Abstract
Background and purpose:
Both ischaemia preconditioning (PC) and the intracoronary infusion of peroxynitrite (PN) suppress ischaemia and reperfusion (I/R)-induced arrhythmias and the generation of nitrotyrosine (NT, a marker of PN). However, it is still unclear whether this latter effect is due to a reduction in nitric oxide (NO) or superoxide (O2−) production.
Experimental approach:
Dogs anaesthetized with chloralose and urethane were infused, twice for 5 min, with either saline (control) or 100 nM PN, or subjected to similar periods of occlusion (PC), 5 min prior to a 25 min occlusion and reperfusion of the left anterior descending coronary artery. Severities of ischaemia and ventricular arrhythmias, as well as changes in the coronary sinus nitrate/nitrite (NOx) levels were assessed throughout the experiment. The production of myocardial NOx, O2− and NT was determined following reperfusion.
Key results:
Both PC and PN markedly suppressed the I/R-induced ventricular arrhythmias, compared to the controls, and increased NOx levels during coronary artery occlusion. Reperfusion induced almost the same increases in NOx levels in all groups, but superoxide production and, consequently, the generation of NT were significantly less in PC- and PN-treated dogs than in controls.
Conclusions and implications:
Since both PC and the administration of PN enhanced NOx levels during I/R, the attenuation of endogenous PN formation in these dogs is primarily due to a reduction in the amount of O2 produced. Thus, the anti-arrhythmic effect of PC and PN can almost certainly be attributed to the preservation of NO availability during myocardial ischaemia.
Keywords: arrhythmias, preconditioning, nitric oxide, peroxynitrite, superoxide
Introduction
Although there is much evidence for the detrimental effects of peroxynitrite (PN), a by-product of the highly reactive radicals nitric oxide (NO) and superoxide, during ischaemia and reperfusion (I/R) (reviewed recently by Pryor and Squadrito, 1995; Ferdinándy and Schulz, 2001, 2003; Lalu et al., 2002; Uppu et al., 2007), there is also evidence that when it is applied exogenously in low (micromolar) concentrations (Nossuli et al., 1997; Altug et al., 2000) or formed as a result of brief I/R insults (Altug et al., 2001), it may induce cardioprotection.
In a recent study (Kiss et al., 2008) designed to examine the effect of brief (5 min) periods of the intracoronary infusion of PN on the consequences of coronary artery occlusion, and especially the severity of arrhythmias in anaesthetized dogs, we showed that PN markedly suppressed the I/R-induced ventricular arrhythmias. This protection was similar to that obtained previously with preconditioning (PC) occlusions of the same duration (Végh et al., 1992a), and was associated with a reduction in the formation of endogenous PN, as assessed by changes in nitrotyrosine (NT) levels following a prolonged (25 min) period of I/R (Kiss et al., 2007; 2008;). It was concluded that the anti-arrhythmic effects of PC and PN administration may, at least in part, result from the attenuation of endogenous PN production (Kiss et al., 2008).
Since PN is generated from equivalent concentrations of NO and superoxide (Miles et al., 1996), theoretically, a decrease in PN formation may result from either a reduced production of NO or superoxide. Although both these radicals are involved in I/R-induced injuries and there is also evidence that they play a role in the cardioprotection induced by PC (reviewed, e.g. in Ferdinándy and Schulz, 2001; 2003; Berges et al., 2003), their exact role and importance in these situations are still not fully understood; indeed, results obtained from various experimental setups are controversial and largely depend on the model used. For example, studies performed mainly under in vitro conditions suggest that in hearts subjected to global ischaemia, NO production is substantially increased (Zweier et al., 1995a), and that PC, by attenuating this harmful overproduction of NO and the subsequent generation of PN, results in cardioprotection (Csonka et al., 1999). In contrast, in vivo studies in large animals, such as dogs and pigs, indicate that NO production is significantly reduced during sustained coronary artery occlusion (Engelman et al., 1995; Mori et al., 1998; Stevens et al., 2002; Prasan et al., 2007). This loss of NO production may certainly contribute to the severe consequences of I/R, such as the occurrence of fatal ventricular arrhythmias. Thus, it is not surprising that manoeuvres which maintain NO synthesis, for example, by enhancing the activation of nitric oxide synthase (NOS) enzymes (Depréet al., 1997; Muscari et al., 2004) or the donation of NO (Lefer, 1995; Rickover et al., 2008; Raat et al., 2009) during ischaemia would result in cardioprotective effects. Indeed, we were the first to show that the protective effect of ischaemic PC involves the generation of NO (Végh et al., 1992b). Although in these experiments, NO production was not directly measured, the results clearly showed that the inhibition of NO synthesis by Lω-nitro-arginine-methyl-ester markedly attenuated the anti-arrhythmic effect of PC (Végh et al., 1992b). Furthermore, the intracoronary administration of NO donors prior to and during ischaemia resulted in anti-arrhythmic protection (Végh et al., 1996; György et al., 2000; Gönczi et al., 2009) which furthers supports the hypothesis that NO is an endogenous cardioprotectant (Parratt, 1993).
More recently, it has been suggested that NO may exert its cardioprotective effect through the attenuation of oxidative stress, mainly by reducing superoxide production (Berges et al., 2003; Jones and Bolli, 2006; Iwase et al., 2007) which is the other component of PN formation. Superoxide generated during I/R (Vanden Hoek et al., 1997) may also contribute to the appearance of fatal ventricular arrhythmias (Aon et al., 2008; Xie et al., 2009).
In most of the studies mentioned above, NO, superoxide and PN were measured separately; only a few attempts have been made to assess the production of these radicals in parallel during I/R (Liu et al., 1997; Falk et al., 2007). Since in our previous studies the anti-arrhythmic effect of PC and the administration of PN was associated with a substantial reduction in endogenous PN generation (Kiss et al., 2007; 2008;), we have now examined whether this effect is due to a reduction in the amount of NO produced or to reduced superoxide production following a prolonged I/R. Therefore, the effects of brief periods of coronary artery occlusion and of PN infusion on plasma nitrate/nitrite levels, superoxide and NT productions, and on the severity of arrhythmias and ischaemic changes during and following a 25 min occlusion of the left anterior descending (LAD) coronary artery, were examined in chloralose–urethane anaesthetized dogs, and compared to those in a control group of dogs that were only subjected to the prolonged I/R. We showed that both PC and the administration of PN enhanced NOx levels during occlusion and attenuated superoxide, and subsequently, NT production following reperfusion. Thus, we conclude, that in anaesthetized dogs, the marked anti-arrhythmic effects of PC and PN can be associated with the preservation of NO availability during myocardial I/R.
Methods
Mongrel dogs of either sex, and with a mean body weight of 20 ± 4 kg were anaesthetized intravenously with a mixture of chloralose and urethane (60 and 200 mg·kg−1, respectively; Sigma, St. Louis, MO, USA). They were ventilated with room air using a Harvard respirator at a rate and volume sufficient to maintain arterial blood gases within normal limits (Végh et al., 1992a). Body temperature was measured from the mid-oesophagus and maintained at 37 ± 0.5°C. The origin and upkeep of these dogs were in accord with Hungarian law (XXVIII, chapter IV, paragraph 31) regarding large experimental animals, which conforms with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication no. 85-23, revised 1996).
Polyethylene catheters were inserted into the right femoral artery for monitoring arterial blood pressure (systolic, diastolic, and mean), and into the left ventricle (LV) for the measurement of LV systolic and end-diastolic pressures and LVdP/dt (isovolumic contraction and relaxation). The right femoral vein was also catheterized for further anaesthetic administration. The arterial catheters were connected through transducers (Statham P23XL, Hugo Sachs Elektronik, March-Hugstetten, Germany) to a six-channel haemodynamic apparatus (SYTEM6, Triton Technology, San Diego, CA, USA).
A thoracotomy was performed at the left fifth intercostal space and the anterior descending branch of the left coronary artery (LAD) prepared for occlusion just proximal to the first main diagonal branch. A smaller side branch of this artery distal to the proposed site of occlusion was catheterized for the intracoronary administration of saline or PN. Another catheter was positioned into the coronary sinus through the right jugular vein to obtain blood samples for the measurement of plasma nitrate/nitrite (NOx) levels.
Epicardial ST-segment changes and the degree of inhomogeneity of electrical activation were measured from the left ventricular wall distal to the occlusion site using unipolar electrodes and a composite electrode previously described (Végh et al., 1992a; Kiss et al., 2008). The greatest delay in activation occurring within the ischaemic area following coronary artery occlusion was expressed in ms. All parameters, together with a chest lead electrocardiogram, were recorded on an eight-channel Medicor R81 recorder (Esztergom, Hungary).
Ventricular arrhythmias were analysed as outlined previously (Végh et al., 1992a; Kiss et al., 2008). This analysis is based on the suggestions made at the ‘Lambeth Conventions’ (Walker et al., 1998). During occlusion, we estimated the total number of ventricular premature beats (VPBs), the incidence and the number of episodes of ventricular tachycardia (VT; defined as a run of four or more VPBs at a rate faster than the resting sinus rate), and the incidence of ventricular fibrillation (VF). During reperfusion, only the incidence of VF was determined. Dogs that were alive 1–2 min after reperfusion were considered to be survivors.
Synthesis of PN
This has been described in detail previously (Beckman et al., 1994; Kiss et al., 2008). In brief, the prepared solution was filtered and the final concentration of the PN sample was measured spectrophotometrically (peak absorbance at 302 nm wavelength). The stock solutions (50–150 mM) were aliquoted and stored at −80°C away from light. Before each experiment, the absorbance of PN was again measured and the concentration was adjusted to 100 nM with pH 8.4 saline (Nossuli et al., 1997).
Assessment of superoxide production
Two or three myocardial tissue samples (size: 0.5 × 0.5 × 2.0 cm) were excised from the ischaemic area within 2 min of the reperfusion, and superoxide production was determined using dihydroethidium (DHE; Sigma) fluorescence staining (Gu et al., 2003). The tissue blocks were embedded in optimal cutting temperature compounds and cryosections (20 µm) were produced, stained with DHE (1 µM) dissolved in phosphate buffer solution (pH 7.4) and incubated at 37°C for 30 min in a dark humidified chamber. A negative control was obtained by blocking the reaction with N-acetyl-L-cysteine (100 mM, Sigma). Both from the stained and negative control samples, 10–15 serial images were captured by a confocal laser scanning microscope (Olympus FV1000, Tokyo, Japan). The intensity of the fluorescent signals was analysed by ImageQuant software (Molecular Dynamics, Sunnyvale, CA, USA) and expressed in arbitrary units.
Measurement of plasma and tissue nitrate/nitrite (NOx) levels
Plasma nitrate/nitrite (NOx) concentrations were determined by means of the Griess reaction (modified by Moshage et al., 1995). Blood samples taken from the coronary sinus at various time intervals (Figure 1) were centrifuged at 10 000×g for 15 min at 4°C. The plasma was mixed with β-nicotinamide adenine dinucleotide phosphate (NADPH), flavin adenine dinucleotide, nitrate reductase (Sigma) and incubated for 30 min at 37°C. Following the enzymatic reduction of nitrate to nitrite, the Griess reagent was added to the mixture and incubated for an additional 10 min at room temperature. The absorbance of the azo compound was measured spectrophotometrically at a wavelength of 540 nm and the total nitrate/nitrite (NOx) concentration (µmol·L−1) was determined using a standard calibration curve of NaNO2 and NaNO3 (Sigma).
Figure 1.
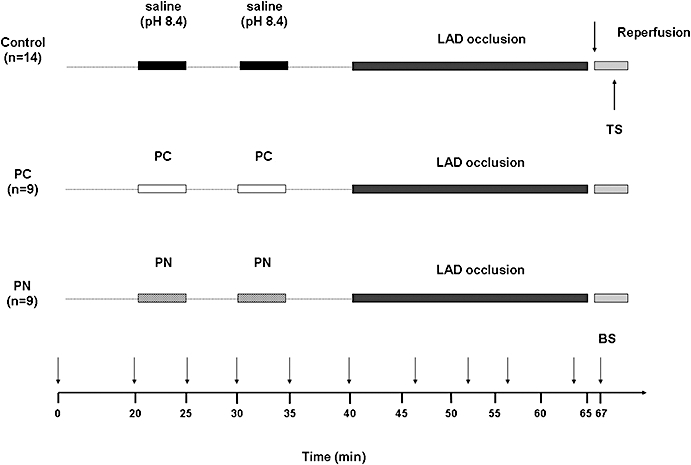
Experimental protocol. After surgery, all animals were allowed to equilibrate for 20 min. Fourteen dogs (control group) were infused with pH 8.4 saline (the solvent of peroxynitrite) at a rate of 0.5 mL·min−1 by the intracoronary route, twice for 5 min, 5 min prior to the 25 min occlusion of the LAD. This was followed by rapid reperfusion. In another two groups of dogs, the prolonged occlusion and reperfusion insult was preceded by either two 5 min preconditioning occlusions (PC group, n = 9) or identical periods of intracoronary infusion of 100 nM peroxynitrite (PN group, n = 9). During the experiment, blood samples (BS) were taken at various time intervals from the coronary sinus for the assessment of plasma nitrate/nitrite (NOx) levels. Following reperfusion, tissue samples (TS) were taken from the ischaemic area for the determination of NO metabolites, superoxide and nitrotyrosine.
Tissue NOx was measured in samples taken from the ischaemic myocardium. Tissue blocks were homogenized in phosphate buffer (pH 7.4) containing Tris–HCl (50 mM), EDTA (0.1 mM), dithiothreitol (0.5 mmol·L−1), phenylmethylsulphonyl fluoride (0.1 mmol·L−1), soybean trypsin inhibitor (10 µg·mL−1) and leupeptin (10 µg·mL−1). The homogenates were centrifuged (20 000×g, for 15 min at 4°C) and the supernatants were collected. The total nitrate/nitrite was determined as outlined above. NOx levels are expressed in nmol·mg−1 protein.
Determination of NT formation
Free NT, as the marker of PN generation, was measured by enzyme-linked immunosorbent assay (Cayman Chemical, Ann Arbor, MI, USA). Myocardial tissue samples were homogenized and centrifuged at 15 000× g. The supernatants were collected and incubated overnight with anti-NT rabbit IgG (Cayman Chemical) and NT acetylcholinesterase tracer in pre-coated (mouse anti-rabbit IgG; Cayman Chemical) microplates, followed by development with Ellman's reagent. NT content was normalized to protein content of cardiac homogenate and expressed in ng·mg−1 protein.
Experimental protocol
This is illustrated in Figure 1. Three groups of dogs were used. Each animal was subjected to a 25 min LAD occlusion followed by rapid reperfusion. In a group of dogs, PC (n = 9) was induced by two 5 min periods of occlusion with a 5 min reperfusion interval between, 5 min prior to the prolonged occlusion/reperfusion insult. In another group of dogs, PN (n = 9), dissolved in pH 8.4 saline to a final concentration of 100 nM, was administered by the intracoronary route (infusion rate: 0.5 mL·min−1) for identical periods to the PC occlusions. This concentration of PN has been shown previously to reduce arrhythmias (Kiss et al., 2008). Control dogs (C; n = 14) were infused with pH 8.4 saline by the same route and time as PN. An additional four dogs served as sham-operated controls (not included into the protocol). These dogs were instrumented, infused locally with saline without being subjected to ischaemia. In the dogs that survived reperfusion, the hearts were stopped within 2 min by administration of an overdose of anaesthetic, and myocardial tissue samples were collected from the ischaemic ventricular wall. In dogs that suddenly fibrillated on reperfusion, these samples were taken when the fibrillation was observed.
The drug and molecular target nomenclature, used in this study, complies with proposals outlined in the British Journal of Pharmacology (Alexander et al., 2008).
Statistical evaluation
All data are expressed as means ± SEM and the differences between means were compared by anova for repeated measures and by the one-way anova as appropriate, using the Fisher post hoc test. VPBs and episodes of VT were compared using the Kruskal–Wallis test. The incidences of arrhythmias (such as VT and VF) and survival from the combined I/R insult were compared by the Fisher's exact test. Differences between groups were considered significant when *P < 0.05.
Results
Haemodynamic effects of saline, PN and subsequent coronary artery occlusion
These are summarized in Tables 1 and 2. Local intracoronary infusions of pH 8.4 saline and PN resulted in no significant haemodynamic effects (Table 1). Occlusion-induced changes were similar in all groups except that in preconditioned dogs and in dogs infused with PN, the decreases in arterial blood pressure, left ventricular systolic pressure and in negative dP/dt as well as the increase in LVEDP were less pronounced than in the controls (Table 2).
Table 1.
Haemodynamic changes following the infusions of saline (pH 8.4) and peroxynitrite
|
Saline (pH 8.4) (n = 14) |
PN (n = 9) |
|||
|---|---|---|---|---|
| Baseline | Max. change | Baseline | Max. change | |
| SABP (mmHg) | 123 ± 4 | 3 ± 3 | 123 ± 5 | −3 ± 2 |
| DABP (mmHg) | 82 ± 7 | 4 ± 1 | 84 ± 3 | −2 ± 2 |
| MABP (mmHg) | 96 ± 6 | 3 ± 3 | 97 ± 4 | −2 ± 2 |
| LVSP (mmHg) | 113 ± 11 | 2 ± 2 | 112 ± 4 | −3 ± 2 |
| LVEDP (mmHg) | 3.8 ± 0.6 | −0.4 ± 0.5 | 4.1 ± 0.6 | −0.4 ± 0.6 |
| +dP/dt (mmHg·s−1) | 2705 ± 166 | 45 ± 100 | 2900 ± 110 | −48 ± 96 |
| −dP/dt (mmHg·s−1) | 2057 ± 109 | 45 ± 28 | 1967 ± 106 | −64 ± 85 |
| HR (beats·min−1) | 151 ± 2 | 2 ± 2 | 145 ± 6 | −1 ± 2 |
Data are means ± SEM calculated from n = 9–14 experiments. Data, presented as changes, were determined 5 min after starting the infusion of saline and PN.
SABP, systolic arterial blood pressure; DABP, diastolic arterial blood pressure; MABP, mean arterial blood pressure; LVSP, left ventricular systolic pressure; LVEDP, left ventricular end-diastolic pressure; HR, heart rate.
Table 2.
Haemodynamic changes during a 25 min occlusion of the LAD
|
Control (n = 14) |
PC (n = 9) |
PN (n = 9) |
||||
|---|---|---|---|---|---|---|
| Baseline | Max. Change | Baseline | Max. Change | Baseline | Max. Change | |
| SABP (mmHg) | 121 ± 6 | −17 ± 2# | 121 ± 5 | −10 ± 1*# | 121 ± 6 | −11 ± 1*# |
| DABP (mmHg) | 81 ± 4 | −15 ± 2# | 84 ± 4 | −11 ± 1*# | 84 ± 4 | −11 ± 1*# |
| MABP (mmHg) | 95 ± 5 | −16 ± 2# | 101 ± 4 | −10 ± 2*# | 96 ± 5 | −10 ± 1*# |
| LVSP (mmHg) | 112 ± 5 | −17 ± 2# | 110 ± 4 | −11 ± 2*# | 110 ± 5 | −13 ± 2# |
| LVEDP (mmHg) | 4.0 ± 0.6 | 7.9 ± 0.4# | 4.0 ± 0.4 | 5.1 ± 0.9*# | 3.6 ± 0.4 | 5.8 ± 0.6*# |
| +dP/dt (mmHg·s−1) | 2638 ± 142 | −558 ± 92# | 2434 ± 178 | −394 ± 60# | 2753 ± 203 | −395 ± 54# |
| −dP/dt (mmHg·s−1) | 2034 ± 92 | −611 ± 76# | 1975 ± 146 | −282 ± 44*# | 1847 ± 46 | −374 ± 46*# |
| HR (beats·min−1) | 154 ± 4 | 3 ± 2 | 142 ± 5 | 1 ± 3 | 147 ± 8 | 1 ± 2 |
Data are means ± SEM calculated from n = 9–14 experiments.
P < 0.05 compared to control
P < 0.05 compared to baseline value.
SABP, systolic arterial blood pressure; DABP, diastolic arterial blood pressure; MABP, mean arterial blood pressure; LVSP, left ventricular systolic pressure; LVEDP, left ventricular end-diastolic pressure; HR, heart rate.
Ventricular arrhythmias during coronary artery occlusion and reperfusion
These are shown in Figures 2 and 3. Both PC and the administration of PN resulted in marked anti-arrhythmic effects. Thus, compared to the control group, in PC- and in PN-treated dogs, there were only a few ectopic beats during the 25 min occlusion (246 ± 75 cp. 24 ± 9 and 53 ± 19; P < 0.05). Similarly, VT occurred in 33% of the PC and PN groups with a mean episode of VT less than 1 (0.5 ± 0.3), compared to 92% incidence and 10.5 ± 3.5 episodes of VT in the controls (Figure 2). Furthermore, fatal VF occurred in 6 of the 14 control dogs (43%) during coronary artery occlusion and all the remaining animals fibrillated on reperfusion; thus, no dog in the control group survived the combined I/R insult (Figure 3). In contrast, no preconditioned and PN-treated dog fibrillated during the occlusion period. Furthermore, 67 and 55%, respectively, of these treated dogs survived reperfusion (Figure 3).
Figure 2.
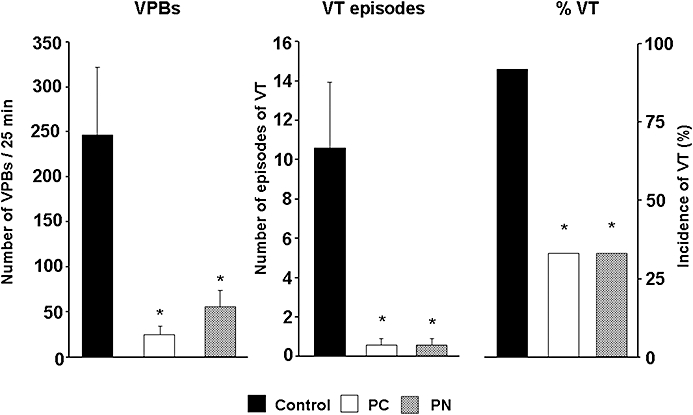
The total number of ventricular premature beats (VPBs), the incidence and the number of episodes of ventricular tachycardia (VT) during a 25 min occlusion of the LAD in control dogs and in dogs subjected to preconditioning (PC) and peroxynitrite (PN) infusion. Compared to the controls, both PC and the administration of PN markedly reduced the ischaemia-induced ventricular arrhythmias. Values are means ± SEM; *P < 0.05 compared to the controls.
Figure 3.
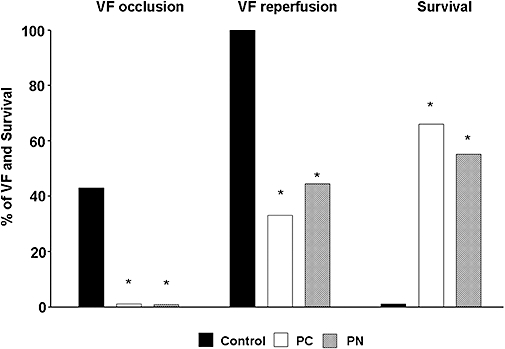
The incidence of ventricular fibrillation (VF) during a 25 min occlusion and reperfusion as well as survival from this combined ischaemia-reperfusion insult. In control dogs, a high incidence of VF occurred during occlusion and all the remaining dogs fibrillated on reperfusion. Thus, no control dog survived the combined occlusion and reperfusion insult. Both PC and PN attenuated these severe ventricular arrhythmias and increased the rate of survival. *P < 0.05 compared to the controls.
Changes in the severity of ischaemia following coronary artery occlusion
This was examined using both epicardial ST-segment mapping and the degree of inhomogeneity of electrical activation (Figure 4). In control dogs, the ST-segment was markedly elevated during the initial 5 min of the occlusion and this was maintained over the entire occlusion period. Both PC and the administration of PN significantly reduced this index of ischaemia severity (Figure 4A). Similarly, the degree of inhomogeneity of electrical activation was rapidly increased (from around 50 ms to around 200 ms) following coronary artery occlusion. These changes were significantly less pronounced in PC dogs and in dogs infused with PN (Figure 4B).
Figure 4.
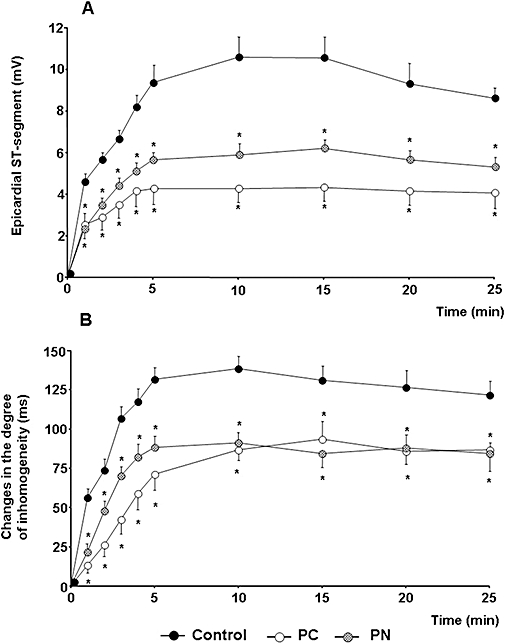
Changes in the epicardial ST segment (A) and in the degree of inhomogeneity of electrical activation (B) during a 25 min occlusion of the LAD. These indices of ischaemia severity were markedly reduced by PC and by the administration of PN. Values are means ± SEM; *P < 0.05 compared to the controls.
Changes in NOx levels during coronary artery occlusion and reperfusion
These are shown in Figure 5. The plasma level of NO metabolites in the coronary sinus blood was 20.4 ± 0.1 µmol·L−1, as determined from 32 dogs at baseline. In control dogs, occlusion of the LAD resulted in significant increases in NOx levels and these reached a maximum at around 7 min of the ischaemia. After this, the concentration of NOx declined, and by the end of the occlusion period, it was significantly lower than values measured at baseline (18.8 ± 0.2 cp. 20.3 ± 0.3 µmol·L−1, P < 0.05). Both the PC procedure and the infusion of PN significantly increased the concentration of NO metabolites; this was especially marked following the first episode of PC occlusion and during PN administration. In these dogs, the pre-occlusion concentrations of NOx were significantly higher than either the baseline values or the pre-occlusion NOx levels in the controls. After these PC and PN dogs had been subjected to prolonged occlusion, the NOx levels were markedly increased (the maximum occurred again at around 7 min of the ischaemia), and they remained significantly higher than in the controls throughout the entire occlusion period. The rapid reperfusion of the ischaemic myocardium resulted in almost similar increases in NOx levels in all groups, although the absolute concentration values were significantly different.
Figure 5.
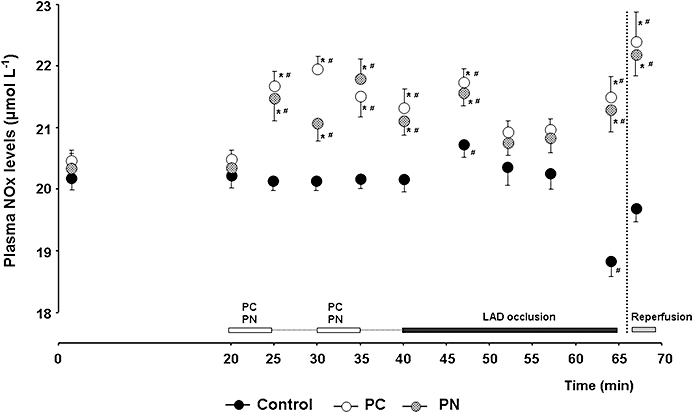
Changes in plasma nitrate/nitrite (NOx) levels in the blood of the coronary sinus. In control dogs, NOx levels were markedly reduced by the end of the coronary artery occlusion. Both PC and the infusion of PN enhanced the formation of NOx and their levels were significantly higher than those in the controls over the entire prolonged period of occlusion. Reperfusion of the myocardium resulted in almost similar increases in NOx in all groups. Values are means ± SEM; *P < 0.05 compared to the controls and #P < 0.05 compared to the initial baseline value.
The myocardial NOx levels determined in the ischaemic tissue samples within 2 min of reperfusion are illustrated in Figure 6A. Compared to the sham-operated dogs, the total NOx was significantly higher in the PC and PN groups than in the controls (4.4 ± 0.2 and 4.0 ± 0.1 cp. 3.4 ± 0.2 nmol·mg−1 protein; P < 0.05).
Figure 6.
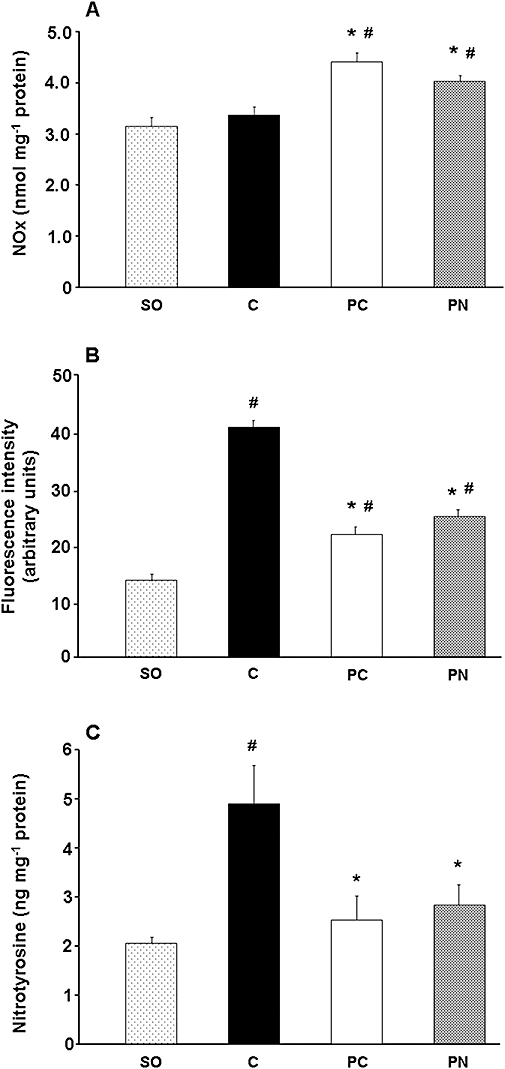
Tissue NOx (A), superoxide (B) and nitrotyrosine (C) production following a 25 min occlusion and reperfusion insult. Compared to the sham-operated (SO) controls, in dogs subjected to ischaemia and reperfusion, tissue NOx levels remained unchanged whereas the production of superoxide and nitrotyrosine was markedly increased. Both PC and PN resulted in marked reductions in superoxide, and consequently in nitrotyrosine production, whereas the tissue NOx levels in these dogs were significantly higher than in the controls. Values are means ± SEM; *P < 0.05 compared to the controls, #P < 0.05 compared to the sham-operated dogs.
The effect of PC and the administration of PN on superoxide production
Myocardial superoxide content was also measured during reperfusion in all dogs no matter whether they had fibrillated or were alive (Figure 6B). Compared to the sham-operated dogs, the myocardial superoxide production was significantly increased in dogs subjected to a 25 min occlusion and reperfusion (14.7 ± 1.5 cp. 40.1 ± 1.8 arbitrary units, P < 0.05). Both PC and the administration of PN significantly reduced this I/R-induced superoxide production (21.4 ± 1.4 and 24.5 ± 2.2 arbitrary units respectively).
The effect of PC and the administration of PN on NT formation
This was also determined in myocardial tissue samples taken from dogs just after (within 2 min) the release of the coronary artery occlusion (Figure 6C). Although in normal hearts (sham-operated dogs), a small amount of NT was detected (2.0 ± 0.14 ng·mg−1 protein), this was markedly increased in the control dogs subjected to I/R (4.9 ± 0.8 ng·mg−1 protein). However, this increase was significantly reduced in dogs infused with PN (2.8 ± 0.4 ng·mg−1 protein) or subjected to PC (2.5 ± 0.5 ng·mg−1 protein).
Discussion and conclusions
There is ongoing debate as to whether the generation of NO increases or decreases during myocardial ischaemia. In in vitro studies, it has been demonstrated, using electron paramagnetic resonance (EPR) for NO spin trapping, that NO production is increased in hearts subjected to 30 min of global ischaemia (Zweier et al., 1995a; Csonka et al., 1999). This enhanced generation of NO was attributed to the formation of non-enzymatic NO during ischaemia when, in the absence of oxygen and in the presence of low pH, nitrite is preferably reduced to form NO (Zweier et al., 1995b). In contrast, results from in vivo studies, using microdialysis and electrochemical techniques to measure NO (EPR has not yet feasible for NO detection in large animals), have shown that the release of NO or NO metabolites rapidly declines during myocardial ischaemia (Mori et al., 1998; Stevens et al., 2002; Prasan et al., 2007). Our present results, showing that in anaesthetized dogs NOx levels were markedly reduced by the end of the prolonged (25 min) coronary artery occlusion, confirm these findings. However, in contrast to these previous data (Stevens et al., 2002; Prasan et al., 2007), we observed that a transient increase in NO metabolites occurred soon after the commencement of the coronary artery occlusion (at around 7 min of the occlusion). We do not know whether this increase in NOx levels results from the activation of NOS, since this was not measured in the present study. There is some evidence that NOS activity can rapidly (within 5 min) be stimulated by ischaemia (Depréet al., 1997), but whether this is maintained over a longer ischaemic period is still a matter of debate (Depréet al., 1997; Wang et al., 1997; Prasan et al., 2007). Nevertheless, the finding, in our experiments, that NOx levels during coronary artery occlusion were, after an initial increase, markedly reduced (Figure 5) supports the hypothesis of a decrease in the overall myocardial NOS activity and NO production (Wang et al., 1997). Another explanation for this transient increase in NO production might be that we measured plasma NOx levels in blood samples taken from the coronary sinus. Since this collects blood from both the ischaemic and non-ischaemic areas, an elevation in NOx may derive from a compensatory increase in NO production occurring within the non-ischaemic myocardium. Such an elevation in NOx levels within the non-ischaemic area has been found by Stevens et al. (2002) in anaesthetized pigs. In their study, when the LAD was occluded either for 15 or 60 min, NOx levels rapidly declined within the occluded area but increased in samples taken from the adjacent circumflex coronary bed. Furthermore, in rabbit isolated hearts, Prasan et al. (2007) found that the concentration of NO metabolites in the coronary sinus was significantly increased during low-flow ischaemia. However, when they calculated the net release of NO within the ischaemic area, this was found to be significantly reduced. Hence, it is possible that this transient increase in the coronary sinus NOx levels may represent an important compensatory mechanism, that is, the enhanced NO production within the normal region attempts to compensate for the loss of NO production within the severely injured myocardium.
In contrast to the controls in which NOx levels were markedly reduced during ischaemia, the production of NO was substantially increased in dogs that were subjected to PC or infused with PN prior to the occlusion. Both interventions enhanced NOx levels, albeit differently. PC increased NOx levels particularly during and after the first episode of the PC occlusion, which might indicate a rapid activation of NOS by ischaemia (Depréet al., 1997; Muscari et al., 2004). In contrast, PN elevated NOx levels only during the infusion periods, suggesting that PN possibly acts as an NO donor (Kiss et al., 2008). Nevertheless, in both groups, the pre-occlusion levels of NO metabolites were significantly higher than in the controls. Occlusion of the LAD in these dogs resulted in further increases in NOx and these levels were maintained over the entire 25 min occlusion period. Thus, in contrast to previous findings (Zweier et al., 1995a; Csonka et al., 1999), our present results suggest that in anaesthetized dogs, the preservation of NO synthesis, and subsequently, the maintenance of NO production during myocardial ischaemia is cardioprotective. This accords with previous experience in rat isolated hearts, that is, the preservation of structures responsible for NO release is important for the cardioprotective and anti-arrhythmic effect of PC (Parikh and Singh, 1999). NO, as we have proposed previously, attenuates the ischaemic burden (indicated by less marked changes in epicardial ST-segment and in the degree of inhomogeneity of electrical activation) and reduces the severity of arrhythmias (Végh et al., 1992b; 1996; Végh and Parratt, 1996), possibly by acting directly on gap junctions (Gönczi et al., 2009).
It is interesting to note that there was a slight decrease in NOx levels during and after the second period of the PC occlusion. This presumably indicates that some of the NO has formed into PN with superoxide, which is, in this species, generated only after the second period of the PC occlusion (Hajnal et al., 2005). This accords with a more recent finding, that a significant increase in NT formation occurs just after two brief PC occlusions (Novalija et al., 2003; Kiss et al., 2007) or PN infusions (Kiss et al., 2008). These results add further support to the hypothesis that PN may play a trigger role in the anti-arrhythmic effect of ischaemic PC (Altug et al., 2000; 2001;). However, our results clearly demonstrated that the NT production, resulting from a prolonged period of I/R, was markedly suppressed in dogs that had been subjected to PC or infused with PN previously (Novalija et al., 2003; Kiss et al., 2007). Furthermore, we have now provided evidence that this reduction in NT formation is primarily due to an attenuation of superoxide production rather than to a decrease in the harmful accumulation of NO, as has previously been suggested (Csonka et al., 1999). The parallel measurement of tissue NOx, superoxide and NT production, which is an advantage of this study, clearly indicated that, in the presence of increased NOx levels, both PC and PN suppressed the generation of superoxide and, subsequently, the formation of endogenous PN (Figure 6). This finding, and also the fact that the infusion of nitrites just prior to reperfusion protected against reperfusion injury (reviewed by Raat et al., 2009), supports the involvement of an NO-mediated mechanism in the generation of superoxide induced by I/R (Iwase et al., 2007; Burwell and Brookes, 2008; Korge et al., 2008). There are, of course, a number of ways by which NO may regulate superoxide production. For example, NO inhibits xanthine oxidase and xanthine dehydrogenase, the major sources of superoxide production, by converting them into a desulpho-type inactive form (Ichimori et al., 1999), NADPH oxidase (Clancy et al., 1992; Fujii et al., 1997). There is also increasing evidence that both NO and PN, by acting directly on the electron transport chain or the uncoupling proteins, reduce mitochondrial superoxide production (Burwell and Brookes, 2008). All these mechanisms may account for the protective effect of NO in vivo.
In summary, our results clearly demonstrate that under in vivo conditions, myocardial ischaemia suppresses the generation of NO. This decrease in NO formation is prevented by PC and by the prior administration of PN, suggesting that the preservation of NO production during myocardial ischaemia is an integral part of the anti-arrhythmic protection. Furthermore, we propose that this enhanced or, at least, maintained NO availability following PC and PN administration plays an important role in the suppression of superoxide production during I/R. If true, this mechanism would explain the marked reduction in endogenous PN production obtained in preconditioned and in PN-treated dogs with enhanced NO production. We think that our present results add further support to the concept that NO plays an important trigger and mediator role in the anti-arrhythmic protection (Végh et al., 1992b; 1996; Végh and Parratt, 1996).
Acknowledgments
This work was supported by the Hungarian Scientific Research Foundation (OTKA; Project number K75281 and NI61092) and by BIO-37-49. We are grateful to Professor Péter Ferdinándy and his colleagues (Department of Biochemistry) for providing PN and helping us with the measurements of the NT formation. We thank Marian Lipták and Csilla Mester (Institute of Surgical Research) for their help in measuring nitrate/nitrite levels. We also acknowledge the excellent technical assistance of Erika Bakó and Irene Biczók.
Glossary
Abbreviations:
- cp
compared to
- DABP
diastolic arterial blood pressure
- DHE
dihydroethidium
- HR
heart rate
- I/R
ischaemia/reperfusion
- LAD
left anterior descending coronary artery
- LVEDP
left ventricular end-diastolic pressure
- LVSP
left ventricular systolic pressure
- MABP
mean arterial blood pressure
- NADPH
nicotinamide adenine dinucleotide phosphate
- NO
nitric oxide
- NOS
nitric oxide synthase
- NT
nitrotyrosine
- PC
preconditioning
- PN
peroxynitrite
- SABP
systolic arterial blood pressure
- VF
ventricular fibrillation
- VPBs
ventricular premature beats
- VT
ventricular tachycardia
Conflict of interest
None declared.
References
- Alexander SPH, Matie A, Peters JA. Guide to receptors and channels (GARC), 3rd edition. Br J Pharmacol. 2008;153(Suppl. 2):S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altug S, Demiryürek T, Kane KA, Kanzik J. Evidence for the involvement of peroxynitrite in ischaemic preconditioning in rat isolated hearts. Br J Pharmacol. 2000;130:125–131. doi: 10.1038/sj.bjp.0703280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altug S, Demiryürek T, Ak D, Tungel M, Kanzik I. Contribution of peroxynitrite to the beneficial effects of preconditioning on ischaemia-induced arrhythmias in rat isolated hearts. Eur J Pharmacol. 2001;415:239–246. doi: 10.1016/s0014-2999(01)00843-3. [DOI] [PubMed] [Google Scholar]
- Aon MA, Cortassa S, O'Rourke B. Mitochondrial oscillations in physiology and pathophysiology. Adv Exp Med Biol. 2008;641:98–117. doi: 10.1007/978-0-387-09794-7_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckman JS, Chen J, Ischiropoulos H, Crow JP. Oxidative chemistry of peroxynitrite. Methods Enzymol. 1994;233:229–240. doi: 10.1016/s0076-6879(94)33026-3. [DOI] [PubMed] [Google Scholar]
- Berges A, VanNassauw L, Bosmans J, Timmermans J-P, Vrints C. Role of nitric oxide and oxidative stress in ischaemic myocardial injury and preconditioning. Acta Cardiol. 2003;58:119–132. doi: 10.2143/AC.58.2.2005264. [DOI] [PubMed] [Google Scholar]
- Burwell LS, Brookes PS. Mitochondria as a target for the cardioprotective effects of nitric oxide in ischemia-reperfusion injury. Antioxid Redox Signal. 2008;10:579–599. doi: 10.1089/ars.2007.1845. [DOI] [PubMed] [Google Scholar]
- Clancy RM, Leszczynska-Piziak J, Abramson SB. Nitric oxide, an endothelial cell relaxing factor, inhibits neutrophil superoxide anion production via a direct action on the NADPH oxidase. J Clin Invest. 1992;90:1116–1121. doi: 10.1172/JCI115929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csonka C, Szilvássy Z, Fülöp F, Páli T, Blasig IE, Tosaki A, et al. Classic preconditioning decreases the harmful accumulation of nitric oxide during ischemia and reperfusion in rat hearts. Circulation. 1999;100:2260–2266. doi: 10.1161/01.cir.100.22.2260. [DOI] [PubMed] [Google Scholar]
- Depré C, Fiérain L, Hue L. Activation of nitric oxide synthase by ischaemia in the perfused heart. Cardiovasc Res. 1997;33:82–87. doi: 10.1016/s0008-6363(96)00176-9. [DOI] [PubMed] [Google Scholar]
- Engelman DT, Watanabe M, Maulik N, Cordis GA, Engelman RM, Rousou JA, et al. L-arginine reduces endothelial inflammation and myocardial stunning during ischemia/reperfusion. Ann Thorac Surg. 1995;60:1275–1281. doi: 10.1016/0003-4975(95)00614-Q. [DOI] [PubMed] [Google Scholar]
- Falk JA, Aune SE, Kutala VK, Kuppusamy P, Angelos MG. Inhibition of peroxynitrite precursors, NO and O2, at the onset of reperfusion improves myocardial recovery. Resuscitation. 2007;74:508–515. doi: 10.1016/j.resuscitation.2007.01.026. [DOI] [PubMed] [Google Scholar]
- Ferdinándy P, Schulz R. Peroxynitrite. Toxic or protective in the heart? Circ Res. 2001;88:e12–e13. doi: 10.1161/01.res.88.2.e12. [DOI] [PubMed] [Google Scholar]
- Ferdinándy P, Schulz R. Nitric oxide, superoxide and peroxynitrite in myocardial ischemia and reperfusion injury. Br J Pharmacol. 2003;138:532–543. doi: 10.1038/sj.bjp.0705080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii H, Ichimori K, Hoshiai K, Nakazawa H. Nitric oxide inactivates NADPH oxidase in pig neutrophils by inhibiting its assembling process. J Biol Chem. 1997;272:32773–32778. doi: 10.1074/jbc.272.52.32773. [DOI] [PubMed] [Google Scholar]
- Gönczi M, Papp R, Kovács M, Seprényi G, Végh A. Modulation of gap junctions by nitric oxide contributes to the anti-arrhythmic effect of sodium nitroprusside. Br J Pharmacol. 2009;156:786–793. doi: 10.1111/j.1476-5381.2008.00089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu W, Weihrauch D, Tanaka K, Tessmer JP, Pagel PS, Kersten JR, et al. Reactive oxygen species are critical mediators of coronary collateral development in a canine model. Am J Physiol. 2003;285:H1582–H1589. doi: 10.1152/ajpheart.00318.2003. [DOI] [PubMed] [Google Scholar]
- György K, Végh Á, Rastegar MA, Papp JG, Parratt JR. Isosorbide-2-mononitrate reduces the consequences of myocardial ischaemia, including arrhythmia severity: implications for preconditioning. J Cardiovasc Drugs Ther. 2000;14:481–488. doi: 10.1023/a:1007832921391. [DOI] [PubMed] [Google Scholar]
- Hajnal Á, Nagy L, Parratt JR, Papp JG, Végh Á. Failure of N-2-mercaptopropionylglycine, a scavanger of reactive oxygen species, to modify the antiarrhythmic effect of ischaemic preconditioning in anaesthetised dogs. J Cardiovasc Drugs Ther. 2005;18:449–459. doi: 10.1007/s10557-004-6222-2. [DOI] [PubMed] [Google Scholar]
- Ichimori K, Fukahori M, Nakazawa H. Inhibition of xanthine oxidase and xanthine dehydrogenase by nitric oxide. J Biol Chem. 1999;274:7763–7768. doi: 10.1074/jbc.274.12.7763. [DOI] [PubMed] [Google Scholar]
- Iwase H, Robin E, Guzy RD, Mungai PT, Vanden Hoek TL, Chandel NS, et al. Nitric oxide during ischemia attenuates oxidant stress and cell death during ischemia and reperfusion in cardiomyocytes. Free Radic Biol Med. 2007;43:590–599. doi: 10.1016/j.freeradbiomed.2007.05.017. [DOI] [PubMed] [Google Scholar]
- Jones SP, Bolli R. The ubiquitous role of nitric oxide in cardioprotection. J Mol Cell Cardiol. 2006;40:16–23. doi: 10.1016/j.yjmcc.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Kiss A, Juhász L, Huliák I, Ferdinándy P, Végh Á. Peroxynitrite induces an antiarrhythmic effect in anaesthetised dogs. J Mol Cell Cardiol. 2007;42(Suppl. 1):S9. abstract. [Google Scholar]
- Kiss A, Juhász L, Huliák I, Végh Á. Peroxynitrite decreases arrhythmias induced by ischaemia reperfusion in anaesthetised dogs, without involving mitochondrial KATP channels. Br J Pharmacol. 2008;155:1015–1024. doi: 10.1038/bjp.2008.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korge P, Ping P, Weiss JN. Reactive oxygen species production in energized cardiac mitochondria during hypoxia/reoxygenation: modulation by nitric oxide. Circ Res. 2008;103:873–880. doi: 10.1161/CIRCRESAHA.108.180869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalu MM, Wang W, Schulz R. Peroxynitrite in myocardial ischemia-reperfusion injury. Heart Fail Rev. 2002;7:359–369. doi: 10.1023/a:1020766502316. [DOI] [PubMed] [Google Scholar]
- Lefer AM. Attenuation of myocardial ischemia-reperfusion injury with nitric oxide replacement therapy. Ann Thorac Surg. 1995;60:847–851. doi: 10.1016/0003-4975(95)00423-I. [DOI] [PubMed] [Google Scholar]
- Liu P, Hock CE, Nagele R, Wong PY-K. Formation of nitric oxide, superoxide, and peroxynitrite in myocardial ischemia-reperfusion injury in rats. Am J Physiol Heart Circ Physiol. 1997;272:H2327–H2336. doi: 10.1152/ajpheart.1997.272.5.H2327. [DOI] [PubMed] [Google Scholar]
- Miles AM, Bohle DS, Glassbrenner A, Hansert B, Wink DA, Grisham MB. Modulation of superoxide-dependent oxidation and hydroxylation reactions by nitric oxide. J Biol Chem. 1996;271:40–47. doi: 10.1074/jbc.271.1.40. [DOI] [PubMed] [Google Scholar]
- Mori E, Haramaki N, Ikeda H, Imaizumi T. Intra-coronary administration of L-arginine aggravates myocardial stunning through production of peroxynitrite in dogs. Cardiovasc Res. 1998;40:113–123. doi: 10.1016/s0008-6363(98)00146-1. [DOI] [PubMed] [Google Scholar]
- Moshage H, Kok B, Huizenga JR, Jansen PL. Nitrite and nitrate determinations in plasma: a critical evaluation. Clin Chem. 1995;41:892–896. [PubMed] [Google Scholar]
- Muscari C, Bonafe' F, Gamberini C, Giordano E, Tantini B, Fattori M, et al. Early preconditioning prevents the loss of endothelial nitric oxide synthase and enhances its activity in the ischemic/reperfused rat heart. Life Sci. 2004;74:127–137. doi: 10.1016/j.lfs.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Nossuli TO, Hayward R, Scalia R, Lefer AM. Peroxynitrite reduces myocardial infarct size and preserves coronary endothelium after ischemia and reperfusion in cats. Circulation. 1997;96:2317–2324. doi: 10.1161/01.cir.96.7.2317. [DOI] [PubMed] [Google Scholar]
- Novalija E, Hogg N, Kevin LG, Camara KS, Stowe DF. Ischemic preconditioning: triggering role of nitric oxide-derived oxidants in isolated hearts. J Cardiovasc Pharmacol. 2003;42:593–600. doi: 10.1097/00005344-200311000-00003. [DOI] [PubMed] [Google Scholar]
- Parikh V, Singh M. Possible role of cardiac mast cell degranulation and preservation of nitric oxide release in isolated rat heart subjected to ischaemic preconditioning. Mol Cell Biochem. 1999;199:1–6. doi: 10.1023/a:1006930011622. [DOI] [PubMed] [Google Scholar]
- Parratt JR. Endogenous myocardial protective substances. Cardiovasc Res. 1993;27:693–702. doi: 10.1093/cvr/27.5.693. [DOI] [PubMed] [Google Scholar]
- Prasan AM, McCarron HC, Zhang Y, Jeremy RW. Myocardial release of nitric oxide during ischaemia and reperfusion: effects of L-arginine and hypercholesterolaemia. Heart Lung Circ. 2007;16:274–281. doi: 10.1016/j.hlc.2007.02.092. [DOI] [PubMed] [Google Scholar]
- Pryor W, Squadrito GL. The chemistry of peroxynitrite: from the reaction of nitric oxide and superoxide. Am J Physiol. 1995;268:699–722. doi: 10.1152/ajplung.1995.268.5.L699. [DOI] [PubMed] [Google Scholar]
- Raat NJH, Shiva S, Gladwin MT. Effects of nitrite on modulating ROS generation following ischemia and reperfusion. Adv Drug Deliv Rev. 2009;61:339–350. doi: 10.1016/j.addr.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Rickover O, Zinman T, Kaplan D, Shainberg A. Exogenous nitric oxide triggers classic ischemic preconditioning by preventing intracellular Ca2+ overload in cardiomyocytes. Cell Calcium. 2008;43:324–333. doi: 10.1016/j.ceca.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Stevens RM, Jahania MS, Stivers JE, Mentzer RM, Jr, Lasley RD. Effects of in vivo myocardial ischemia and reperfusion on interstitial nitric oxide metabolites. Ann Thorac Surg. 2002;4:1261–1266. doi: 10.1016/s0003-4975(02)03372-6. [DOI] [PubMed] [Google Scholar]
- Uppu RM, Nossaman BD, Greco AJ, Fohin A, Murthy SN, Fonseca VA, et al. Cardiovascular effects of peroxynitrite. Clin Exp Pharmacol Physiol. 2007;34:933–937. doi: 10.1111/j.1440-1681.2007.04641.x. [DOI] [PubMed] [Google Scholar]
- Vanden Hoek TL, Li C, Shao Z, Schumacker PT, Becker LB. Significant levels of oxidants are generated by isolated cardiomyocytes during ischemia prior to reperfusion. J Mol Cell Cardiol. 1997;29:2571–2583. doi: 10.1006/jmcc.1997.0497. [DOI] [PubMed] [Google Scholar]
- Végh Á, Parratt JR. Ischaemic preconditioning markedly reduces the severity of ischaemia and reperfusion-induced arrhythmias; role of endogenous myocardial protective substances. In: Wainwright CL, Parratt JR, editors. Myocardial Preconditioning. Berlin: Springer; 1996. pp. 35–60. [Google Scholar]
- Végh Á, Komori S, Szekeres L, Parratt JR. Antiarrhythmic effects of preconditioning in anaesthetised dogs and rats. Cardiovasc Res. 1992a;26:487–495. doi: 10.1093/cvr/26.5.487. [DOI] [PubMed] [Google Scholar]
- Végh Á, Szekeres L, Parratt JR. Preconditioning of the ischaemic myocardium; involvement of the L-arginine – nitric oxide pathway. Br J Pharmacol. 1992b;107:648–652. doi: 10.1111/j.1476-5381.1992.tb14501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Végh Á, György K, Papp JG, Sakai K, Parratt JR. Nicorandil suppressed ventricular arrhythmias in a canine model of myocardial ischaemia. Eur J Pharmacol. 1996;305:163–168. doi: 10.1016/0014-2999(96)00166-5. [DOI] [PubMed] [Google Scholar]
- Walker MJA, Curtis MJ, Hearse DJ, Campbell RWF, Janse MJ, Yellon DM, et al. The Lambeth Conventions: guidelines for the study of arrhythmias in ischaemia, infarction, and reperfusion. Cardiovasc Res. 1998;22:447–455. doi: 10.1093/cvr/22.7.447. [DOI] [PubMed] [Google Scholar]
- Wang Q, Morcos E, Wiklund P, Pernow J. L-arginine enhances functional recovery and Ca2+-dependent nitric oxide synthase activity after ischemia and reperfusion in the rat heart. J Cardiovasc Pharmacol. 1997;29:291–296. doi: 10.1097/00005344-199702000-00020. [DOI] [PubMed] [Google Scholar]
- Xie L-H, Chen F, Karagueuzian HS, Weiss JN. Oxidative stress-induced after depolarisations and calmodulin kinase II signaling. Circ Res. 2009;104:79–86. doi: 10.1161/CIRCRESAHA.108.183475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweier JL, Wang P, Kuppusamy P. Direct measurement of nitric oxide generation in the ischemic heart using electric paramagnetic resonance spectroscopy. J Biol Chem. 1995a;270:304–307. doi: 10.1074/jbc.270.1.304. [DOI] [PubMed] [Google Scholar]
- Zweier JL, Wang P, Samouilov A, Kuppusamy P. Enzyme-independent formation of nitric oxide in biological tissues. Nat Med. 1995b;1:804–809. doi: 10.1038/nm0895-804. [DOI] [PubMed] [Google Scholar]


