Abstract
Context and Objective: Adult-onset idiopathic hypogonadotropic hypogonadism (AHH) is a rare disorder characterized by an isolated failure of gonadotropin secretion occurring after an otherwise normal sexual maturation in men. This study aims to examine the etiology and long-term natural history of this disorder.
Design and Setting: Long-term follow up, including detailed clinical, biochemical, and genetic examinations, were performed and compared with those at diagnosis.
Patients: Patients included 10 men with AHH [serum testosterone (T) <125 ng/dl].
Interventions: Overnight neuroendocrine studies, semen fluid analyses, and genetic screening were performed (KAL1, FGFR1, PROK2, PROKR2, NELF, TAC3, TACR3, and GNRH1) over a decade of longitudinal follow up.
Results: Follow-up evaluations were conducted 10.6 ± 5.9 yr after initial studies and revealed that the clinical characteristics and seminal fluid analyses of AHH men (body mass index, 28.8 ± 4.1 vs. 27.0 ± 4.3 kg/m2; testicular volume, 18 ± 6 vs. 19 ± 6 ml) do not change over a decade with no spontaneous reversals. Several men exhibited some variability in their endogenous GnRH-induced LH secretory patterns, including emergence of endogenous pulsatility in three individuals. However, all remained hypogonadal (T ≤130 ng/dl). A single heterozygous DNA sequence change in PROKR2 (V317L) was identified, although this rare sequence variant did not prove to be functionally abnormal in vitro. Seven days of pulsatile GnRH therapy in this subject nearly normalized his serum T, supporting that the site of the defect is hypothalamic and not pituitary.
Conclusions: 1) AHH in men appears to be a long-lasting condition. 2) Although minor changes in the abnormal pattern of endogenous GnRH-induced LH secretion occurred in some AHH patients, all remained frankly hypogonadal.
Adult-onset, idiopathic GnRH deficiency in men is not reversible during a decade of follow-up, unlike its female counterpart, i.e. hypothalamic amenorrhea.
Isolated GnRH deficiency underlies a broad spectrum of human reproductive disorders in males and females. The spectrum extends from the more severe phenotype, i.e. congenital forms of idiopathic hypogonadotropic hypogonadism (IHH) with and without anosmia (1,2), to less complete forms of GnRH dysfunction, including constitutional delay of puberty (3), functional hypothalamic amenorrhea (HA) (4), and adult-onset idiopathic hypogonadotropic hypogonadism (AHH) in men (5). Although this family of GnRH-related disorders typically presents to clinicians as distinct clinical entities, considerable clinical and phenotypic variability and overlap can occur in each over time. For example, men and women with IHH and anosmia (Kallmann syndrome) are typically characterized by absent or incomplete puberty, infertility, otherwise normal pituitary hormone testing, normal cranial magnetic resonance images, and responsiveness to physiologic regimens of exogenous GnRH, demonstrating a hypothalamic defect of isolated GnRH secretion/action (1,2,4). However, we recently reported that approximately 10% of IHH men can experience a spontaneous recovery of GnRH-induced LH pulsatility and maintain adult serum testosterone (T) levels after therapy (6). Women with functional HA have typically undergone a normal GnRH-induced activation of their hypothalamic-pituitary-gonadal (HPG) axis culminating in normal pubertal development but subsequently experience temporary suppression of normal GnRH secretion during periods of significant metabolic and/or psychosocial stress (6,7) and typically recover after this perturbation has been eliminated (8,9).
In contrast to congenital IHH, AHH is a rare form of GnRH deficiency presenting in otherwise healthy adult men after completion of normal pubertal development and often proven fertility (5). Acquired hypogonadotropic hypogonadism can result from anatomic etiologies, including infiltrative processes, e.g. granulomatous disease (10,11,12), lymphocytic hypophysitis (13), space-occupying lesions such as pituitary adenomas, craniopharyngiomas, and other central nervous system tumors (14,15,16), as well as genetic disorders such as hemochromatosis (17). However, in AHH, most of these causes are ruled out through imaging studies and blood chemistries at diagnosis. Furthermore, there has been little evidence for functional causes of AHH such as psychosocial factors (e.g. severe stress) and/or metabolic disruptions (e.g. extreme exercise and/or malnutrition) as occurs in women with HA, and no long-term studies have addressed its reversibility. Moreover, we have documented a robust gonadotropin response to physiologic regimens of exogenous GnRH administration in the 90% of AHH cases, confirming that the level of defect is the function of the GnRH neuron and not at the pituitary (5).
Thus, men with AHH can have biochemical and clinical features similar to those observed in IHH and Kallmann syndrome save for their age of onset and completeness of previous pubertal development (i.e. TV) before the onset of this condition. The completion of a normal sexual maturation in AHH indicates a developmentally appropriate period of normal GnRH secretion during puberty and points to a potentially different pathophysiology. Additionally, little is known about the natural history of this disorder such as whether this defect is permanent or whether AHH men undergo spontaneous recovery of their HPG axis as we reported in the congenital forms of GnRH deficiency (6) and as frequently occurs in women with HA (9).
Since our initial report of AHH, considerable evidence has emerged supporting genetic etiologies to this condition. A number of genetic loci have been implicated in congenital GnRH deficiency, with approximately 40% of IHH and Kallmann syndrome cases now exhibiting one or more genetic mutations (18,19,20,21,22,23,24,25,26,27,28). The majority of these genes are expressed in the adult hypothalamus, suggesting a potential role in GnRH neuronal maturation, migration, function, and survival as might be suggested by the adult-onset nature of AHH. Recently, rare DNA variants were identified in two AHH men [GnRH receptor (GNRHR) Q106R and fibroblast growth factor 8 (FGF8) T229M] (20,26). Although biallelic GNRHR mutations can underlie some cases of isolated GnRH deficiency (IHH) (24), the reported heterozygous loss-of-function mutation in GNRHR in AHH is of unknown significance. Conversely, a heterozygous loss-of-function mutation in FGF8 alone has been demonstrated to underlie IHH (20). Thus, the T229M heterozygous loss-of-function mutation in FGF8 appears to create a more compelling story for rare gene variants underlying this disorder. These reports led us to search for other mutations in genetic loci causing GnRH deficiency in our AHH cohort.
Thus, this study aims to define the natural history of AHH through detailed prospective clinical and biochemical analyses, to document changes in endogenous GnRH secretion signaling spontaneous recovery, and to further the search for additional genetic defects underlying AHH.
Materials and Methods
This research was approved by the ethics committee of the Massachusetts General Hospital, and all subjects provided written, informed consent before the initiation of any study procedures.
AHH cohort
Ten Caucasian men with AHH (mean age, 38.6 ± 9.4 yr at diagnosis) were available for this prospective long-term follow-up study. Of the 10 men appearing in the initial report (5), we were able to locate seven who agreed to return for follow up, and three additional men meeting the criteria were included in the study (Table 1). Diagnosis of AHH was based on the following criteria: 1) complete pubertal development by age 18 yr; 2) clinical symptoms of hypogonadism, including sexual dysfunction and/or idiopathic infertility; 3) frankly hypogonadal serum T levels (<130 ng/dl) in the presence of low or normal gonadotropins on multiple assessments; 4) otherwise normal anterior pituitary function; 5) normal ferritin concentrations; 6) normal radiographic imaging of the sella turcica; and 7) absence of predisposing anatomical or functional factors for hypogonadotropic hypogonadism (5).
Table 1.
Clinical characteristics of AHH men at baseline and follow up
| Patient number | Baseline
|
Follow up
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age at baseline (yr) | BMI (kg/m2) | Presenting symptoms | Pubertal history | Previous paternity | Comorbid conditions | Age at follow up (yr) | BMI (kg/m2) | Comorbid conditions | Interim medications (yr) | |
| 1 | 48 | 22.4 | Impotence | Delayed | Yes | 52 | 22.9 | TDT (4) | ||
| 2 | 32 | 24.4 | Impotence and infertility | Normal | No | 36 | 23.1 | GnRH (4) | ||
| 3a | 40 | 35.2 | Infertility | Normal | No | Hypertension and obesity | 48 | 35.4 | Hypertension and obesity | GnRH (8) |
| 4a | 48 | 22.6 | Infertility and loss of libido | Delayed | No | 56 | 22.5 | hCG (8) | ||
| 5a | 57 | 27.9 | Loss of libido | Normal | Yes | Depression | 66 | 29.2 | Depression | GnRH (0.5), hCG (1.5), TE (3), TDT (4) |
| 6 | 37 | 24.1 | Loss of libido and impotence | Normal | Yes | 46 | 26.0 | hCG (2), TE (1) | ||
| 7a | 37 | 24.3 | Loss of libido | Normal | Yes | Previous history of depression | 48 | 25.7 | Migraines | hCG (0.5), TDT (7.5) |
| 8a | 41 | 28.4 | Loss of libido | Normal | Yes | 53 | 32.5 | Type 2 diabetes | GnRH (12) | |
| 9a | 28 | 21.8 | Infertility | Delayed | Yes | 47 | 25.2 | GnRH (2), hCG (2), TE (4), TDT (11) | ||
| 10a | 29 | 28.4 | Infertility | Normal | No | 51 | 27.9 | GnRH (2), hCG (9), TDT (11) | ||
Baseline evaluation was reported previously (5).
TE, Testosterone enanthate injections; TDT, transdermal testosterone (patch or gel); hCG, human chorionic gonadotropin; GnRH, pulsatile GnRH.
Clinical and neuroendocrine evaluation
All subjects underwent a repeat detailed evaluation at least 4 yr after diagnosis, including repeat thyroid function, liver function, prolactin, and iron binding studies and magnetic resonance imaging (MRI) when possible to reassess for potential causes of acquired hypogonadism. Reproductive hormonal therapy was discontinued in all subjects for an appropriate washout period (pulsatile GnRH ≥2 wk; transdermal T ≥4 wk; intramuscular T or gonadotropin injections ≥8 wk) before follow up.
History and physical examination
A complete physical examination was performed on all subjects, including calculation of body mass index (BMI) and measurement of TV using a Prader orchidometer. Quantitative smell testing was performed as described previously (29), and a detailed interval history was taken, including symptoms of hypogonadism, review of medications, use of illicit drugs, history of pubertal development, and family history of hypogonadism.
Neuroendocrine studies and biochemical profiling
Subjects underwent a detailed neuroendocrine evaluation, including an overnight frequent blood sampling study (every 10 min for 12 h) to determine endogenous LH pulse pattern. Serum FSH, inhibin B, T, and SHBG levels were measured from the study pool. In men able to produce an ejaculate, a semen sample was obtained and analyzed using standard World Health Organization criteria (30). Because none of the subjects exhibited any evidence of an anatomic defect on MRI at the time of diagnosis and insurance carriers denied requests for repeated MRIs, reevaluations were only performed on a subset of three men (all of which were negative).
Hormone assays
All assay methods used in this study have been described previously (31,32). Individual samples from the frequent sampling studies were assayed for LH, whereas all other assays were performed on pooled samples constituted from equal aliquots of the 10-min samples. Serum LH and FSH concentrations were determined by microparticle enzyme immunoassay using the automated Abbott AxSYM system (Abbott Laboratories, Chicago, IL). The Second International Reference Preparation was used as the reference standard. The assay sensitivity for both LH and FSH was 1.6 IU/liter based on a HMG standard, which is equivalent to 0.34 mIU/ml LH or 0.66 mIU/ml FSH based on pituitary standards (80/552 and 92/510, respectively). We elected to use the HMG standard because we have published using this reference over the past decades and chose to keep it for consistency. The intraassay coefficient of variation (CV) values for LH and FSH were less than 7% and less than 6%, respectively, with interassay CVs for both hormones of less than 7.4%. Serum T concentrations were measured using the DPC Coat-A-Count RIA kit (Diagnostics Products Corporation, Los Angeles, CA), which has intraassay and interassay CVs of less than 10%. Inhibin B was measured using a previously described, commercially available (Serotec, Oxford, UK) double-antibody ELISA, which has a clinical detection limit of 15.6 pg/ml, an intraassay CV of less than 6%, and an interassay CV of less than 18%. SHBG was measured using a fully automated system (Immulite; Siemens Diagnostics, Deerfield, IL) with intraassay and interassay CVs of less than 7%.
Statistical analysis
A modified version of the Santen and Bardin algorithm was used to analyze LH pulsatility (33,34). When biochemical data fell below the assay limit of detection, the group analysis was conservatively completed using the limit of detection. For normally distributed data, a paired t test was used, and, for data not passing normality, the Mann-Whitney rank sum test was used for comparing group follow-up data with baseline. The data are expressed as mean ± sd unless otherwise stated, and P < 0.05 was considered statistically significant.
Genetic studies
All 10 subjects provided a blood sample for genetic studies, and the following genes (including GenBank accession numbers) were screened for mutations: FGFR1, NM_023110 (22); KAL1, NM_000216 (21); NELF, NM_015537 (24); PROK2, NM_ 021935 (23); PROKR2, NM_144773 (18); TAC3, NM_013251 (28); TACR3, NM_001059 (28); and GNRH1, NM_000825 (27). Mutations in FGF8 (NM_033163) (20), GNRHR, (NM_000406) (26), and GPR54 (NM_032551) (25) were previously screened in this cohort (20,26). Exon and exon-intron boundaries were screened as reported previously (20,23).
Nonsense changes resulting in a truncated protein, frame shift, insertion, or deletion were categorized as definitive mutations. Nucleotide changes, which were 1) absent from the Single Nucleotide Polymorphism database (http://www.ncbi.nlm.nih.gov/SNP/) and expressed sequence tags and 2) absent in at least 170 ethnically matched healthy controls were identified as mutations. All genes and proteins are described using standard nomenclature (35).
Healthy control population
A Caucasian healthy adult male control cohort (n = 192) was screened to determine whether observed base pair changes in the screened genes were normal variants.
Results
Clinical characteristics
The clinical characteristics of the 10 AHH men at baseline and after long-term follow up are compared in Table 1. Follow-up evaluation was performed 10.6 ± 5.9 yr after baseline (range, 4–22 yr), and the mean age of the follow-up cohort was 50.3 ± 7.7 yr. No significant changes were seen in their BMI (28.8 ± 4.1 vs. 27.0 ± 4.3 kg/m2) or TV (18 ± 6 vs. 19 ± 6 ml) from their initial diagnosis. In the interim, all subjects had received hormone replacement therapy with exogenous T, human chorionic gonadotropin, and/or pulsatile GnRH. Before follow up, only two subjects were on no therapy (subjects 6 and 7), both of whom reported recurrent sexual dysfunction off therapy. Subject 3 remained hypertensive and obese at follow up, depression had resolved in subject 7, although he now reported recurrent migraines, and subject 5 was still on antidepressant therapy. Subject 8 had become obese and developed type 2 diabetes mellitus. Nine of 10 subjects completed quantitative smell testing scoring within normal limits (mean score, 34.1 ± 3 of 40; range, 15th to 82nd percentile by age), whereas the other subject reported a normal sense of smell and could identify common items. Thus, all remained normosmic at follow up.
Neuroendocrine studies and biochemical profiling
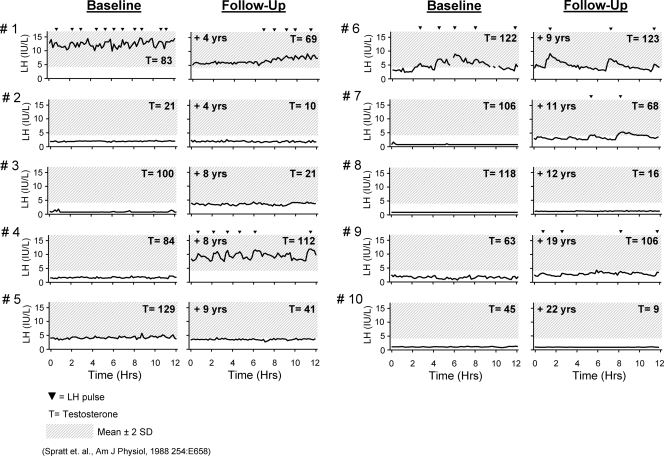
Figure 1 displays the overnight frequent sampling studies originally obtained at baseline compared with those during a mean decade of follow up. The mean LH levels at follow up did not differ significantly from baseline (P = 0.2). However, some variability was seen within individual subjects. Five subjects exhibited some degree of enfeebled pulsatile LH secretion at follow up (subjects 1, 4, 6, 7, and 9) compared with only two at their baseline evaluation. However, in each case, this degree of endogenous GnRH-induced LH secretion was insufficient to normalize their testicular secretion of T just as had been documented during baseline of patients 1 and 6. Of the five subjects who were apulsatile at their repeat evaluation (subjects 2, 3, 5, 8, and 10), two had developed undetectable LH levels (subjects 8 and 10) compared with four at the baseline evaluation. All subjects remained frankly hypogonadal at follow up.
Figure 1.
Baseline and follow up in 12-h overnight frequent sampling studies of 10 men with AHH to compare the endogenous LH pulse patterns. LH pulses were assessed using a modification of the Santen and Bardin algorithm (33,34).
The biochemical characteristics of the cohort are listed in Table 2. As a group, all parameters remained unchanged. The subjects’ semen analyses were quite consistent between baseline and follow up with four subjects remaining azoospermic (subjects 1, 2, 8, and 10). However, we cannot completely rule out that the azoospermia at follow up may reflect years of suppressed gonadotropins during interim treatment. One subject remained severely oligospermic at follow up (subject 6), and two individuals who were unable to produce an ejaculate at baseline were oligospermic (subject 3) and had a normal sperm concentration (subject 7), respectively. Given the low serum T level and hypogonadotropic state of patient 7, this normal semen concentration was interesting and suggests that spermatogenesis may be maintained by relatively low levels of serum LH and intratesticular T levels (36).
Table 2.
Biochemical characteristics of AHH men at baseline and follow up
| Patient number | Interim (yr) | LH (IU/liter)
|
T (ng/dl)
|
FSH (IU/liter)
|
Inhibin B (pg/ml)
|
Sperm count (×106/ml)
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| BL | FU | BL | FU | BL | FU | BL | FU | BL | FU | ||
| 1 | 4 | 12.5 | 6.2 | 83 | 69 | 14.2 | 8.6 | 43 | 12 | 0 | 0 |
| 2 | 4 | 2.1 | 2.2 | 21 | 10 | 1.6 | 1.6 | 160 | 183 | 0 | 0 |
| 3a | 8 | <1.6 | 3.6 | 100 | 21 | 2.2 | 2.4 | 147 | 134 | X | <1 |
| 4a | 8 | 1.8 | 9.3 | 84 | 112 | 4.9 | 8.6 | 60 | 32 | X | X |
| 5a | 9 | 4.3 | 3.4 | 129 | 41 | 4.9 | 4.1 | 124 | 51 | X | X |
| 6 | 9 | 4.8 | 5.1 | 122 | 123 | 3.2 | 4.0 | 189 | 168 | <1 | <1 |
| 7a | 11 | <1.6 | 3.5 | 106 | 68 | 2.9 | 3.6 | 114 | 85 | X | 84 |
| 8a | 12 | <1.6 | <1.6 | 118 | 16 | 1.9 | 1.7 | 63 | 73 | 0 | 0 |
| 9a | 19 | 1.6 | 2.9 | 63 | 106 | 1.8 | 1.8 | 228 | 266 | 0 | X |
| 10a | 22 | <1.6 | <1.6 | 45 | 9 | <1.6 | <1.6 | 80 | 66 | 0 | 0 |
| Mean ± sem | 10.6 ± 5.9 | 2.3 ± 0.4 | 3.9 ± 0.8 | 84 ± 12 | 58 ± 14 | 2.8 ± 0.4 | 3.8 ± 0.9 | 121 ± 19 | 118 ± 25 | ||
Baseline evaluation has been reported previously (5).
BL, Baseline at diagnosis; FU, long-term follow up; X, subject unable to provide specimen.
Genetics studies
One potentially novel DNA sequence change was identified in subject 6, who carries a heterozygous PROKR2 mutation (c.949 G > C, p.V317L). The valine 317 amino acid is conserved across species and was absent in 384 ethnically matched control alleles. In vitro studies did not demonstrate a consistent loss of function with this mutant when compared with controls.
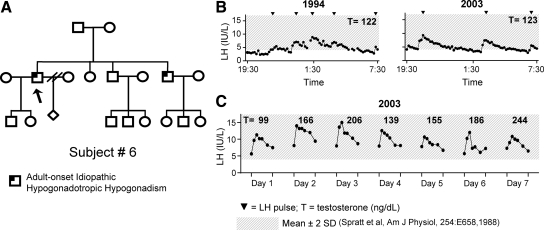
Subject 6, PROKR2 (V317L)
The 46-yr-old proband of mixed European descent harbors a heterozygous mutation in PROKR2. At age 37 yr, this proband presented to clinical care for decreased libido and erectile dysfunction (Table 1). He reported undergoing normal pubertal development at age 14 yr, and his history was notable for natural paternity at age 22 yr. He also reported having a brother who developed hypogonadism in adulthood (Fig. 2A) but unfortunately was unavailable for study. On physical examination, proband 6 was well virilized, with a BMI of 24.1 kg/m2, no gynecomastia, and a TV of 20 ml bilaterally. Multiple hypogonadal T assessments and low/normal gonadotropins in the setting of no predisposing factors led to a diagnosis of AHH. He then underwent a baseline frequent sampling study, which revealed hypogonadal T (122 ng/dl), detectable gonadotropins, and a pulsatile pattern of LH secretion (Fig. 2B). Seminal fluid analysis revealed azoospermia in the setting of a normal inhibin B level (189 pg/ml). During the 12 yr interim before reevaluation, he had received T treatment and also fathered a second child on gonadotropin therapy. His follow-up study revealed three LH pulses in 12 h of normal amplitude (5 IU/liter) yet hypogonadal T (Fig. 2B; Table 2).
Figure 2.
Clinical phenotype of subject 6 (denoted by arrow). A, Pedigree of subject 6. B, LH secretory pattern at baseline and follow-up frequent sampling studies. C, LH and T response to 7 d of exogenous pulsatile GnRH therapy.
In light of the potential role of the prokineticin system in GnRH pacemaking (37), he agreed to return for 7 d of treatment with pulsatile GnRH (25 ng/kg per bolus sc, every 2 h via microinfusion pump) to assess the effect of regulating LH frequency on the HPG axis (2). During treatment with pulsatile GnRH, his serum LH and T progressively increased to 244 ng/dl, approaching the healthy adult male normal range, by d 7 (Fig. 2C).
Discussion
Because the natural history, genetics, and long-term follow up of AHH patients are unknown, we report the prospective, long-term neuroendocrine follow up of a cohort of 10 men with AHH. Although repeat neuroendocrine evaluations demonstrated interesting and varying endogenous patterns of GnRH induced LH secretion, none were sufficient to mount a spontaneous recovery of their HPG axis. These findings suggest that AHH is a lasting condition; this feature stands in sharp contrast to the reversibility we have documented in other men with IHH and Kallmann syndrome (6) and the recovery typically seen in HA women with a functional defect in GnRH secretion (9,38). However, given our small sample size, we cannot completely rule out the possibility of some ultimate recovery, however small. We also identify a novel heterozygous PROKR2 DNA sequence change in a subject with a defective LH pulse pattern who responded robustly to exogenous GnRH therapy. His 7-d study demonstrates normal pituitary gonadotrope responsiveness, strongly suggesting a GnRH pacemaker defect. This rare sequence variant did not prove to be functionally abnormal in vitro (data not shown).
After initially describing AHH as a rare acquired hypogonadotropic hypogonadism and a treatable form of male infertility, we now provide support that this disorder in men appears to be an enduring rather than a transient suppression of the HPG axis as occurs in women with HA. Although half of the cohort showed no difference in their LH secretion pattern at follow up, three subjects developed some evidence of endogenous GnRH-induced LH pulses, and one subject exhibited a dampening of his secretion pattern that was limited to the early morning hours. However, these “enfeebled” patterns of endogenous GnRH secretion were consistently abnormal in terms of their amplitude and frequency when compared with healthy controls (39). Moreover, their GnRH-induced LH secretion was insufficient to elicit an increase in serum T levels, thus resulting in an abiding hypogonadism.
Although we have identified several new cases of AHH since our initial report, the etiology of this condition remains unclear other than consistently pointing to the GnRH “pulse generator” as the source of the defect. All subjects had negative central nervous system imaging studies at diagnosis as well as three repeat studies. None had developed any signs suggestive of an infiltrative or metabolic process at follow up, and all demonstrated consistently normal thyroid function, prolactin, liver function, and iron binding studies. AHH thus differs from the congenital forms of isolated GnRH deficiency, i.e. IHH and Kallmann syndrome, in that full pubertal development and occasional fertility has been achieved before the disruption of the HPG axis and as such the prognosis for sperm development is much more optimistic (31). Interestingly, three of our AHH subjects reported a somewhat delayed puberty (subjects 1, 4, and 9), yet all had completed sexual maturation by their late teens without any treatment. Although AHH is considered an acquired disorder, this subtle history of a somewhat delayed puberty in several AHH men suggests the possibility of an underlying preexisting, mild defect of GnRH ontogeny. This hypothesis is now supported by the identification of heterozygous PROKR2, GNRHR, and FGF8 variants, all of which are genetic loci impacting on the ontogeny of GnRH neuronal development (19,20,40).
PROKR2 expression has been identified in the arcuate and suprachiasmic nuclei, olfactory track, and hypothalamus during development and adulthood (41,42). Both human and murine PROKR2 deficiency result in absent olfactory bulbs and no sexual maturation (18,40,43,44), and several loss-of-function PROKR2 mutations have been reported in IHH subjects with an apparently normal olfactory system (18). In the present study, iterative functional studies (Egr1-LUC gene transcription assay and aequorin-based Ca2+ flux assay) failed to clearly demonstrate the V317L PROKR2 mutation to be loss of function (data not shown). However, the subject harboring this rare variant provides potentially interesting functional insight with his response to pulsatile GnRH. Despite exhibiting pulsatile LH secretion at both diagnosis and follow up, this gonadotropin secretory activity was insufficient to elicit a testicular response; thus, he remained frankly hypogonadal. It is possible that the amplitude of GnRH secretion is reduced because the number of GnRH secreting cells in the hypothalamus is decreased, similar to some of the knockout murine models (44). Interestingly, when the amplitude and frequency of GnRH secretion was normalized via 7 d of a physiologic regimen of exogenous pulsatile GnRH, the subject responded dramatically with T levels approaching the lower limit of normal for healthy adult males. One might thus speculate a link between the observed GnRH response to various circadian queues and the hypothesized role of PROK2 secretion as a potential output protein messenger from the suprachiasmatic nucleus to regulate peripheral pacemakers such as the GnRH neuronal network pacemaking (37,45). However, because PROKR2 is not expressed in murine GnRH neurons themselves (23) and this mutation was not consistently decreased in vitro, the effects of the PROK2 ligand, if any, on GnRH neuron pulsatility are likely indirect and hence must remain speculative.
In summary, AHH appears to be a rare form of acquired GnRH deficiency in men that may be lifelong as attested to by the observation that no spontaneous recoveries during a decade of follow up in this cohort. The discovery of an increasing number of rare monogenic DNA sequence changes not seen in controls in loci involved in GnRH ontogeny support the notion that there may well be a genetic contribution in the etiology of AHH that might occur in concert with other epigenetic or environmental influences. Although AHH is a rare subset of hypogonadotropic hypogonadism, it is important in that it not only remains one of the few medically treatable forms of male infertility but may well provide future biological lessons about the ontogeny of the GnRH neuronal network.
Acknowledgments
We wish to thank Dr. Hang Lee for his statistical advice and the nurses of the General Clinical Research Center for their excellent patient care.
Footnotes
This work was supported by National Institutes of Health (NIH) Grants R01 HD15788 and RO1 HD056264, Eunice Kennedy Shriver National Institute of Child Health and Human Development/NIH Cooperative Agreement U54 HD028138 as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research, and NIH National Center for Research Resources, General Clinical Research Centers Program Grant M01-RR-01066.
Disclosure Summary: The authors have nothing to disclose.
First Published Online June 30, 2010
Abbreviations: AHH, Adult-onset idiopathic hypogonadotropic hypogonadism; BMI, body mass index; CV, coefficient of variation; FGF, fibroblast growth factor; GNRHR, GnRH receptor; HA, hypothalamic amenorrhea; HPG, hypothalamic-pituitary-gonadal; IHH, idiopathic hypogonadotropic hypogonadism; MRI, magnetic resonance imaging; T, testosterone; TV, testicular volume.
References
- Seminara SB, Hayes FJ, Crowley Jr WF 1998 Gonadotropin-releasing hormone deficiency in the human (idiopathic hypogonadotropic hypogonadism and Kallmann’s syndrome): pathophysiological and genetic considerations. Endocr Rev 19:521–539 [DOI] [PubMed] [Google Scholar]
- Hoffman AR, Crowley Jr WF 1982 Induction of puberty in men by long-term pulsatile administration of low-dose gonadotropin-releasing hormone. N Engl J Med 307:1237–1241 [DOI] [PubMed] [Google Scholar]
- Boyar RM 1978 Control of the onset of puberty. Annu Rev Med 29:509–520 [DOI] [PubMed] [Google Scholar]
- Santoro N, Filicori M, Crowley Jr WF 1986 Hypogonadotropic disorders in men and women: diagnosis and therapy with pulsatile gonadotropin-releasing hormone. Endocr Rev 7:11–23 [DOI] [PubMed] [Google Scholar]
- Nachtigall LB, Boepple PA, Pralong FP, Crowley Jr WF 1997 Adult-onset idiopathic hypogonadotropic hypogonadism—a treatable form of male infertility. N Engl J Med 336:410–415 [DOI] [PubMed] [Google Scholar]
- Raivio T, Falardeau J, Dwyer A, Quinton R, Hayes FJ, Hughes VA, Cole LW, Pearce SH, Lee H, Boepple P, Crowley Jr WF, Pitteloud N 2007 Reversal of idiopathic hypogonadotropic hypogonadism. N Engl J Med 357:863–873 [DOI] [PubMed] [Google Scholar]
- Couzinet B, Young J, Brailly S, Le Bouc Y, Chanson P, Schaison G 1999 Functional hypothalamic amenorrhoea: a partial and reversible gonadotrophin deficiency of nutritional origin. Clin Endocrinol (Oxf) 50:229–235 [DOI] [PubMed] [Google Scholar]
- Welt CK, Chan JL, Bullen J, Murphy R, Smith P, DePaoli AM, Karalis A, Mantzoros CS 2004 Recombinant human leptin in women with hypothalamic amenorrhea. N Engl J Med 351: 987–997 [DOI] [PubMed] [Google Scholar]
- Perkins RB, Hall JE, Martin KA 2001 Aetiology, previous menstrual function and patterns of neuro-endocrine disturbance as prognostic indicators in hypothalamic amenorrhoea. Hum Reprod 16:2198–2205 [DOI] [PubMed] [Google Scholar]
- Braunstein GD, Kohler PO 1981 Endocrine manifestations of histiocytosis. Am J Pediatr Hematol Oncol 3:67–75 [PubMed] [Google Scholar]
- Cariski AT 1981 Isolated CNS sarcoidosis. JAMA 245:62–63 [PubMed] [Google Scholar]
- Ranjan A, Rajshekhar V, Joseph T, Chandy MJ, Chandi SM 1993 Nondiagnostic CT-guided stereotactic biopsies in a series of 407 cases: influence of CT morphology and operator experience. J Neurosurg 79:839–844 [DOI] [PubMed] [Google Scholar]
- Thodou E, Asa SL, Kontogeorgos G, Kovacs K, Horvath E, Ezzat S 1995 Clinical case seminar: lymphocytic hypophysitis: clinicopathological findings. J Clin Endocrinol Metab 80:2302–2311 [DOI] [PubMed] [Google Scholar]
- Jenkins JS, Gilbert CJ, Ang V 1976 Hypothalamic-pituitary function in patients with craniopharyngiomas. J Clin Endocrinol Metab 43:394–399 [DOI] [PubMed] [Google Scholar]
- Snyder PJ 1987 Gonadotroph cell pituitary adenomas. Endocrinol Metab Clin North Am 16:755–764 [PubMed] [Google Scholar]
- Takeuchi J, Handa H, Nagata I 1978 Suprasellar germinoma. J Neurosurg 49:41–48 [DOI] [PubMed] [Google Scholar]
- Charbonnel B, Chupin M, Le Grand A, Guillon J 1981 Pituitary function in idiopathic haemochromatosis: hormonal study in 36 male patients. Acta Endocrinol (Copenh) 98:178–183 [DOI] [PubMed] [Google Scholar]
- Cole LW, Sidis Y, Zhang C, Quinton R, Plummer L, Pignatelli D, Hughes VA, Dwyer AA, Raivio T, Hayes FJ, Seminara SB, Huot C, Alos N, Speiser P, Takeshita A, Van Vliet G, Pearce S, Crowley Jr WF, Zhou QY, Pitteloud N 2008 Mutations in prokineticin 2 and prokineticin receptor 2 genes in human gonadotrophin-releasing hormone deficiency: molecular genetics and clinical spectrum. J Clin Endocrinol Metab 93:3551–3559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Roux N, Young J, Misrahi M, Genet R, Chanson P, Schaison G, Milgrom E 1997 A family with hypogonadotropic hypogonadism and mutations in the gonadotropin-releasing hormone receptor. N Engl J Med 337:1597–1602 [DOI] [PubMed] [Google Scholar]
- Falardeau J, Chung WC, Beenken A, Raivio T, Plummer L, Sidis Y, Jacobson-Dickman EE, Eliseenkova AV, Ma J, Dwyer A, Quinton R, Na S, Hall JE, Huot C, Alois N, Pearce SH, Cole LW, Hughes V, Mohammadi M, Tsai P, Pitteloud N 2008 Decreased FGF8 signaling causes deficiency of gonadotropin-releasing hormone in humans and mice. J Clin Invest 118:2822–2831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira LM, Seminara SB, Beranova M, Hayes FJ, Valkenburgh SB, Schipani E, Costa EM, Latronico AC, Crowley Jr WF, Vallejo M 2001 The importance of autosomal genes in Kallmann syndrome: genotype-phenotype correlations and neuroendocrine characteristics. J Clin Endocrinol Metab 86:1532–1538 [DOI] [PubMed] [Google Scholar]
- Pitteloud N, Acierno Jr JS, Meysing AU, Dwyer AA, Hayes FJ, Crowley Jr WF 2005 Reversible kallmann syndrome, delayed puberty, and isolated anosmia occurring in a single family with a mutation in the fibroblast growth factor receptor 1 gene. J Clin Endocrinol Metab 90:1317–1322 [DOI] [PubMed] [Google Scholar]
- Pitteloud N, Zhang C, Pignatelli D, Li JD, Raivio T, Cole LW, Plummer L, Jacobson-Dickman EE, Mellon PL, Zhou QY, Crowley Jr WF 2007 Loss-of-function mutation in the prokineticin 2 gene causes Kallmann syndrome and normosmic idiopathic hypogonadotropic hypogonadism. Proc Natl Acad Sci U S A 104:17447–17452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitteloud N, Quinton R, Pearce S, Raivio T, Acierno J, Dwyer A, Plummer L, Hughes V, Seminara S, Cheng YZ, Li WP, Maccoll G, Eliseenkova AV, Olsen SK, Ibrahimi OA, Hayes FJ, Boepple P, Hall JE, Bouloux P, Mohammadi M, Crowley W 2007 Digenic mutations account for variable phenotypes in idiopathic hypogonadotropic hypogonadism. J Clin Invest 117:457–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno Jr JS, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O'Rahilly S, Carlton MB, Crowley Jr WF, Aparicio SA, Colledge WH 2003 The GPR54 gene as a regulator of puberty. N Engl J Med 349:1614–1627 [DOI] [PubMed] [Google Scholar]
- Cerrato F, Shagoury J, Kralickova M, Dwyer A, Falardeau J, Ozata M, Van Vliet G, Bouloux P, Hall JE, Hayes FJ, Pitteloud N, Martin KA, Welt C, Seminara SB 2006 Coding sequence analysis of GNRHR and GPR54 in patients with congenital and adult-onset forms of hypogonadotropic hypogonadism. Eur J Endocrinol 155 (Suppl 1):S3–S10 [DOI] [PubMed] [Google Scholar]
- Bouligand J, Ghervan C, Tello JA, Brailly-Tabard S, Salenave S, Chanson P, Lombès M, Millar RP, Guiochon-Mantel A, Young J 2009 Isolated familial hypogonadotropic hypogonadism and a GNRH1 mutation. N Engl J Med 360:2742–2748 [DOI] [PubMed] [Google Scholar]
- Topaloglu AK, Reimann F, Guclu M, Yalin AS, Kotan LD, Porter KM, Serin A, Mungan NO, Cook JR, Ozbek MN, Imamoglu S, Akalin NS, Yuksel B, O'Rahilly S, Semple RK 2009 TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for Neurokinin B in the central control of reproduction. Nat Genet 41:354–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doty RL, Shaman P, Kimmelman CP, Dann MS 1984 University of Pennsylvania Smell Identification Test: a rapid quantitative olfactory function test for the clinic. Laryngoscope 94:176–178 [DOI] [PubMed] [Google Scholar]
- World Health Organization 1999 WHO laboratory manual for the examination of human semen and sperm-cervical mucus interaction. London: Cambridge University Press [Google Scholar]
- Pitteloud N, Hayes FJ, Dwyer A, Boepple PA, Lee H, Crowley Jr WF 2002 Predictors of outcome of long-term GnRH therapy in men with idiopathic hypogonadotropic hypogonadism. J Clin Endocrinol Metab 87:4128–4136 [DOI] [PubMed] [Google Scholar]
- Pitteloud N, Mootha VK, Dwyer AA, Hardin M, Lee H, Eriksson KF, Tripathy D, Yialamas M, Groop L, Elahi D, Hayes FJ 2005 Relationship between testosterone levels, insulin sensitivity, and mitochondrial function in men. Diabetes Care 28:1636–1642 [DOI] [PubMed] [Google Scholar]
- Santen RJ, Bardin CW 1973 Episodic luteinizing hormone secretion in man. Pulse analysis, clinical interpretation, physiologic mechanisms. J Clin Invest 52:2617–2628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes FJ, McNicholl DJ, Schoenfeld D, Marsh EE, Hall JE 1999 Free α-subunit is superior to luteinizing hormone as a marker of gonadotropin-releasing hormone despite desensitization at fast pulse frequencies. J Clin Endocrinol Metab 84:1028–1036 [DOI] [PubMed] [Google Scholar]
- Antonarakis SE 1998 Recommendations for a nomenclature system for human gene mutations. Nomenclature Working Group. Hum Mutat 11:1–3 [DOI] [PubMed] [Google Scholar]
- Achard C, Courtillot C, Lahuna O, Méduri G, Soufir JC, Lière P, Bachelot A, Benyounes H, Schumacher M, Kuttenn F, Touraine P, Misrahi M 2009 Normal spermatogenesis in a man with mutant luteinizing hormone. N Engl J Med 361:1856–1863 [DOI] [PubMed] [Google Scholar]
- Prosser HM, Bradley A, Caldwell MA 2007 Olfactory bulb hypoplasia in Prokr2 null mice stems from defective neuronal progenitor migration and differentiation. Eur J Neurosci 26:3339–3344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins RB, Hall JE, Martin KA 1999 Neuroendocrine abnormalities in hypothalamic amenorrhea: spectrum, stability, and response to neurotransmitter modulation. J Clin Endocrinol Metab 84:1905–1911 [DOI] [PubMed] [Google Scholar]
- Spratt DI, Crowley Jr WF 1988 Pituitary and gonadal responsiveness is enhanced during GnRH-induced puberty. Am J Physiol 254:E652–E657 [DOI] [PubMed] [Google Scholar]
- Dodé C, Teixeira L, Levilliers J, Fouveaut C, Bouchard P, Kottler ML, Lespinasse J, Lienhardt-Roussie A, Mathieu M, Moerman A, Morgan G, Murat A, Toublanc JE, Wolczynski S, Delpech M, Petit C, Young J, Hardelin JP 2006 Kallmann syndrome: mutations in the genes encoding prokineticin-2 and prokineticin receptor-2. PLoS Genet 2:e175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng MY, Leslie FM, Zhou QY 2006 Expression of prokineticins and their receptors in the adult mouse brain. J Comp Neurol 498:796–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soga T, Matsumoto S, Oda T, Saito T, Hiyama H, Takasaki J, Kamohara M, Ohishi T, Matsushime H, Furuichi K 2002 Molecular cloning and characterization of prokineticin receptors. Biochim Biophys Acta 1579:173–179 [DOI] [PubMed] [Google Scholar]
- Abreu AP, Trarbach EB, de Castro M, Frade Costa EM, Versiani B, Matias Baptista MT, Garmes HM, Mendonca BB, Latronico AC 2008 Loss-of-function mutations in the genes encoding prokineticin-2 or prokineticin receptor-2 cause autosomal recessive Kallmann syndrome. J Clin Endocrinol Metab 93:4113- 4118 [DOI] [PubMed] [Google Scholar]
- Matsumoto S, Yamazaki C, Masumoto KH, Nagano M, Naito M, Soga T, Hiyama H, Matsumoto M, Takasaki J, Kamohara M, Matsuo A, Ishii H, Kobori M, Katoh M, Matsushime H, Furuichi K, Shigeyoshi Y 2006 Abnormal development of the olfactory bulb and reproductive system in mice lacking prokineticin receptor PKR2. Proc Natl Acad Sci U S A 103:4140–4145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JD, Hu WP, Boehmer L, Cheng MY, Lee AG, Jilek A, Siegel JM, Zhou QY 2006 Attenuated circadian rhythms in mice lacking the prokineticin 2 gene. J Neurosci 26:11615–11623 [DOI] [PMC free article] [PubMed] [Google Scholar]