Abstract
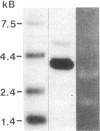
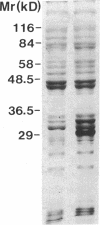
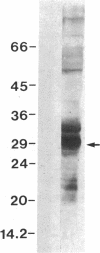
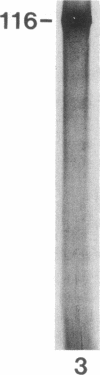
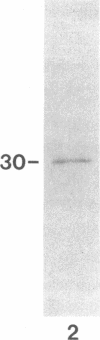
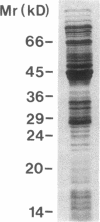
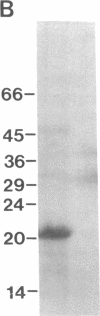
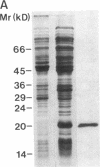
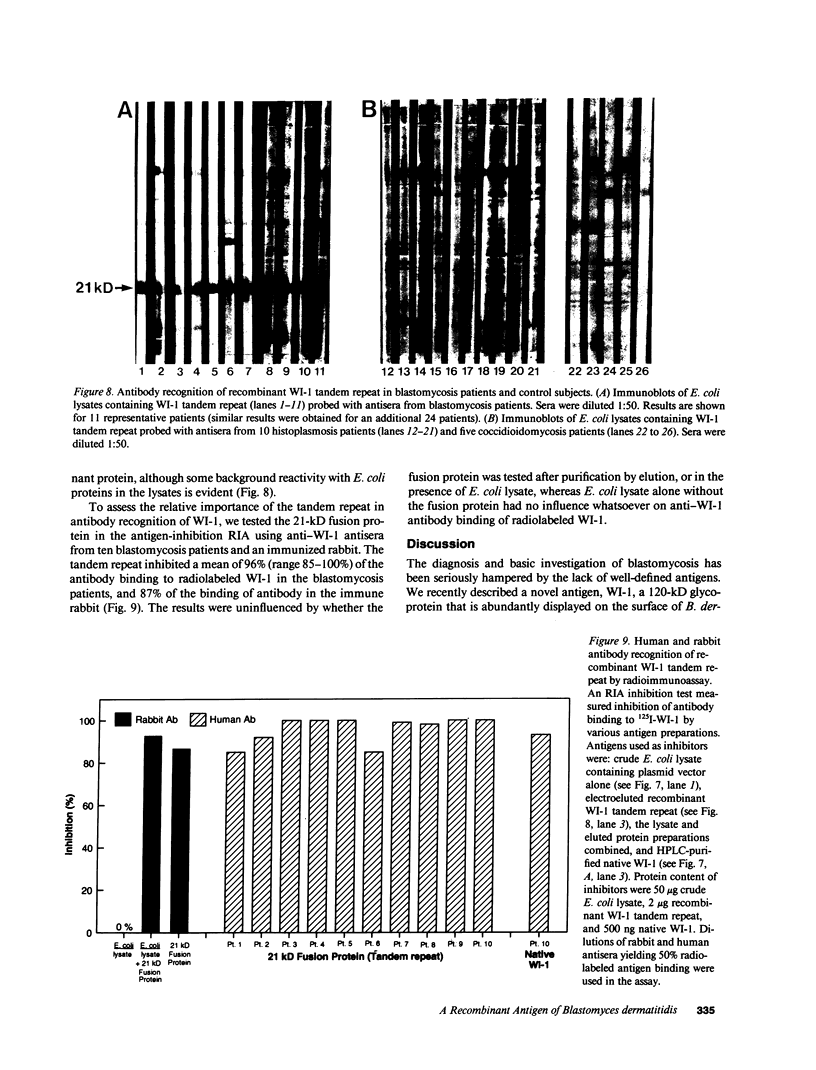
A 120-kD glycoprotein antigen abundantly expressed on Blastomyces dermatitidis yeasts is a target of cellular and humoral immune responses in human infection. To investigate the antigen and immune response more carefully at the molecular level, we screened an expression library from B. dermatitidis to identify clones that encode this antigen, designated WI-1. A 942-bp cDNA was isolated by immunologic screening with polyclonal, rabbit anti-WI-1 antiserum. Northern hybridization analysis showed that the cDNA hybridized to yeast message approximately equal to 3.9 kb. DNA and deduced protein sequence analysis of the clone demonstrated a 25-amino acid repeat arrayed in tandem, present in 4.5 copies near the 5' end, and rich in predicted antigenic epitopes. Further analysis showed strong homology in these tandem repeats with invasin, an adhesin of Yersiniae. Cloned cDNA was used to express a 30-kD fusion protein strongly recognized in western blots by rabbit anti-WI-1 antiserum, and by sera from all 35 blastomycosis patients studied. The fusion protein product of subcloned cDNA encoding only the tandem repeat also was strongly recognized in western blots by sera from the 35 blastomycosis patients, but not by sera from 10 histoplasmosis and 5 coccidioidomycosis patients. An antigen-inhibition radioimmunoassay showed that the tandem repeat alone completely eliminated rabbit and human anti-WI-1 antibody binding to radiolabeled native WI-1. From these results, we conclude that the 25-amino acid repeat of WI-1 displays an immunodominant B cell epitope, and that the carboxyl-terminus of the molecule exhibits an architecture that may promote adhesion of Blastomyces yeasts to host cells or extracellular matrix proteins and ultimately provide a clearer picture of the molecular pathogenesis of blastomycosis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Badley J. E., Bishop G. A., St John T., Frelinger J. A. A simple, rapid method for the purification of poly A+ RNA. Biotechniques. 1988 Feb;6(2):114–116. [PubMed] [Google Scholar]
- Burns J. M., Jr, Shreffler W. G., Rosman D. E., Sleath P. R., March C. J., Reed S. G. Identification and synthesis of a major conserved antigenic epitope of Trypanosoma cruzi. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1239–1243. doi: 10.1073/pnas.89.4.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonzi W. A., Sypherd P. S. Expression of the gene for ornithine decarboxylase of Saccharomyces cerevisiae in Escherichia coli. Mol Cell Biol. 1985 Jan;5(1):161–166. doi: 10.1128/mcb.5.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden D. W., Kronstad J. W., Leong S. A. Mutation in a heat-regulated hsp70 gene of Ustilago maydis. EMBO J. 1989 Jul;8(7):1927–1934. doi: 10.1002/j.1460-2075.1989.tb03596.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopp T. P. Protein surface analysis. Methods for identifying antigenic determinants and other interaction sites. J Immunol Methods. 1986 Apr 3;88(1):1–18. doi: 10.1016/0022-1759(86)90045-1. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992 Apr 3;69(1):11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Isberg R. R. Discrimination between intracellular uptake and surface adhesion of bacterial pathogens. Science. 1991 May 17;252(5008):934–938. doi: 10.1126/science.1674624. [DOI] [PubMed] [Google Scholar]
- Isberg R. R., Leong J. M. Multiple beta 1 chain integrins are receptors for invasin, a protein that promotes bacterial penetration into mammalian cells. Cell. 1990 Mar 9;60(5):861–871. doi: 10.1016/0092-8674(90)90099-z. [DOI] [PubMed] [Google Scholar]
- Isberg R. R., Voorhis D. L., Falkow S. Identification of invasin: a protein that allows enteric bacteria to penetrate cultured mammalian cells. Cell. 1987 Aug 28;50(5):769–778. doi: 10.1016/0092-8674(87)90335-7. [DOI] [PubMed] [Google Scholar]
- Klein B. S., Jones J. M. Isolation, purification, and radiolabeling of a novel 120-kD surface protein on Blastomyces dermatitidis yeasts to detect antibody in infected patients. J Clin Invest. 1990 Jan;85(1):152–161. doi: 10.1172/JCI114406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein B. S., Sondel P. M., Jones J. M. WI-1, a novel 120-kilodalton surface protein on Blastomyces dermatitidis yeast cells, is a target antigen of cell-mediated immunity in human blastomycosis. Infect Immun. 1992 Oct;60(10):4291–4300. doi: 10.1128/iai.60.10.4291-4300.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leong J. M., Fournier R. S., Isberg R. R. Identification of the integrin binding domain of the Yersinia pseudotuberculosis invasin protein. EMBO J. 1990 Jun;9(6):1979–1989. doi: 10.1002/j.1460-2075.1990.tb08326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann K., Deutzmann R., Aumailley M., Timpl R., Raimondi L., Yamada Y., Pan T. C., Conway D., Chu M. L. Amino acid sequence of mouse nidogen, a multidomain basement membrane protein with binding activity for laminin, collagen IV and cells. EMBO J. 1989 Jan;8(1):65–72. doi: 10.1002/j.1460-2075.1989.tb03349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennica D., Kohr W. J., Kuang W. J., Glaister D., Aggarwal B. B., Chen E. Y., Goeddel D. V. Identification of human uromodulin as the Tamm-Horsfall urinary glycoprotein. Science. 1987 Apr 3;236(4797):83–88. doi: 10.1126/science.3453112. [DOI] [PubMed] [Google Scholar]
- Siegelman M. H., van de Rijn M., Weissman I. L. Mouse lymph node homing receptor cDNA clone encodes a glycoprotein revealing tandem interaction domains. Science. 1989 Mar 3;243(4895):1165–1172. doi: 10.1126/science.2646713. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Nhieu G. T., Isberg R. R. The Yersinia pseudotuberculosis invasin protein and human fibronectin bind to mutually exclusive sites on the alpha 5 beta 1 integrin receptor. J Biol Chem. 1991 Dec 25;266(36):24367–24375. [PubMed] [Google Scholar]
- Zaret K. S., Sherman F. DNA sequence required for efficient transcription termination in yeast. Cell. 1982 Mar;28(3):563–573. doi: 10.1016/0092-8674(82)90211-2. [DOI] [PubMed] [Google Scholar]