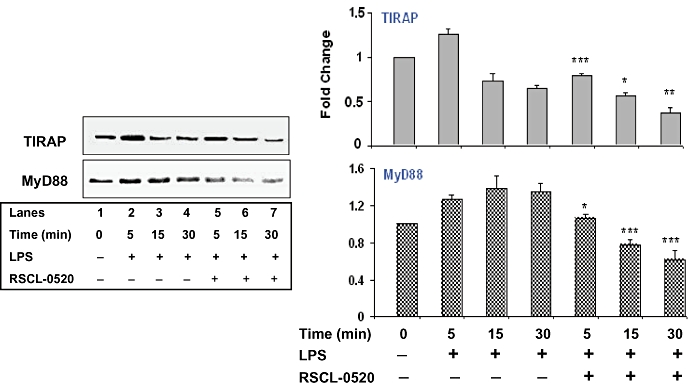
RSCL-0520 inhibited the protein expression of TIRAP and MyD88. Serum-starved RSCL-0520-pretreated THP-1 cells were stimulated with LPS for the indicated time. Immunoblotting of total protein was carried out as before to determine protein levels of TIRAP and MyD88. Quantification of bands TIRAP and MyD88 was normalized by ERK-1/2, and data presented are from three independent experiments. ***P < 0.001, **P < 0.01, *P < 0.05; LPS treated versus RSCL-0520 + LPS treated at 5, 15, 30 min respectively.
Effect of RSCL-0520 on LPS-induced cytokine production in vivo
As a consequence of our in vitro results, we tested the effect of RSCL-0520 in a murine model of LPS-induced inflammation. Mice were injected i.p. with 10 or 20 mg·kg−1 RSCL-0520, 30 min prior to LPS injection. Blood samples were collected via retro-orbital route under anaesthesia 1 h after LPS injection for TNF-α analysis. As shown in Figure 12, there was ∼66 and ∼70% inhibition of LPS-induced TNF-α production at 10 and 20 mg·kg−1 respectively.
Figure 12.
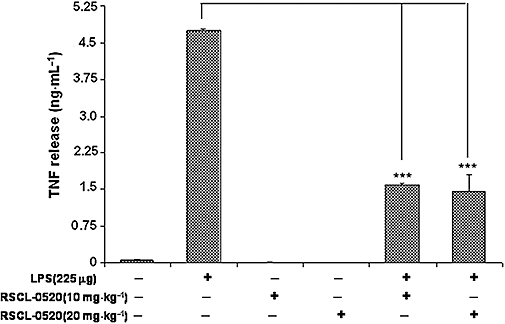
RSCL-0520 suppresses LPS-induced TNF-α release in Balb/c mice. Balb/c mice (five animals per group) weighing ∼25–30 g were injected i.p. with LPS (225 µg·mL−1) with and without pretreatment of RSCL-0520 (10 and 20 mg·kg−1). RSCL-0520 was injected i.p., 30 min before LPS injection. Then, 1 h after LPS injection, blood was collected retro-orbitally under anaesthesia and serum analysed for TNF-α (***P < 0.001; LPS treated versus RSCL-0520 + LPS treated).
Effect of RSCL-0520 treatment on the carrageenan-induced inflammation in rat paw
We studied the ability of RSCL-0520 at 10 and 20 mg·kg−1 to exert protection against carrageenan-induced oedema in rats. Along with the untreated control group where only carrageenan was injected to the hind paw, the test group was given RSCL-0520, 30 min prior to carrageenan treatment. The paw volume was measured 0, 2, 4 and 6 h after carrageenan using a plethysmometer. Analysis (Figure 13) revealed that in rats pretreated with RSCL-0520, the carrageenan-induced inflammation was reduced by about 30%, by either dose of RSCL-0520, over the experimental period.
Figure 13.
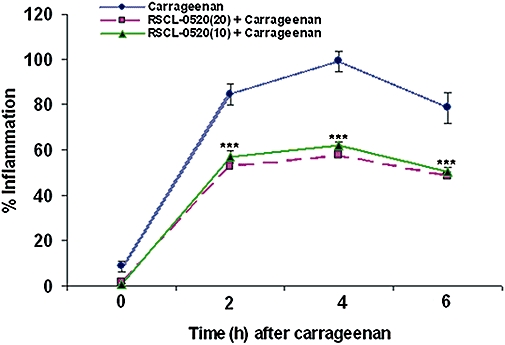
RSCL-0520 inhibits carrageenan-induced paw oedema in Wistar rats. The hind paws of rats (weighing ∼180–200 g) were injected with 1% carrageenan in saline (100 µL) to induce oedema. Then, 100 µL of vehicle (PBS) without carrageenan served as a vehicle control. RSCL-0520 (10 or 20 mg·kg−1) was injected i.p., 30 min prior to carrageenan injection in the test animals. Using a plethysmometer, the oedema induced was measured as increase in paw volume, at 0, 2, 4 and 6 h after carrageenan. They were compared with the pre-injection volume of the same paw against the untreated foot of the same rat which served as a reference. % Inflammation was then calculated in control and in drug-treated animals. Values are means ± SEM from two independent experiments with five rats in each experiment. ***P < 0.001, significantly different from values without RSCL-0520.
Discussion and conclusions
Recent work carried out in our laboratory on the isolation of antioxidant molecules from medicinal plants based on free-radical scavenging activity has led to the identification of a novel anti-inflammatory compound from the tubers of an orchid E. ochreata. The compound RSCL-0520 was isolated to ∼99% purity and chemically characterized as a phenanthrene, RSCL-0520, based on spectroscopic analysis (Kshirsagar et al., unpubl. exp.). Although phenanthrenes have been isolated from various species of orchids (Tuchinda et al., 1988; Estrada et al., 1999a,b;, Majumder et al., 2001), no antioxidant or anti-inflammatory activity has been reported for any of these molecules. The only known report is from crude tuber extracts of Eulophia nuda (an allied species of E. ochreata), which has been reported to inhibit carrageenan-induced paw oedema (Tuchinda et al., 1988). However, the mechanism has not been elucidated nor any active ingredient identified.
This study, to our knowledge, provides the first evidence of a phenanthrene showing anti-inflammatory activity exerted through TLR4-mediated signalling. Here, we show that RSCL-0520 inhibited TNF-α production by monocytic cells in response to LPS. The observed effects on TNF-α production appeared to be mediated by inhibition of TLR signalling pathways.
Due to its strong antioxidant properties (Kshirsagar et al., unpubl. exp.), we postulated that RSCL-0520 could exhibit anti-inflammatory activity. So, we checked its ability to inhibit TNF-α release, which involves an oxidant-responsive mechanism, that is, NF-κB signalling, in an in vitro model, human monocytes stimulated by LPS. Our results clearly demonstrated that RSCL-0520 inhibited TNF-α secretion in a concentration-dependent manner, and the inhibitory effects were similar in cell lines of monocytic lineage (THP-1, PBMCs and RAW264.7 cells) without affecting cell viability (data not shown).
Our studies further demonstrate that RSCL-0520 selectively down-regulated TLR4-mediated TNF-α production, rather than TLR2- or TLR6-mediated signalling, indicating that RSCL-0520 acted primarily on a signalling pathway from TLR4 in THP-1 monocytic cells. Further studies are in progress to delineate how RSCL-0520 blocks, specifically, TLR4-mediated LPS signalling and not that activated by signalling through TLR2 or TLR6. Further, because RSCL-0520 is a small molecule (MW about 272), it is unlikely that such a small molecule could directly inhibit interactions between large proteins. It may be possible that RSCL-0520 might suppress or activate an unknown molecule that is uniquely required to regulate TLR4 signalling. Similar results from TNF-α output following stimulation with a TLR4 ligand were also observed in human PBMCs. In addition, we also observed inhibition of TLR1-2-stimulated TNF-α. It is possible that RSCL-0520 inhibits TNF-α through multiple pathways in PBMC, and this needs further evaluation. In all our in vitro experiments involving LPS, we have used LPS (from E. coli serotype O55:B5). However, in the TLR experiments (Figure 3A,B), we have used the LPS supplied along with the kit which is from S. minnesota. So, we see different levels of TNF release as compared to that seen in Figure 2A–C. For all the other in vitro and in vivo experiments, we have used the LPS from E. coli.
Because NF-κB has a central role in regulation of the pro-inflammatory molecules inhibited by RSCL-0520, we postulated that RSCL-0520 must suppress NF-κB activation. Investigating the mechanism involved, we found that RSCL-0520 inhibited NEMO, IκB-α degradation and phosphorylation of the p65 subunit of NF-κB. To rule out any possible intrinsic effect of RSCL-0520 on NEMO, we have evaluated its effect in the absence of the stimulant LPS, and we did not observe any effect in the absence of LPS (data not shown). Studies have shown that the phosphorylation and acetylation of p65 play a major role in DNA binding and trans-activation of NF-κB (Egan et al., 1999; Sakurai et al., 1999; Chen et al., 2001). Further, IKK has been shown to phosphorylate p65 (Fargnoli et al., 1995, Egan et al., 1999; Sizemore et al., 2002), and it is possible that RSCL-0520 inhibits p65 phosphorylation through inhibition of IKK.
TLR4 ligation with LPS induces activation of specific intracellular pathways through receptor dimerization and recruitment of different adaptor molecules MyD88, TIRAP (Fitzgerald et al., 2001; Horng et al., 2001), TRIF (Yamamoto et al., 2002; Oshiumi et al., 2003) and TRAM (Fitzgerald et al., 2003). Studies have shown that TLR4 signals through two different pathways, the MyD88-dependent pathway leading directly to NF-κB activation, and the second pathway, MyD88-independent pathway, involving the adaptor proteins TRIF and TRAM which trigger an interferon response, as well as late NF-κB activation (Kawai et al., 2001; Hoebe et al., 2003; Yamamoto et al., 2003). We checked the mRNA expression of the genes involved in these signalling processes after stimulation with LPS. Our preliminary results show down-regulation of TIRAP, MyD88 and IRAK-1 mRNA levels. This observation, to our knowledge, is the first documented evidence of a phenanthrene showing down-regulation of TLR-associated genes in any cell line in an in vitro study. Investigations are underway to elucidate the molecular mechanisms and delineate the signalling pathways involved in this inhibitory process.
LPS injection can elicit the release of a series of inflammatory mediators, including cytokines and prostaglandins (PGs). Both cytokine and PGE2 production are key factors in inflammatory diseases such as arthritis, sepsis and inflammatory bowel disease (Laufer, 2003; Meyer, 2003; Stokkers and Hommes, 2004; Ulloa and Tracey, 2005). As a result of the promising in vitro evidence, we tested its anti-inflammatory efficacy in an in vivo model. Our findings clearly demonstrated that pretreatment with RSCL-0520 effectively prevented the release of TNF-αin vivo in response to LPS injection, in mice. Carrageenan-induced paw oedema is known to elicit a typical biphasic oedema, with peaking observed between 6 and 72 h (Levy, 1969; Di Rosa et al., 1971; Salvemini et al., 1996; Posadas et al., 2004; Rocha et al., 2006). However, in our studies, the edema peaked at 4 h followed by a decline. Pretreatment with RSCL-0520 was able to reduce the carrageenan paw oedema in rats. We would like to mention that responses in this model are known to be dependent on the age, weight and strain of rats used (Sprague-Dawley or Wistar). In addition, the biphasic pattern reported is based on 72 h monitoring. In our study, we have monitored for a maximum of 24 h, and over this period we saw a decline in the response. It may be that the biphasic phenomenon would be observed if the monitoring was for 72 h.
In conclusion, this study demonstrates that a naturally occurring phenanthrene, RSCL-0520, down-regulates expression of LPS-stimulated, NF-κB-mediated, inflammatory cytokines via a TLR-mediated process. The involvement of other signal mediators or other transcription factors, however, cannot be ruled out. As RSCL-0520 inhibited the output of several pro-inflammatory mediators, particularly pro-inflammatory cytokines, it shows promise as an anti-inflammatory agent.
Acknowledgments
The authors gratefully acknowledge the encouragement and support of Reliance Life Sciences Pvt Ltd, in carrying out this research work.
P.D. and S.U. were employees of Reliance Life Sciences Pvt Ltd when the work was undertaken. Their current affiliation (EID Parry India Ltd) mentioned is indicative of their current address only and does not indicate collaborative work.
Glossary
Abbreviations:
- EA
ethyl acetate
- IL-1β
interleukin-1β
- IL-6
interleukin-6
- IRAK
IL-1R-associated kinase-1
- MyD88
myeloid differentiation primary response gene (88)
- NEMO
NF-κB essential modulator
- NF-κB
nuclear factor-κB
- PE
petroleum ether
- TAB1 and TAB2
TAK1-binding protein 1 and 2
- TAK1
transforming growth factor-β-activated kinase 1
- TIR
Toll/interleukin-1 receptor
- TIRAP
Toll receptor IL-1R domain-containing adapter protein
- TLRs
Toll-like receptors
- TNF-α
tumour necrosis factor-α
- TRAF6
TNF-α receptor-associated factor-6
Conflict of interest
None.
References
- Aggarwal BB. Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol. 2003;3:745–756. doi: 10.1038/nri1184. [DOI] [PubMed] [Google Scholar]
- Alexander C, Rietschel ET. Bacterial lipopolysaccharides and innate immunity. J Endotoxin Res. 2001;7:167–202. [PubMed] [Google Scholar]
- Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- Chen LF, Fischle W, Verdin E, Greene WC. Duration of nuclear NF-κB action regulated by reversible acetylation. Science. 2001;293:1653–1657. doi: 10.1126/science.1062374. [DOI] [PubMed] [Google Scholar]
- Darveau RP, Pham TT, Lemley K, Reife RA, Bainbridge BW, Coats SR, et al. Porphyromonas gingivalis lipopolysaccharide contains multiple lipid A species that functionally interact with both Toll-like receptors 2 and 4. Infect Immun. 2004;72:5041–5051. doi: 10.1128/IAI.72.9.5041-5051.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauphinee SM, Karsan A. Lipopolysaccharide signaling in endothelial cells. Lab Invest. 2006;86:9–22. doi: 10.1038/labinvest.3700366. [DOI] [PubMed] [Google Scholar]
- Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303:1529–1531. doi: 10.1126/science.1093616. [DOI] [PubMed] [Google Scholar]
- Dignam JD, Lebovitz RM, Roeder RG. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983;11:1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Rosa M, Giroud JP, Willoughby DA. Studies on the mediators of the acute inflammatory response induced in rats in different sites by carrageenan and turpentine. J Pathol. 1971;104:15–29. doi: 10.1002/path.1711040103. [DOI] [PubMed] [Google Scholar]
- Egan LJ, Mays DC, Huntoon CJ, Bell MP, Pike MG, Sandborn WJ, et al. Inhibition of interleukin-1-stimulated NF-κB RelA/p65 phosphorylation by mesalamine is accompanied by decreased transcriptional activity. J Biol Chem. 1999;274:26448–26453. doi: 10.1074/jbc.274.37.26448. [DOI] [PubMed] [Google Scholar]
- Estrada S, Toscano RA, Mata R. New phenanthrene derivatives from Maxillaria densa. J Nat Prod. 1999a;62:1175–1178. doi: 10.1021/np990061e. [DOI] [PubMed] [Google Scholar]
- Estrada S, Rojas A, Mathison Y, Israel A, Mata R. Nitric oxide/cGMP mediates the spasmolytic action of 3,4′-dihydroxy-5,5′-dimethoxybibenzyl from Scaphyglottis livida. Planta Med. 1999b;65:109–114. doi: 10.1055/s-1999-14056. [DOI] [PubMed] [Google Scholar]
- Fargnoli J, Burkhardt AL, Laverty M, Kut SA, van Oers NS, Weiss A, et al. Protein Syk mutation in Jurkat E6-derived clones results in lack of p72syk expression. J Biol Chem. 1995;270:26533–26537. doi: 10.1074/jbc.270.44.26533. [DOI] [PubMed] [Google Scholar]
- Fitzgerald KA, Palsson-McDermott EM, Bowie AG, Jefferies CA, Mansell AS, Brady G, et al. Mal (MyD88-adapter-like) is required for Toll-like receptor-4 signal transduction. Nature. 2001;413:78–83. doi: 10.1038/35092578. [DOI] [PubMed] [Google Scholar]
- Fitzgerald KA, Rowe DC, Barnes BJ, Caffrey DR, Visintin A, Latz E, et al. LPS–TLR4 signaling to IRF-3/7 and NF-kappaB involves the Toll adapters TRAM and TRIF. J Exp Med. 2003;198:1043–1055. doi: 10.1084/jem.20031023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanos C, Gumenscheimer M, Mühlradt P, Jirillo E, Freudenberg M. MALP-2, a mycoplasma lipopeptide with classical endotoxic properties: end of an era of LPS monopoly? J Endotoxin Res. 2000;6:471–476. [PubMed] [Google Scholar]
- Guha M, Mackman N. LPS induction of gene expression in human monocytes. Cell Signal. 2001;13:85–94. doi: 10.1016/s0898-6568(00)00149-2. [DOI] [PubMed] [Google Scholar]
- Hoebe K, Du X, Georgel P, Janssen E, Tabeta K, Kim SO, et al. Identification of Lps2 as a key transducer of MyD88-independent TIR signalling. Nature. 2003;424:743–748. doi: 10.1038/nature01889. [DOI] [PubMed] [Google Scholar]
- Horng T, Barton GM, Medzhitov R. TIRAP: an adapter molecule in the Toll signaling pathway. Nat Immunol. 2001;2:835–841. doi: 10.1038/ni0901-835. [DOI] [PubMed] [Google Scholar]
- Jiang Z, Ninomiya-Tsuji J, Qian Y, Matsumoto K, Li X. Interleukin-1 (IL-1) receptor-associated kinase-dependent IL-1- induced signaling complexes phosphorylate TAK1 and TAB2 at the plasma membrane and activate TAK1 in the cytosol. Mol Cell Biol. 2002;22:7158–7167. doi: 10.1128/MCB.22.20.7158-7167.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai T, Akira S. TLR signaling. Cell Death Differ. 2006;3:816–825. doi: 10.1038/sj.cdd.4401850. [DOI] [PubMed] [Google Scholar]
- Kawai T, Takeuchi O, Fujita T, Inoue J, Muhlradt PF, Sato S, et al. Lipopolysaccharide stimulates the MyD88-independent pathway and results in activation of IFN-regulatory factor 3 and the expression of a subset of lipopolysaccharide-inducible genes. J Immunol. 2001;167:5887–5894. doi: 10.4049/jimmunol.167.10.5887. [DOI] [PubMed] [Google Scholar]
- Laufer S. Role of eicosanoids in structural degradation in osteoarthritis. Curr Opin Rheumatol. 2003;15:623–627. doi: 10.1097/00002281-200309000-00017. [DOI] [PubMed] [Google Scholar]
- Levy L. Carrageenan-induced paw edema in the mouse. Life Sci. 1969;8:601–606. doi: 10.1016/0024-3205(69)90021-6. [DOI] [PubMed] [Google Scholar]
- Li S, Strelow A, Fontana EJ, Wesche H. IRAK-4: a novel member of the RAK family with the properties of an IRAK-kinase. Proc Natl Acad Sci USA. 2002;99:5567–5572. doi: 10.1073/pnas.082100399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumder PL, Sen S, Majumder S. Phenanthrene derivatives from the orchid Coelogyne cristata. Phytochemistry. 2001;58:581–586. doi: 10.1016/s0031-9422(01)00287-4. [DOI] [PubMed] [Google Scholar]
- Meyer O. Role of TNF-α and cytokines in the physiopathology of rheumatoid arthritis – therapeutic perspectives. Bull Acad Natl Med. 2003;187:935–954. [PubMed] [Google Scholar]
- Nishimura M, Naito S. Tissue-specific mRNA expression profiles of human Toll-like receptors and related genes. Biol Pharm Bull. 2005;28:886–892. doi: 10.1248/bpb.28.886. [DOI] [PubMed] [Google Scholar]
- O'Neill LA, Dunne A, Edjeback M, Gray P, Jefferies C, Wietek C. Mal and MyD88: adapter proteins involved in signal transduction by Toll-like receptors. J Endotoxin Res. 2003;9:55–59. doi: 10.1179/096805103125001351. [DOI] [PubMed] [Google Scholar]
- Oshiumi H, Matsumoto M, Funami K, Akazawa T, Seya T. TICAM-1, an adaptor molecule that participates in Toll-like receptor 3-mediated interferon-beta induction. Nat Immunol. 2003;4:161–167. doi: 10.1038/ni886. [DOI] [PubMed] [Google Scholar]
- Posadas I, Bucci M, Roviezzo F, Rossi A, Parente L, Sautebin L, et al. Carrageenan-induced mouse paw oedema is biphasic, age-weight dependent and displays differential nitric oxide cyclooxygenase-2 expression. Br J Pharmacol. 2004;142:331–338. doi: 10.1038/sj.bjp.0705650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha AC, Fernandes ES, Quintão NL, Campos MM, Calixto JB. Relevance of tumour necrosis factor-alpha for the inflammatory and nociceptive responses evoked by carrageenan in the mouse paw. Br J Pharmacol. 2006;148:688–695. doi: 10.1038/sj.bjp.0706775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai H, Chiba H, Miyoshi H, Sugita T, Toriumi W. IkB kinases phosphorylate NF-B p65 subunit on serine 536 in the transactivation domain. J Biol Chem. 1999;274:30353–30356. doi: 10.1074/jbc.274.43.30353. [DOI] [PubMed] [Google Scholar]
- Salvemini D, Wang ZQ, Wyatt PS, Bourdon DM, Marino MH, Manning PT, et al. Nitric oxide: a key mediator in the early and late phase of carrageenan-induced rat paw inflammation. Br J Pharmacol. 1996;118:829–838. doi: 10.1111/j.1476-5381.1996.tb15475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandor F, Latz E, Re F, Mandell L, Repik G, Golenbock DT, et al. Importance of extra- and intracellular domains of TLR1 and TLR2 in NFkappa B signaling. J Cell Biol. 2003;162:1099–1110. doi: 10.1083/jcb.200304093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierro F, Dubois B, Coste A, Kaiserlian D, Kraehenbuhl JP, Sirard JC. Flagellin stimulation of intestinal epithelial cells triggers CCL20-mediated migration of dendritic cells. Proc Natl Acad Sci USA. 2001;98:13722–13727. doi: 10.1073/pnas.241308598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sizemore N, Lerner N, Dombrowski N, Sakurai H, Stark GR. Distinct roles of the IkB kinase alpha and beta subunits in liberating nuclear factor-κB (NF-κB) from IκB and in phosphorylating the p65 subunit of NF-κB. J Biol Chem. 2002;277:3863–3869. doi: 10.1074/jbc.M110572200. [DOI] [PubMed] [Google Scholar]
- Stokkers PC, Hommes DW. New cytokine therapeutics for inflammatory bowel disease. Cytokine. 2004;28:167–173. doi: 10.1016/j.cyto.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Takeda K, Akira S. TLR signaling pathways. Semin Immunol. 2004;16:3–9. doi: 10.1016/j.smim.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Tuchinda P, Udchachon J, Khumtaveeporn K, Taylor WC, Engelhardt LM, White AH. Phenanthrenes of Eulophia nuda. Phytochemistry. 1988;27:3267–3271. [Google Scholar]
- Ulloa L, Tracey KJ. The ‘cytokine profile’: a code for sepsis. Trends Mol Med. 2005;11:56–63. doi: 10.1016/j.molmed.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Vollmer J, Weeratna R, Payette P, Jurk M, Schetter C, Laucht M, et al. Characterization of three CpG oligodeoxynucleotide classes with distinct immunostimulatory activities. Eur J Immunol. 2004;34:251–262. doi: 10.1002/eji.200324032. [DOI] [PubMed] [Google Scholar]
- Wang C, Deng L, Hong M, Akkaraju GR, Inoue J, Chen ZJ. TAK1 is an ubiquitin-dependent kinase of MKK and IKK. Nature. 2001;412:346–351. doi: 10.1038/35085597. [DOI] [PubMed] [Google Scholar]
- Wesche H, Henzel WJ, Shillinglaw W, Li S, Cao Z. MyD88: an adapter that recruits IRAK to the IL-1 receptor complex. Immunity. 1997;7:837–847. doi: 10.1016/s1074-7613(00)80402-1. [DOI] [PubMed] [Google Scholar]
- Winter CA, Risley EA, Nuss GW. Carrageenan-induced oedema in hind paw of rat as assay for anti-inflammatory drugs. Proc Soc Exp Biol Med. 1962;111:544–547. doi: 10.3181/00379727-111-27849. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Sato S, Mori K, Hoshino K, Takeuchi O, Takeda K, et al. Cutting edge: a novel Toll/IL-1 receptor domain-containing adapter that preferentially activates the IFN-beta promoter in the Toll-like receptor signaling. J Immunol. 2002;169:6668–6672. doi: 10.4049/jimmunol.169.12.6668. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, et al. Role of adaptor TRIF in the MyD88-independent Toll-like receptor signaling pathway. Science. 2003;301:640–643. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]


