Abstract
Background and purpose:
Our recent experiments demonstrated that the Sphingosine-1-phosphate (S1P) receptor agonist FTY720 (2-amino-2-[2-(4-octylphenyl)ethyl]-1,3-propanediol hydrochloride) improves recovery of function after myocardial ischaemia–reperfusion ex vivo. Therefore, we tested the hypothesis that pharmacological post-conditioning with FTY720 reduces infarct size after myocardial ischaemia–reperfusion in vivo.
Experimental approach:
Myocardial ischaemia was induced in Wistar rats by ligation of the left coronary artery for 45 min. FTY720 (0.5 mg·kg−1) was applied i.p. either once, before reperfusion, or twice, 24 h before myocardial ischaemia and before reperfusion. After 24 h reperfusion, we determined infarct size by triphenyltetrazolium chloride staining and granulocyte infiltration by immunohistochemistry. Tumour necrosis factor-α (TNF)-α concentration was determined by elisa. S1P receptor expression was studied by Western blot. Calcium transients were evaluated in Indo-1-loaded cardiomyocytes.
Key results:
In both groups, FTY720 significantly reduced lymphocyte count in peripheral blood. FTY720 treatment attenuated granulocyte infiltration and TNF-α protein expression in reperfused myocardium. However, both treatment regimens were not able to reduce infarct size. FTY720 increased mortality due to induction of fatal ventricular tachyarrhythmias when administered once before reperfusion, but protected against reperfusion arrhythmias when given 24 h prior to ischaemia. Pretreatment selectively down-regulated S1P1 receptor expression within the myocardium. S1P receptor agonists did not induce calcium deregulation in cardiomyocytes.
Conclusions and implications:
FTY720 applied during reperfusion did not reduce infarct size but increased mortality during myocardial ischaemia–reperfusion due to induction of arrhythmias. Pretreatment with FTY720 before ischaemia abrogated the deleterious pro-arrhythmic effects without reducing infarct size.
Keywords: ischaemia–reperfusion, sphingosine-1-phosphate, FTY720
Introduction
With the finding that the same cardioprotective pathways described for preconditioning can effectively be induced by both ischaemic and pharmaceutical post-conditioning, there is now growing interest in developing pharmacological strategies to reduce infarct size and improve clinical outcome after reperfusion of acute myocardial infarction (Yellon and Downey, 2003). Recently, lysophospholipids and their receptors came into focus as therapeutic targets for improving tolerance to ischaemia and limiting reperfusion injury. Sphingosine-1-phosphate (S1P) is currently the best-characterized member of the growing family of lysophospholipid mediators. S1P is a physiological high-nanomolar constituent of serum and other extracellular fluids. S1P binds with low-nanomolar affinity to five related G-protein-coupled receptors S1P1–5 (An et al., 1998; Jongsma et al., 2009). S1P receptors are widely expressed on different cell types, and S1P affects multiple biological functions including cell migration, cytoskeletal organization, cell survival and differentiation (Spiegel and Milstien, 2003). S1P receptors mediate a wide array of immunological effects, such as lymphocyte migration (Brinkmann et al., 2004; Singer et al., 2005; Chiba et al., 2006), leukocyte adhesion and pro-inflammatory cytokine secretion (Bolick et al., 2005; Theilmeier et al., 2006; Whetzel et al., 2006).
In the cardiovascular system, S1P was first characterized as modulator of endothelial cell function, such as vasopermeability (Gon et al., 2005), vascular tone (Dantas et al., 2003) and angiogenesis (English et al., 2002). In cardiac myocytes, the S1P1 receptor is the predominant subtype expressed and the activation of this receptor attenuates adrenergic receptor-mediated contractility (Landeen et al., 2008). Stimulation of the S1P3 receptor results in bradykardia by activating an inward rectifying K+ current in atrial myocytes (Sanna et al., 2004).
Sphingosine-1-phosphate confers protection against cardiomyocytes ischaemia–reperfusion injury both ex vivo (Karliner et al., 2001; Tao et al., 2007) and, especially as a physiological constituent of HDL particles,in vivo (Theilmeier et al., 2006; Means et al., 2007). Signals from S1P receptors activate pro-survival pathways, especially Akt signalling via the PI3 kinase pathway. S1P receptor signalling limits apoptosis and improves cardiomyocytes survival during hypoxia (Zhang et al., 2007). Studies using pharmacological inhibitors or genetically modified mice demonstrated that protection occurred by S1P1, S1P2 or S1P3 receptors (Means and Brown, 2009). Besides anti-apoptotic effects, experimental studies have further demonstrated that the S1P receptor agonist FTY720 (2-amino-2-[2-(4-octylphenyl)ethyl]-1,3-propanediol hydrochloride) reduces endothelial permeability (Sanchez et al., 2003; Dudek et al., 2007). Increased permeability during ischaemia–reperfusion leads to tissue oedema, which hampers microvascular reperfusion and tissue oxygenation. Therefore pharmacological S1P receptor agonists might also favourably modulate the outcome after ischaemia–reperfusion by maintaining the endothelial barrier integrity.
For their high therapeutic potential as anti-inflammatory immune modulators several S1P receptor agonists have been developed. FTY720 is a substrate of sphingosine kinases and the phosphorylated metabolite acts as an agonist at four of the five S1P receptors, namely S1P1, S1P3, S1P4 and S1P5, but not S1P2 (Brinkmann, 2004). The compound is currently under investigation for solid organ transplantation and autoimmunity (Zhang et al., 2007; Mansoor and Melendez, 2008). FTY720 further reduces both hepatic and renal ischaemia–reperfusion injury when administered before ischaemia (Anselmo et al., 2002; Awad et al., 2006). Pharmacological post-conditioning with FTY720 improves recovery of function after ischaemia–reperfusion in both rat and human myocardium (Hofmann et al., 2009).
As the principle of pharmacological post-conditioning is highly attractive for clinical application, we tested the hypothesis that treatment with FTY720 reduces myocardial infarct size in vivo. However, we found that both pre- and post-conditioning with FTY720 were not able to reduce infarct size. Instead, treatment with FTY720 induced fatal arrhythmias when administered before reperfusion.
Methods
Animals and surgery
Male Wistar rats (body weight 350–400 g) underwent left anterior descending artery ligation for 45 min with reperfusion for 24 h. Briefly, after induction of anaesthesia (inhalation of 5% isoflurane) and intubation, animals were ventilated with a volume-cycled rodent respirator (1–2% isoflurane/air). Body temperature was maintained by placing animals on an adjustable heating pad. Ligation of the left coronary artery with ‘soft pledges’ on top of the vessel was performed with a 6-0 suture. After 45 min of ischaemia, reperfusion was allowed by opening the knot. Proper reperfusion was determined by visual inspection, changes in ventricular function and ECG changes (ST-segment elevation). Buprenophine (0.05 mg·kg−1, Temgesic®, Essex Pharma, Munich, Germany) was given routinely i.p. for analgesia during isoflurane anaesthesia. The chest wall was closed with a continuous 6-0 prolene suture.
As malignant arrhythmias are very common in the rat model, animals were monitored by continuous ECG monitoring during the first hour. Every R-R interval corresponding to a heart rate <300 beats·min−1 or to a heart rate >500 min−1 was evaluated individually as bradykardia or tachycardia respectively. Ventricular tachycardia was defined as >3 consecutive ventricular premature beats, ventricular fibrillation was defined as signal that changed from beat to beat in configuration or rate or where QRS deflections could not easily be distinguished from another. (Opitz et al., 1998)
The local governmental Committee on Animal Research has reviewed and approved the animal study protocol (Ref. Nr. 64/05).
Experimental design
Rats (n = 109) were randomly assigned to control treatment (control: NaCl 0.9%) or FTY720 (post) 0.5 mg·kg−1 (diluted in NaCl 0.9%) given i.p. to anaesthetized animals after ligature of the left coronary artery. The timing should allow resorption of the compounds into systemic circulation until start of reperfusion without reaching the area at risk (AAR) as long as the left coronary artery was ligated. We selected the dose based on the review of published experimental studies, which demonstrated protection in other ischaemia–reperfusion models. In pilot experiments, we treated sham-operated animals with increasing doses up to 5 mg·kg−1 and found no obvious toxicity, but a dose-dependant lymphopenic effect. In a third group (pre + post) of animals 0.5 mg·kg−1 FTY720 was given twice i.p., 24 h before infarct surgery and immediately before reperfusion.
After 24 h of reperfusion, infarct size was determined by planimetry after triphenyltetrazolium chloride (TTC) staining. Animals, which demonstrated an AAR < 30%, were excluded from further analysis (Table 1).
Table 1.
Distribution of the animals to the study groups
| Treatment | Infarct-operated n= | Surviving 24 h n= | Excluded n= |
|---|---|---|---|
| Control | 38 | 24 | 3 |
| Post | 42 | 22 | 5 |
| Pre + post | 22 | 19 | 1 |
In six additional animals of each treatment group the infarcted area of the left ventricle (LV) was sectioned perpendicular to the long axis in three parts. The apical section was fixed in 10% formalin for histology. The mid ventricular ring was separated in infarct zone, border zone and septum and harvested in liquid nitrogen. The basal section was harvested in OCT compound (Tissue Tec, Sakura Finetek, Staufen, Germany) for cryo-sectioning
Assessment of the AAR and infarct size
Area at risk and infarct size were assessed as recently described (Frantz et al., 2005). After 24 h, the animals were intubated again; the suture was re-occluded, and 5% Evans blue was injected through the jugular vein. Hearts were excised and sectioned transversely into five to six sections after freezing in OCT compound for 30 min at −20°C. Sections were then incubated in 1.5% TTC at 37°C. After TTC staining viable myocardium stains red and the infarcted areas appear pale. Sections were weighed, digitally photographed and the area of infarction of each section was determined by computerized planimetry (ImageJ imaging software). Tracing was performed under direct comparison with the original tissue sample for optimal delineation of different areas. All measurements were done within 24 h after staining for optimal colours and contrast of the specimen. The size of infarction was determined by the following equations: Weight of infarction = (A1× Wt1) + … + (An × Wtn), where A is percent area of infarction of the section determined by planimetry and Wt is the weight of the individual section. For each heart, n = 6–7 sections were analysed. Percentage of infarcted LV = (weight of infarction/weight of LV) × 100. AAR as a percentage of LV = (weight of LV − weight of LV stained blue)/weight of LV. Animals developing an infarct size less than 30% of the AAR were excluded from the study.
Blood counts
After 24 h of reperfusion blood was drawn from the left A. carotis and harvested in EDTA-coated tubes. Differential leukocyte count was done by an automated analyser (Sysmex, XE2100, Norderstedt, Germany).
Tumour necrosis factor-αelisa assay
Heart tissue stored at −80°C prior to analysis was lysed in standard radio-immunopreciptitation buffer (RIPA) with protease inhibitor (Cocktail III), phosphatase inhibitor (Cocktail II, each from Calbiochem, Darmstadt, Germany). Protein concentration in tissue lysates was determined by a commercial Bradford assay (Biorad, Munich, Germany). Serum and border-zone tissue tumour necrosis factor-α (TNF-α) protein was quantified by a commercial elisa kit, as indicated by the manufacturer (Biosource, KRC 3011, distributed by Invitrogen, Darmstadt, Germany).
Immunohistochemistry
Cryostat sections (5 µm) were fixed in acetone. After quenching peroxidases by 0.3% H2O2 for 30 min the slides were immersed in 1% blocking serum. Then, the primary monoclonal mouse anti-rat granulocyte antibody (Clone HIS48, Acris, Herford, Germany), or a control immunoglobulin was applied over night. A biotin-conjugated goat-anti-mouse secondary antibody was applied for labelling by a peroxidase anti-peroxidase complex with diaminobenzidine as chromogen (Vectastain ABC Kit, Vector Laboratories, distributed by LINARS GmbH, Wertheim, Germany). Granulocyte infiltration in the infarct border zone was analysed by counting positive labelled cells on 4–5 high-power fields (400×). Results were presented as the mean count·mm−2 of two independent observers.
Western blot analysis
For Western blotting analysis, the tissue specimens from the septal myocardium were lysed in standard radio-immunoprecipitation buffer (RIPA) with protease inhibitor (Cocktail III), phosphatase inhibitor (Cocktail II, each from Calbiochem). After homogenization, protein determination was performed by a commercial Bradford assay (Biorad). The samples were heated at 90°C for 10 min. Forty microlitres of each sample was separated on 10% sodium dodecylsulfate polyacrylamide electrophoresis gels. Proteins were then transferred electrophoretically onto nitrocellulose membranes, while immersed in transfer buffer [25 mM, Tris(hydroxymethyl)-aminomethan, 192 mM glycine, 20% methanol, 0.037% SDS]. After transfer, non-specific binding was blocked by incubating membranes in blocking buffer (0.1% Tween-20, 5.0% dry milk) for at least 1 h. Protein samples were probed with a purified polyclonal anti-S1P1 antibody (1:100), with a purified monoclonal anti-S1P3 antibody (1:100) and a monoclonal anti-GAPDH antibody (1:5000), followed by horseradish peroxidase (HRP)-conjugated anti-mouse IgG (1:5000) or HRP-conjugated anti-rabbit (1:5000) for 1 h. Bands were visualized via enhanced chemiluminescence (Amersham Biosciences, distributed by GE Healthcare, Freiburg, Germany). Signals from scanned immunoblots were quantified desitometrically by Image J 1.32 software package (National Institute of Health, Bethesda, MD, USA). Results were normalized for GAPDH expression.
Measurement of cardiomyocyte Ca2+ transients
Adult ventricular mouse myocytes were isolated by liberase/trypsin digestion [for detailed procedure see Protocol PP00000125 from The Alliance for Cellular Signalling (AfCS)]. Whole-cell Ca2+ transients were measured in Indo-1-loaded, electrically paced (0.5 Hz) cardiomyocytes as described before (Kilic et al., 2005). Excitation was at 365 nm, and the emitted fluorescence was recorded at 405 and 495 nm. The ratio of fluorescence at the two wavelengths was used as an index of the cytosolic Ca2+ concentration. Data were collected at 20 Hz, and acquisition and processing were supported by Felix software (Felix version 1.1, Photon Technologies, Seefeld, Germany). After obtaining basal recordings for 10 min, myocytes were exposed to the indicated compounds and transients were continuously recorded for at least 15 min. For positive control, myocytes were stimulated with 100 nM isoproterenol at the end of the experiment.
Materials
FTY720 was provided by Novartis Pharma (Basel, Switzerland). Unless otherwise stated, all compounds were from Sigma (Deissenhofen, Germany). Animals were purchased from Harlan (Borchen, Germany). Antibodies: anti-S1P1 and anti-S1P3 were from Acris, anti-GAPDH from Milipore (HRP)-conjugated anti-rabbit (Milipore, Schwalbach, Germany) and (HRP)-conjugated anti-mouse were from GE Healthcare.
The drug and molecular target nomenclature conforms to the Guide to Receptors and Channels as recently published in revised form (Alexander et al., 2009).
Statistical analysis
All data are expressed as mean and standard error of mean (SEM). Statistical analysis was performed using WinStat software (Benecke & Schwippert; Staufen, Germany), on Excel spreadsheets. Multiple comparisons were done by anova analysis for repeated measurements. Comparison of variables between two groups was performed by using the unpaired Student's t-test. P-values <0.05 were considered significant. Mortality rates were compared between groups by a χ2-test. Survival was analysed by a Cox-Mantel Log-rank test.
Results
Blood count
FTY720 induces a dose-dependent lymphopenia in peripheral blood. Therefore, we performed differential blood counts 24 h after ischaemia–reperfusion to verify the effectiveness of treatment. In all experiments, FTY720 (0.5 mg·kg−1) induced leukopenia by significantly reducing the number of lymphocytes in peripheral blood (Figure 1). Lymphocyte count was not different between pre + post and post group. When compared with the control group, FTY720 did not affect absolute neutrophile counts in peripheral blood.
Figure 1.
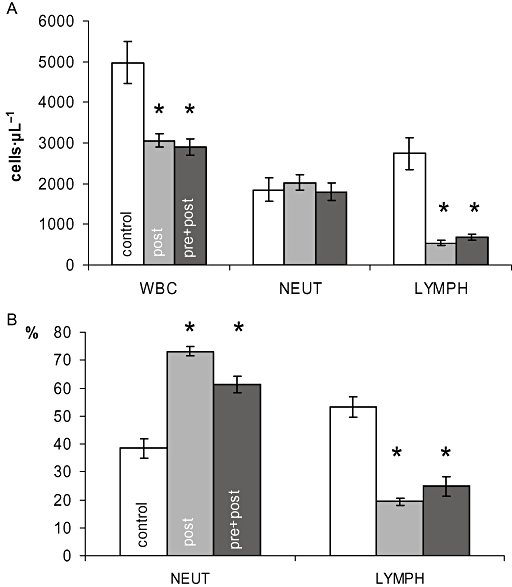
Differential blood count. Blood was drawn after 24 h and analysed for absolute leukocyte numbers (A) in control (white bars), post (grey bars) and pre + post (black bars) treated animals. The percentage of granulocytes (NEUT) and lymphocytes (LYMPH) of the total leukocyte count (WBC) was calculated from these data (B). *P < 0.05 versus control
Mortality and infarct size
Survival was significantly different between groups due to differences in mortality during ischaemia and early reperfusion (Figure 2A). Overall mortality was 37% (n = 38), 50% (n = 42) and 14% (n = 22) in control, post and pre + post group respectively. All post animals that have died within 1 h after ligation showed fatal ventricular tachycardias or ventricular fibrillation (Figure 2C and D). Importantly, most arrhythmias occurred in the post group before initiation of reperfusion. Mortality was 24%, 39%, 14% for control, post and pre + post group during the first hour from start of ligation (45 min of ischaemia + 15 min of reperfusion). During the following 23 h of reperfusion two animals died in the control and three in the pre + post group.
Figure 2.
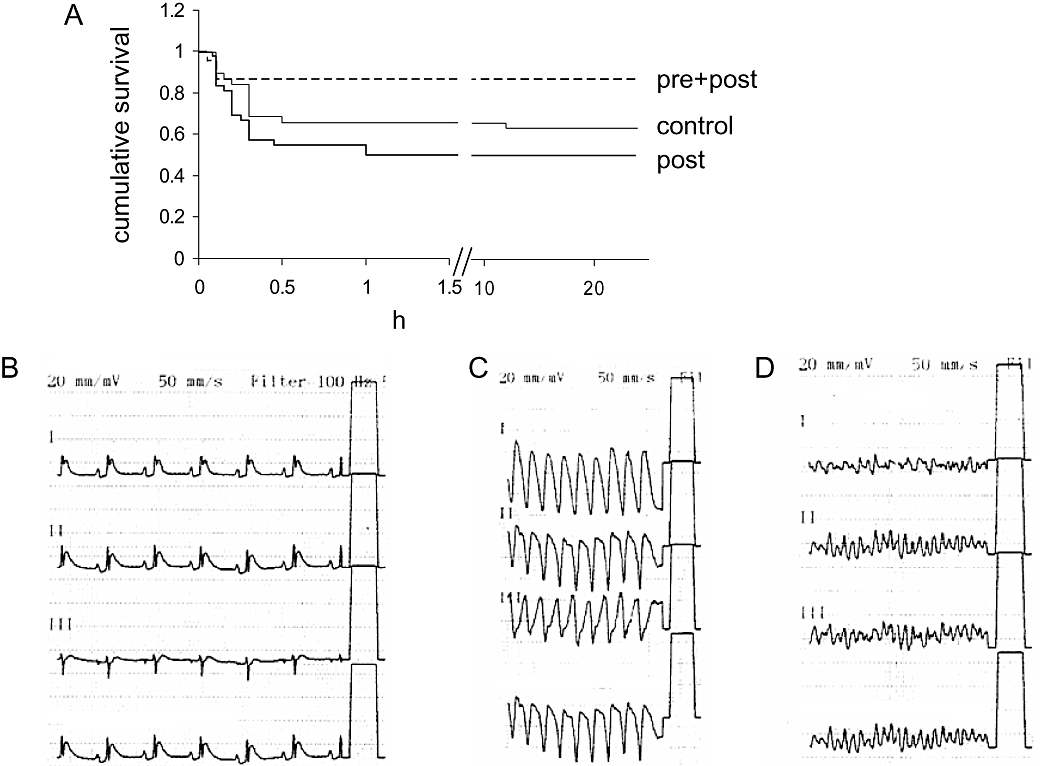
Survival. (A) Kaplan-Meier survival plot for rats treated with 0.9% NaCl (control) and 0.5 mg·kg−1 FTY720 (2-amino-2-[2-(4-octylphenyl)ethyl]-1,3-propanediol hydrochloride) once, before reperfusion (post), or twice, 24 h before ischaemia and before reperfusion (pre + post). Survival was significantly different between groups (P < 0.05). (B–D) Representative ECG tracings from infarcted animals showing ST-segment elevation (B). FTY720 when administered for the first time after coronary ligature (post regimen) increased the incidence of ventricular tachycardias (C) and ventricular fibrillation (D).
After 45 min of ligation and 24 h of reperfusion, the left ventricular (LV) area affected by coronary ligation (AAR) was not significantly different between the experimental groups (AAR/LV: 41%, 43% and 41% in control, post and pre + post). Neither post-conditioning nor combined the pre + post treatment procedures were able to reduce infarct size (infarct area/AAR: 58%, 59% and 61% in control, post and pre + post) (Figure 3).
Figure 3.
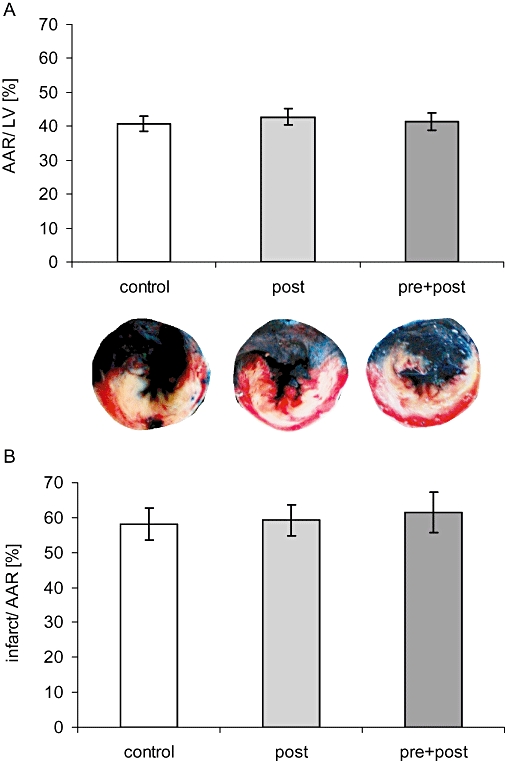
Infarct size. (A) Staining negative for Evans blue delineates the area at risk (AAR) within the left ventricle (LV). (B) triphenyltetrazolium chloride stains vital tissue red and enables calculation of the infracted tissue within the AAR. Neither AAR/LV nor infarct area/AAR was different between groups.
Myocardial inflammation
During early reperfusion, granulocytes make up the major leukocyte population in the infarct border zone and substantially contribute to local expression of pro-inflammatory cytokines. Therefore, we quantified granulocyte numbers in the infarct border zone. FTY720 treatment significantly reduced granulocyte infiltration in the infarct border zone 24 h after I/R (295.9± 37.6 mm−2 post, 345.0± 22.3 mm−2 pre + post, P < 0.05 vs. 432.6± 30.3 mm−2 control, Figure 4).
Figure 4.
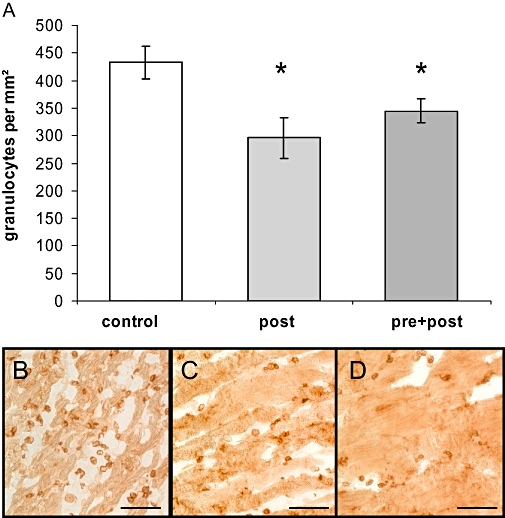
Granulocyte tissue infiltration was evaluated by counting positively stained cells within the infarct border zone of control (B), pre + post (C) and post (D) treated animals. Bar represents 100 µm. FTY720 (2-amino-2-[2-(4-octylphenyl)ethyl]-1,3-propanediol hydrochloride) significantly reduced number of granulocytes/400× field (*P < 0.05 vs. control) (A).
The pre + post regimen significantly reduced TNF-α protein expression in the infarct border zone (pre + post vs. control 0.69 ± 0.04 vs. 0.94 ± 0.10 pg·mg−1, P < 0.05, Figure 5A). Serum TNF-α was quantified by elisa from blood drawn after 24 h. There was no significant difference between groups, albeit the pre + post regimen tended to reduce serum levels (Figure 5B).
Figure 5.
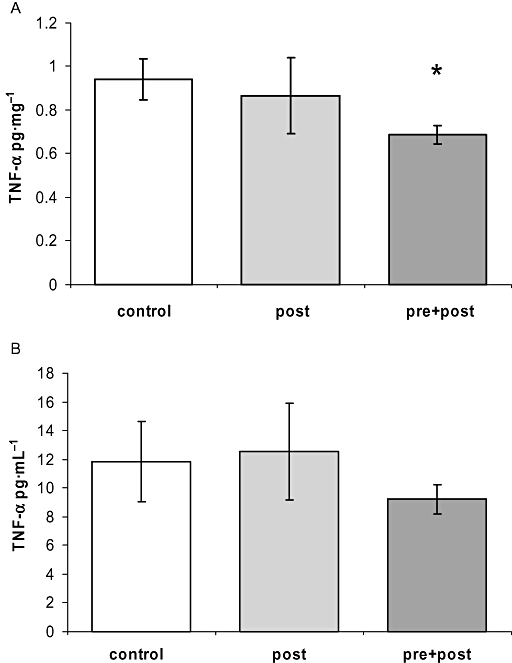
Tumour necrosis factor (TNF)-α protein expression. (A) The pre + post regimen significantly reduced TNF-α protein levels in the infarct border zone (*P < 0.05 vs. control). (B) Serum TNF-α was quantified by elisa from peripheral blood drawn after 24 h from the left A. carotis.
S1P receptor expression
As FTY720 is well known for modulating S1P receptor signalling by receptor down-regulation, we analysed S1P1 and S1P3 receptor expression in non-infarcted left ventricular myocardium. Western blotting demonstrated that FTY720 treatment significantly reduced S1P1 expression (Figure 6A). Expression of S1P3 was not different between groups after 24 h of reperfusion (Figure 6B).
Figure 6.
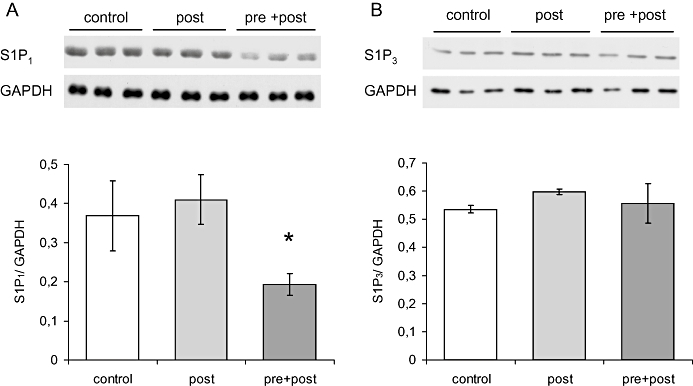
Sphingosine-1-phosphate (S1P) receptor expression after 24 h of reperfusion was analysed by Western blotting. S1P1 receptor expression (A) was significantly lower in the pre + posts treatment group (*P < 0.05 vs. post group). S1P3 receptor expression was not different between groups (B).
Effect of S1P receptor agonist on cardiomyocyte calcium metabolism
S1P was reported to induce both a rise in diastolic calcium and spontaneous calcium transients in neonatal cardiomyocytes, just as can be observed during ischaemia–reperfusion (Nakajima et al., 2000). Therefore, we studied the effect of FTY720 on intracellular calcium transients in adult cardiomyocytes. FTY720, up to 500 nM, did neither affect the diastolic calcium level nor the calcium transient amplitude. We further tested its phosphorylated active form (FTY720-P concentration range tested: 5–500 nM), the selective S1P1 agonist SEW2871 (10 µM) and S1P (5–500 nM). None influenced intracellular calcium transients in electrically paced cardiomyocytes. We also studied intracellular calcium in unstimulated resting cardiomyocytes and found no change in response to S1P receptor stimulation. When the compounds were applied to cardiomyocytes in buffer supplemented with 0.1% bovine serum albumin, each induced a considerable rise in diastolic calcium. However, the effect could be reproduced by 0.1% bovine serum albumin alone. Representative calcium transient recordings are presented in Figure S1.
Discussion and conclusions
Reducing infarct size by pharmaceutical interventions as an adjunct to classical reperfusion interventions would be an attractive therapeutical principle. Therefore, we investigated whether FTY720, the first orally bioavailable S1P receptor agonist, might be effective in reducing infarct size. As expected, FTY720 treatment reduced peripheral lymphocyte counts. However, in contrast to our previous ex vivo findings (Hofmann et al., 2009), the compound was not able to reduce infarct size. Instead, in animals post-conditioned with FTY720 a significantly increased mortality was found. This was mainly caused by induction of fatal arrhythmias during ischaemia and early reperfusion. As the pro-arrhythmic effect might only be a first dose effect and preconditioning treatment might rather be more effective than pharmacological post-conditioning, we decided to treat another group of animals (pre + post group) with FTY720 24 h before surgery. Indeed, this treatment regimen reduced mortality, but still failed to reduce infarct size. In either group most animals died within the first hour from ventricular tachycardia. Unfortunately, we did not comprehensively assess incidence and characteristics of arrhythmias, but our analysis clearly indicated that the mortality from arrhythmias was significantly higher in the post group.
Besides its agonistic effect, FTY720 down-regulates S1P1 receptors but not S1P3 receptors (Graler and Goetzl, 2004). Accordingly, we could demonstrate that S1P1 receptor expression is down-regulated in the pre + post group after 24 h of reperfusion. S1P1 receptor stimulation has previously been reported to aggravate reperfusion arrhythmias (Tsukada et al., 2007). Therefore, the dichotomous effect of FTY720, S1P1 receptor stimulation with first administration and S1P1 receptor down-regulation after repeated administration, offers a plausible explanation for our finding that FTY720 increased mortality in the post but reduced mortality in the pre + post treatment group: After first dosing FTY720 likely aggravates reperfusion arrhythmias by stimulating S1P1 receptors on cardiomyocytes. After repeated administration FTY720 protects against pro-arrhythmic S1P1 receptor-mediated effects of S1P, which is endogenously released by local ischaemia and thrombocyte activation (Sano et al., 2002; Dahm et al., 2006).
It remains unclear however, by which mechanism S1P1 agonists induce arrhythmias. Potential cardiac side effects of the drug FTY720 have been studied extensively in animal model as well in man. FTY720 induces a moderate reduction in heart rate but does not affect repolarization in healthy subjects (Schmouder et al., 2006). Therefore, we did only pilot experiments in sham-operated animals mainly for assessment of the dose- and time-dependent effect of i.p. delivered doses on lymphocyte count. Here we could not find any indication that FTY720 induced arrhythmias. However, there is some experimental evidence that S1P receptors might elicit cardio-depressant and pro-arrhythmogenic effects, which might become relevant in ischaemia-reperfused myocardium. S1P1 receptor stimulation induces calcium overload in neonatal cardiomyocytes (Nakajima et al., 2000). Intracellular calcium overload due to Na+/Ca2+ exchanger reverse mode activity and sarcoplasmatic reticulum dysfunction is generally supposed to be a main cause for induction of arrhythmias in ischaemia-reperfused myocardium. The exact effects of S1P receptor subtypes on ion conductance and calcium homeostasis are still incompletely understood, but the existing literature (Means and Brown, 2009) indicates that a S1P1 receptor-mediated effect on cardiomyocytes calcium metabolism was the most likely mechanism for the observed pro-arrhythmic effect. Therefore, we hypothesized that S1P1 receptor agonism during early reperfusion adds to reperfusion arrhythmias by aggravating calcium overload, whereas pretreatment with FTY720 attenuates pro-arrhythmic effects by S1P1 receptor down-regulation. In order to test this hypothesis, we studied calcium transients in adult cardiomyocytes stimulated with S1P, FTY720, its active form FTY720 phosphate, and with the selective S1P1 agonist SEW2871. However, we were not able to observe an effect on both diastolic calcium and calcium transient amplitude, as has been reported for neonatal cardiomyocytes (Nakajima et al., 2000). The discrepancy might earliest rely on differences in intracellular signalling between neonatal and adult cardiomyocytes. The response to S1P1 receptor ligands might also be different in the in vivo ischaemia-reperfused myocardium, but we would rather conclude from our data, that S1P1 receptor induced calcium deregulation is not the most likely mechanism for the pro-arrhythmogenic effect of FTY720.
Ischaemic (Zatta et al., 2006) as well as pharmacological post-conditioning (Huhn et al., 2008) has been demonstrated to be able to reduce infarct size in in vivo ischaemia–reperfusion models in the rat. Furthermore, Egom et al. could demonstrate previously that FTY720 reduces infarct size and protects against reperfusion arrhythmias ex vivo (Egom et al., 2009). However, we could not find an infarct size limiting effect of FTY720 in our in vivo model. There are several reasonable explanations for this: first, reperfusion after 45 min of ischaemia produces a great infarct within the AAR. If we would have studied shorter periods of ischaemia, as has often been done in comparable in vivo studies, we might have detected an infarct sparing effect. In our opinion however, longer periods of ischaemia are clinically more relevant.
Second, it could be a matter of dosing. However, most studies showing a beneficial effect of FTY720 against renal ischaemia–reperfusion injury used comparable doses in rats or mice (Ortiz et al., 2003; Kaudel et al., 2006; Delbridge et al., 2007). We did not further try higher doses for post-conditioning, as we would expect even higher mortality due to pro-arrhythmic effects.
Third, proper timing might be crucial for efficacy. Our initial protocol with FTY720 application just before start of reperfusions reflects pharmacological post-conditioning. However, this clinically most promising therapeutical principle might not work with S1P receptor modulators. The protective effects of S1P receptor stimulation were mainly demonstrated in ex vivo models for preconditioning. Therefore, we performed another series of experiments in which animals were additionally pretreated 24 h before ischaemia (pre + post). In this group, we could demonstrate significantly reduced mortality. However, there was also no significant effect on infarct size. We would not suggest that the negative results have to be ascribed to inappropriate timing of treatment, as both regimens (post and pre + post) were ineffective in reducing infarct size.
Fourth, the immuno-modulating properties of the compound might be rather disadvantageous. Induction of T-cell lymphopenia might be unfavourable, because there are particular subsets of T-cells that can be able to favourably modulate the immune response in ischaemia–reperfusion (Liesz et al., 2009; Monteiro et al., 2009). We suggest that these immunological effects contribute to the obvious discrepancy between ex and in vivo effects on cardiac injury. In conclusion, treatment with the S1P receptor modulator FTY720 was not able to reduce infarct size after myocardial ischaemia–reperfusion in vivo. FTY720 increased mortality due to induction of arrhythmias as first dose effect when administered during myocardial ischaemia. Therefore, pharmacological S1P receptor agonists in general might not be the ideal post-conditioning agent. Despite the promising effects in ex vivo models, especially the potential confounding effects on cardiomyocyte electrophysiology might pose a major obstacle for the therapeutic use of S1P receptor agonists in myocardial ischaemia–reperfusion.
Acknowledgments
FTY720 was kindly provided by Novartis Pharma. We thank Dr Obergfell from the Institute of Clinical Biochemistry and Pathobiochemistry for the help with the differential blood count analysis, M. Klaiber and M. Kuhn for the calcium measurements, and Katharina Meder for her excellent technical support. SF was supported by the Deutsche Forschungsgemeinschaft (SFB 688, TP A10).
Glossary
Abbreviations:
- AAR
area at risk
- FTY720
S1P receptor agonist, 2-amino-2-[2-(4-octylphenyl)ethyl]-1,3-propanediol hydrochloride
- LV
left ventricle
- S1P
sphingosine-1-phosphate
- TNF-α
tumour necrosis factor-α
- TTC
triphenyltetrazolium chloride
Conflicts of interest
None.
Supporting information
Additional Supporting Information may be found in the online version of this article:
Figure S1 Calcium transients were recorded in Indo-1-loaded cardiomyocytes under baseline conditions (control) and after stimulation with S1P1 receptor agonists. Representative transients were generated by averaging the Indo-1 signal ratio of 10 consecutive single transients. (A) FTY720 did neither affect diastolic nor systolic signal height, unless cardiomyocytes were superfused with buffer containing 0.1% BSA. (B) An analogous rise in diastolic calcium could be demonstrated by supplementing buffer with 0.1% BSA. (C) The active phosphorylated compound FTY720-P, akin to the other S1P1 receptor agonists tested (SEW2871 and S1P, data not shown), did not influence transients. Isoproterenol stimulation at the end of the experiment always confirmed that myocytes' intracellular calcium transients were responsive to exogenous stimulation. FTY720, S1P receptor agonist, 2-amino-2-[2-(4-octylphenyl)ethyl]-1,3-propanediol hydrochloride; S1P, sphingosine-1-phosphate.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 4th edn. Br J Pharmacol. 2009;158(Suppl 1):S1–S254. doi: 10.1111/j.1476-5381.2009.00499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An S, Goetzl E, Lee H. Signaling mechanisms and molecular characteristics of G protein-coupled receptors for lysophosphatidic acid and sphingosine 1-phosphate. J Cell Biochem Suppl. 1998;30–31:147–157. [PubMed] [Google Scholar]
- Anselmo D, Amersi FF, Shen XD, Gao F, Katori M, Ke B, et al. FTY720: a novel approach to the treatment of hepatic ischemia-reperfusion injury. Transplant Proc. 2002;34:1467–1468. doi: 10.1016/s0041-1345(02)02933-0. [DOI] [PubMed] [Google Scholar]
- Awad AS, Ye H, Huang L, Li L, Foss FW, MacDonald TL, et al. Selective sphingosine 1-phosphate 1 receptor activation reduces ischemia-reperfusion injury in mouse kidney. Am J Physiol Renal Physiol. 2006;290:F1516–F1524. doi: 10.1152/ajprenal.00311.2005. [DOI] [PubMed] [Google Scholar]
- Bolick D, Srinivasan S, Kim K, Hatley M, Clemens J, Whetzel A. Sphingosine-1-phosphate prevents tumor necrosis factor-alpha-mediated monocyte adhesion to aortic endothelium in mice. Arterioscler Thromb Vasc Biol. 2005;25:976–981. doi: 10.1161/01.ATV.0000162171.30089.f6. [DOI] [PubMed] [Google Scholar]
- Brinkmann V. FTY720: mechanism of action and potential benefit in organ transplantation. Yonsei Med J. 2004;45:991–997. doi: 10.3349/ymj.2004.45.6.991. [DOI] [PubMed] [Google Scholar]
- Brinkmann V, Cyster J, Hla T. FTY720: sphingosine 1-phosphate receptor-1 in the control of lymphocyte egress and endothelial barrier function. Am J Transplant. 2004;4:1019–1025. doi: 10.1111/j.1600-6143.2004.00476.x. [DOI] [PubMed] [Google Scholar]
- Chiba K, Matsuyuki H, Maeda Y, Sugahara K. Role of sphingosine 1-phosphate receptor type 1 in lymphocyte egress from secondary lymphoid tissues and thymus. Cell Mol Immunol. 2006;3:11–19. [PubMed] [Google Scholar]
- Dahm F, Nocito A, Bielawska A, Lang KS, Georgiev P, Asmis LM, et al. Distribution and dynamic changes of sphingolipids in blood in response to platelet activation. J Thromb Haemost. 2006;4:2704–2709. doi: 10.1111/j.1538-7836.2006.02241.x. [DOI] [PubMed] [Google Scholar]
- Dantas A, Igarashi J, Michel T. Sphingosine 1-phosphate and control of vascular tone. Am J Physiol Heart Circ Physiol. 2003;284:H2045–H2052. doi: 10.1152/ajpheart.01089.2002. [DOI] [PubMed] [Google Scholar]
- Delbridge MS, Shrestha BM, Raftery AT, El Nahas AM, Haylor JL. Reduction of ischemia-reperfusion injury in the rat kidney by FTY720, a synthetic derivative of sphingosine. Transplantation. 2007;84:187–195. doi: 10.1097/01.tp.0000269794.74990.da. [DOI] [PubMed] [Google Scholar]
- Dudek S, Camp S, Chiang E, Singleton P, Usatyuk P, Zhao Y, et al. Pulmonary endothelial cell barrier enhancement by FTY720 does not require the S1P1 receptor. Cell Signal. 2007;19:1754–1764. doi: 10.1016/j.cellsig.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egom E, Ke Y, Musa H, Mohamed T, Wang T, Cartwright E, et al. FTY720 prevents ischemia/reperfusion injury-associated arrhythmias in an ex vivo rat heart model via activation of Pak1/Akt signaling. J Mol Cell Cardiol. 2009;48:406–414. doi: 10.1016/j.yjmcc.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English D, Brindley D, Spiegel S, Garcia J. Lipid mediators of angiogenesis and the signalling pathways they initiate. Biochim Biophys Acta. 2002;1582:228–239. doi: 10.1016/s1388-1981(02)00176-2. [DOI] [PubMed] [Google Scholar]
- Frantz S, Calvillo L, Tillmanns J, Elbing I, Dienesch C, Bischoff H, et al. Repetitive postprandial hyperglycemia increases cardiac ischemia/reperfusion injury: prevention by the a-glucosidase inhibitor acarbose. FASEB J. 2005;19:2459–2472. doi: 10.1096/fj.04-2459fje. [DOI] [PubMed] [Google Scholar]
- Gon Y, Wood MR, Kiosses WB, Jo E, Sanna MG, Chun J, et al. S1P3 receptor-induced reorganization of epithelial tight junctions compromises lung barrier integrity and is potentiated by TNF. Proc Natl Acad Sci USA. 2005;102:9270–9275. doi: 10.1073/pnas.0501997102. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Graler M, Goetzl E. The immunosuppressant FTY720 down-regulates sphingosine 1-phosphate G-protein-coupled receptors. FASEB J. 2004;18:551–553. doi: 10.1096/fj.03-0910fje. [DOI] [PubMed] [Google Scholar]
- Hofmann U, Burkard N, Vogt C, Thoma A, Frantz S, Ertl G, et al. Protective effects of sphingosine-1-phosphate receptor agonist treatment after myocardial ischaemia-reperfusion. Cardiovasc Res. 2009;83:285–293. doi: 10.1093/cvr/cvp137. [DOI] [PubMed] [Google Scholar]
- Huhn R, Heinen A, Weber N, Hollmann M, Schlack W, Preckel B. Hyperglycaemia blocks sevoflurane-induced postconditioning in the rat heart in vivo: cardioprotection can be restored by blocking the mitochondrial permeability transition pore. Br J Anaesth. 2008;100:465–471. doi: 10.1093/bja/aen022. [DOI] [PubMed] [Google Scholar]
- Jongsma M, van Unen J, van Loenen P, Michel M, Peters S, Alewijnse A. Different response patterns of several ligands at the sphingosine-1-phosphate receptor subtype 3 (S1P3) Br J Pharmacol. 2009;156:1305–1311. doi: 10.1111/j.1476-5381.2009.00134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karliner J, Honbo N, Summers K, Gray M, Goetzl E. The lysophospholipids sphingosine-1-phosphate and lysophosphatidic acid enhance survival during hypoxia in neonatal rat cardiac myocytes. J Mol Cell Cardiol. 2001;33:1713–1717. doi: 10.1006/jmcc.2001.1429. [DOI] [PubMed] [Google Scholar]
- Kaudel CP, Schmiddem U, Frink M, Bergmann S, Pape HC, Krettek C, et al. FTY720 for treatment of ischemia-reperfusion injury following complete renal ischemia in C57/BL6 mice. Transplant Proc. 2006;38:679–681. doi: 10.1016/j.transproceed.2006.01.033. [DOI] [PubMed] [Google Scholar]
- Kilic A, Velic A, De Windt L, Fabritz L, Voss M, Mitko D, et al. Enhanced activity of the myocardial Na+/H+ echanger NHE-1 contributes to cardiac remodelling in atrial natriuretic peptide receptor-deficient mice. Circulation. 2005;112:2307–2311. doi: 10.1161/CIRCULATIONAHA.105.542209. [DOI] [PubMed] [Google Scholar]
- Landeen L, Dederko D, Kondo C, Hu B, Aroonsakool N, Haga J. Mechanisms of the negative inotropic effects of sphingosine-1-phosphate on adult mouse ventricular myocytes. Am J Heart Circ Physiol. 2008;294:H736–H749. doi: 10.1152/ajpheart.00316.2007. [DOI] [PubMed] [Google Scholar]
- Liesz A, Suri-Payer E, Veltkamp C, Doerr H, Sommer C, Rivest S, et al. Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nat Med. 2009;15:192–199. doi: 10.1038/nm.1927. [DOI] [PubMed] [Google Scholar]
- Mansoor M, Melendez AJ. Recent trials for FTY720 (fingolimod): a new generation of immunomodulators structurally similar to sphingosine. Rev Recent Clin Trials. 2008;3:62–69. doi: 10.2174/157488708783330486. [DOI] [PubMed] [Google Scholar]
- Means C, Brown J. Sphingosine-1-phosphate receptor signalling in the heart. Cardiovasc Res. 2009;82:193–200. doi: 10.1093/cvr/cvp086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Means CK, Xiao CY, Li Z, Zhang T, Omens JH, Ishii I, et al. Sphingosine 1-phosphate S1P2 and S1P3 receptor-mediated Akt activation protects against in vivo myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2007;292:H2944–H2951. doi: 10.1152/ajpheart.01331.2006. [DOI] [PubMed] [Google Scholar]
- Monteiro RM, Camara NO, Rodrigues MM, Tzelepis F, Damiao MJ, Cenedeze MA, et al. A role for regulatory T cells in renal acute kidney injury. Transpl Immunol. 2009;21:50–55. doi: 10.1016/j.trim.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Nakajima N, Cavalli A, Biral D, Glembotski C, McDonough P, Ho P, et al. Expression and characterization of Edg-1 receptors in rat cardiomyocytes: calcium deregulation in response to sphingosine 1-phosphate. Eur J Biochem. 2000;267:5679–5686. doi: 10.1046/j.1432-1327.2000.01656.x. [DOI] [PubMed] [Google Scholar]
- Opitz C, Finn P, Pfeffer M, Mitchell G, Pfeffer J. Effects of reperfusion on arrhythmias and death after coronary artery occlusion in the rat: increased electrical stability independent of myocardial salvage. J Am Coll Cardiol. 1998;32:261–267. doi: 10.1016/s0735-1097(98)00173-9. [DOI] [PubMed] [Google Scholar]
- Ortiz AM, Troncoso P, Kahan BD. Prevention of renal ischemic reperfusion injury using FTY 720 and ICAM-1 antisense oligonucleotides. Transplant Proc. 2003;35:1571–1574. doi: 10.1016/s0041-1345(03)00374-9. [DOI] [PubMed] [Google Scholar]
- Sanchez T, Estrada-Hernandez T, Paik J, Wu M, Venkataraman K, Brinkmann V, et al. Phosphorylation and action of the immunomodulator FTY720 inhibits vascular endothelial growth factor-induced vascular permeability. J Biol Chem. 2003;278:47281–47290. doi: 10.1074/jbc.M306896200. [DOI] [PubMed] [Google Scholar]
- Sanna M, Liao J, Jo E, Alfonso C, Ahn M, Peterson M. Sphingosine-1-phosphate (S1P) receptor subtypes S1P1 and S1P3, respectively, regulate lymphocyte recirculation and heart rate. J Biol Chem. 2004;279:13839–13848. doi: 10.1074/jbc.M311743200. [DOI] [PubMed] [Google Scholar]
- Sano T, Baker D, Virag T, Wada A, Yatomi Y, Kobayashi T, et al. Multiple mechanisms linked to platelet activation result in lysophosphatidic acid and sphingosine 1-phosphate generation in blood. J Biol Chem. 2002;277:21197–21206. doi: 10.1074/jbc.M201289200. [DOI] [PubMed] [Google Scholar]
- Schmouder R, Serra D, Wang Y, Kovarik J, DiMarco J, Hunt T. FTY720: placebo-controlled study of the effect on cardiac rate and rhythm in healthy subjects. J Clin Pharmacol. 2006;46:895–904. doi: 10.1177/0091270006289853. [DOI] [PubMed] [Google Scholar]
- Singer II, Tian M, Wickham LA, Lin J, Matheravidathu SS, Forrest MJ, et al. Sphingosine-1-phosphate agonists increase macrophage homing, lymphocyte contacts, and endothelial junctional complex formation in murine lymph nodes. J Immunol. 2005;175:7151–7161. doi: 10.4049/jimmunol.175.11.7151. [DOI] [PubMed] [Google Scholar]
- Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat Rev Mol Cell Biol. 2003;4:397–407. doi: 10.1038/nrm1103. [DOI] [PubMed] [Google Scholar]
- Tao R, Zhang J, Vessey D, Honbo N, Karliner J. Deletion of the sphingosine kinase-1 gene influences cell fate during hypoxia and glucose deprivation in adult mouse cardiomyocytes. Cardiovasc Res. 2007;74:56–63. doi: 10.1016/j.cardiores.2007.01.015. [DOI] [PubMed] [Google Scholar]
- Theilmeier G, Schmidt C, Herrmann J, Keul P, Schafers M, Herrgott I. High-density lipoproteins and their constituent, sphingosine-1-phosphate, directly protect the heart against ischemia/reperfusion injury in vivo via the S1P3 lysophospholipid receptor. Circulation. 2006;114:1403–1409. doi: 10.1161/CIRCULATIONAHA.105.607135. [DOI] [PubMed] [Google Scholar]
- Tsukada T, Sanna M, Rosen H, Gottlieb R. S1P1-selective agonist SEW2871 exacerbate reperfusion arrhthmias. J Cardiovasc Pharmacol. 2007;50:660–669. doi: 10.1097/FJC.0b013e318157a5fe. [DOI] [PubMed] [Google Scholar]
- Whetzel A, Bolick D, Srinivasan S, Macdonald T, Morris M, Ley L. Sphingosiene-1-phosphate prevents monocyte/endotheliel interactions in type 1 diabetic NOD mice through activation of the S1P1 receptor. Circ Res. 2006;99:731–739. doi: 10.1161/01.RES.0000244088.33375.52. [DOI] [PubMed] [Google Scholar]
- Yellon D, Downey J. Preconditioning the myocardium: from cellular physiology to clinical cardiology. Physiol Rev. 2003;83:1113–1151. doi: 10.1152/physrev.00009.2003. [DOI] [PubMed] [Google Scholar]
- Zatta A, Kin H, Lee G, Wang N, Jiang R, Lust R, et al. Infarct-sparing effect of myocardial postconditioning is dependent on protein kinase C signalling. Cardiovasc Res. 2006;70:315–324. doi: 10.1016/j.cardiores.2005.11.030. [DOI] [PubMed] [Google Scholar]
- Zhang J, Honbo N, Goetzl EJ, Chatterjee K, Karliner JS, Gray MO. Signals from type 1 sphingosine 1-phosphate receptors enhance adult mouse cardiac myocyte survival during hypoxia. Am J Physiol Heart Circ Physiol. 2007;293:H3150–H3158. doi: 10.1152/ajpheart.00587.2006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


