Abstract
Background: Resveratrol have been shown to exert an antiinflammatory and antiaging effects in vitro and in animal models.
Objective: The objective of the study was to investigate the effect of a Polygonum cuspidatum extract (PCE) containing resveratrol on oxidative and inflammatory stress in normal subjects.
Research Design and Methods: Two groups (10 each) of normal-weight healthy subjects were randomized to placebo or PCE containing 40 mg resveratrol daily for 6 wk. Fasting blood samples were obtained prior to and after treatment at 1, 3, and 6 wk. Mononuclear cells were prepared for reactive oxygen species generation, RNA isolation, nuclear extract, and total cell homogenate preparation. Indices of oxidative and inflammatory stress, suppressor of cytokine signaling-3, phosphotyrosine phosphatase-1B, jun-N-terminal kinase-1, and inhibitor of κB-kinase-β were measured by RT-PCR and Western blotting.
Results: The extract induced a significant reduction in reactive oxygen species generation, the expression of p47phox, intranuclear nuclear factor-κB binding, and the expression of jun-N-terminal kinase-1, inhibitor of κB-kinase-β, phosphotyrosine phosphatase-1B, and suppressor of cytokine signaling-3 in mononuclear cells when compared with the baseline and the placebo. PCE intake also suppressed plasma concentrations of TNF-α, IL-6, and C-reactive protein. There was no change in these indices in the control group given placebo.
Conclusions: The PCE-containing resveratrol has a comprehensive suppressive effect on oxidative and inflammatory stress.
The intake of an extract of Polygonum cuspidatum containing resveratrol suppresses oxidative stress and inflammation in normal subjects.
Oxidative stress and inflammation are involved in the pathogenesis of atherogenesis, micro- and macrovascular complications of diabetes, insulin resistance, and aging. Deletion of genes inducing oxidative and inflammatory stress reduces atherogenesis and restores insulin sensitivity and prolongs life in animal models (1,2,3). Transgenic animals, like the Drosophila with an excess of genes that reduce oxidative stress-catalase and superoxide dismutase, also have a prolonged life span (4,5). Caloric restriction has also been shown to extend life span in small mammalian species (6,7). Our work and that of others have demonstrated that caloric restriction leads to a marked reduction in oxidative and inflammatory stress in humans (8,9,10).
Recent work shows that resveratrol has been shown to exert an antiinflammatory and antioxidative stress in vitro and in animal models (11,12). Resveratrol has also been shown to prolong life expectancy and reduce the rate of aging in the yeast and lower animals like yeast, Caenorhabtidis elegans and Drosophila (13,14). Resveratrol actions are thought to be mediated by increased expression of sirtuin (SIRT)-1 (13), a gene associated with longevity. SIRT-1 overexpression and resveratrol cause a reduction in the expression of phosphotyrosine phosphatase (PTP)-1B (15,16). PTP-1B has been shown to be induced by inflammation and plays a major role in insulin resistance (17,18). Thus, it is important to establish whether such a compound has properties that reduce oxidative and inflammatory stress in human.
Because there are no data demonstrating the effect of resveratrol on oxidative and inflammatory stress, in vivo, we have now hypothesized that Polygonum cuspidatum extract (PCE)-containing resveratrol reduces the level of oxidative and inflammatory stress in the human. Because several of the key mediators of insulin resistance that interfere with insulin signal transduction are also proinflammatory, we also investigated the effect of PCE intake on their expression.
Subjects and Methods
Subjects
Two groups (10 each) of normal-weight, age-matched healthy subjects (aged 36 ± 5 yr, body mass index 21.8 ± 0.5 kg/m2) were randomized to receive either 200 mg of PCE standardized to contain 20% trans-resveratrol (equivalent to 40 mg/d trans-resveratrol; Pure Encapsulations Inc., Sudbury, MA) or placebo daily for 6 wk. The total phenolics and flavonoid content of PCE is approximately 60% (wt/wt) and 6% (wt/wt), respectively (19). The subjects were not on any antiinflammatory drugs. They presented at the Clinical Research Center of the Diabetes Endocrinology Center of Western New York after an overnight fast at 0800–0900 h. Fasting blood samples were collected at baseline and at 1, 3, and 6 wk of treatment. The experimental protocol was approved by the Human Research Committee of the State University of New York at Buffalo, and each subject signed an informed consent.
Mononuclear cell (MNC) isolation
Blood samples were collected in Na-EDTA as an anticoagulant. Three to five milliliters of anticoagulated blood sample are carefully layered over 3.5 ml of Lympholyte medium (Cedarlane Laboratories, Hornby, Ontario, Canada) and centrifuged to separate the cells. A top band consists of MNCs and a bottom consists of polymorphonuclear cells. They were carefully collected. This method provides yields of greater than 95% pure polymorphonuclear cell and MNC suspensions.
Reactive oxygen species (ROS) generation measurement by chemiluminescence
Five hundred microliters of MNCs (2 × 105 cells) were delivered into a Chronolog Lumi-aggregometer cuvette. Luminol was then added, followed by 1.0 μl of 10 mm formylmethionyl leucinyl phenylalanine. In this assay system, the release of superoxide radical, as measured by chemiluminescence, has been shown to be linearly correlated with that measured by the ferricytochrome C method. The interassay coefficient of variation of this assay is 8%. We further established that the biological variation in ROS generation in normal subjects is approximately 6% for readings obtained 1–2 wk apart.
Western blotting
MNC total cell lysates were prepared and electrophoresis and immunoblotting was carried as described before (20). Polyclonal or monoclonal antibodies against p47phox, jun-N-terminal kinase (JNK)-1, inhibitor of κB-kinase (IKK)-β (BD Biosciences, San Jose, CA), PTP-1B, suppressor of cytokine signaling (SOCS)-3 (Abcam Inc., Cambridge, MA), insulin receptor substrate (IRS)-1, SIRT-1, and actin (Santa Cruz Biotechnology, Santa Cruz, CA) were used, and the membranes were developed using chemiluminescence reagent (Pierce Chemical, Rockford, IL). Densitometry was performed using molecular analyst software (Bio-Rad, Hercules, CA) and all values were corrected for loading with actin.
Nuclear factor-κB (NFκB) DNA binding activity
Nuclear NFκB DNA binding activity was measured by EMSA. Nuclear extracts were prepared from MNCs and by high-salt extraction. The specificity of the bands was confirmed by super shifting these bands with specific antibodies against Rel-A (p65) and p50 (Santa Cruz Biotechnology) and by competition with cold oligonucleotides.
Total RNA isolation and real-time RT-PCR
Total RNA was isolated using commercially available RNAqueous-4PCR kit (Ambion, Austin, TX). Real-time RT-PCR [intraassay coefficient of variation (CV) of 5–8%, interassay CV of 8–12%] was performed using Cepheid Smart Cycler (Sunnyvale, CA), Sybergreen master mix (QIAGEN, Valencia, CA), and gene expression of IKKβ, JNK-1, SOCS-3, PTP-1B, IRS-1, Toll-like receptor (TLR)-4, IL6, IL-1β, and TNF-α mRNA was measured using specific primers (Invitrogen, Carlsbad, CA). The specificity and size of the PCR products were tested by adding a melt curve at the end of the amplifications and running it on a 2% agarose gel. All values were normalized to expression of three housekeeping genes (β-actin, ubiquitin C, and cyclophilin A).
Plasma measurements
Insulin concentrations (intraassay CV of 2.6%, interassay CV of 6.2%) were measured from plasma samples using an ELISA kit (Diagnostics Systems Laboratories, Inc., Webster, TX). Free fatty acid (FFA) concentrations (intraassay CV of 4.6%, interassay CV of 9.2%) were measured using the Half-Micro calorimetric kit (Roche Diagnostic, Indianapolis, IN). Leptin and TNF-α concentrations (intraassay CV of 3.1 and 8%, interassay CV of 5.4 and 10.6%, respectively) were measured from serum by an immunoassay kit from R&D Systems (Minneapolis, MN). C-reactive protein (CRP; intraassay CV of 3%, interassay CV of 3–6%) was measured by immunoassay kit from American Diagnostica Inc. (Stamford, CT).
Statistical analysis
Statistical analysis was conducted using SigmaStat software version 3.1 (SPSS Inc., Chicago, IL). Sample size was calculated based on expected difference of 30% between the groups in NFκB DNA binding as observed in our previous studies (21,22) with a sd of 20% and desired power of 80%. Data are represented as mean ± se. Percent change from baseline was calculated and statistical analysis for change from baseline was carried out using one-way, repeated-measures ANOVA (RMANOVA) followed by Holm-Sidak post hoc test. Two-factor RMANOVA analysis followed by Tukey’s post hoc test was used for all multiple comparisons between the two treatment groups.
Results
Effect of PCE on plasma glucose, insulin, leptin, and lipid concentrations
The intake of the extract for 6 wk in normal subjects did not alter plasma insulin or glucose concentrations, nor did it alter homeostatic model on insulin resistance index (HOMA-IR). There was no significant change in plasma FFAs, triglycerides, low-density lipoprotein, high-density lipoprotein, cholesterol, or leptin concentrations after either treatment (Table 1). Serum creatinine and transaminase levels did not alter.
Table 1.
Changes in metabolic parameters before and after 200 mg/d intake of PCE or placebo for 6 wk in normal healthy subjects
| Marker/wk | Group | 0 | 1 | 3 | 6 |
|---|---|---|---|---|---|
| Glucose (mg/dl) | Placebo | 80.6 ± 4.1 | 79.6 ± 3.7 | 81.1 ± 4.7 | 80.7 ± 4.1 |
| PCE | 83.1 ± 3.3 | 84.6 ± 3.2 | 84.1 ± 2.6 | 85.3 ± 2.3 | |
| Insulin (μIU/ml) | Placebo | 5.3 ± 0.5 | 5.5 ± 0.6 | 5.1 ± 0.5 | 5.0 ± 0.5 |
| PCE | 4.7 ± 0.4 | 4.1 ± 0.4 | 4.7 ± 0.7 | 4.5 ± 0.3 | |
| HOMA-IR | Placebo | 0.95 ± 0.21 | 0.90 ± 0.20 | 0.94 ± 0.22 | 0.92 ± 0.18 |
| PCE | 0.98 ± 0.22 | 0.94 ± 0.24 | 0.97 ± 0.30 | 0.93 ± 0.19 | |
| FFA (mm) | Placebo | 0.31 ± 0.04 | 0.32 ± 0.03 | 0.33 ± 0.03 | 0.32 ± 0.04 |
| PCE | 0.25 ± 0.03 | 0.29 ± 0.03 | 0.26 ± 0.03 | 0.26 ± 0.04 | |
| Triglyceride (mg/dl) | Placebo | 82 ± 15 | 84 ± 19 | 87 ± 20 | 84 ± 18 |
| PCE | 78 ± 17 | 77 ± 18 | 81 ± 18 | 80 ± 17 | |
| Cholesterol (mg/dl) | Placebo | 153 ± 13 | 159 ± 13 | 155 ± 12 | 158 ± 14 |
| PCE | 159 ± 14 | 161 ± 12 | 156 ± 12 | 159 ± 14 | |
| HDL (mg/dl) | Placebo | 46 ± 7 | 45 ± 7 | 47 ± 6 | 47 ± 7 |
| PCE | 45 ± 6 | 44 ± 6 | 45 ± 7 | 45 ± 6 | |
| LDL (mg/dl) | Placebo | 93 ± 12 | 101 ± 14 | 103 ± 13 | 98 ± 12 |
| PCE | 104 ± 15 | 106 ± 16 | 98 ± 14 | 108 ± 15 | |
| Leptin (ng/ml) | Placebo | 8.32 ± 1.12 | 8.51 ± 1.21 | 8.27 ± 1.14 | 8.38 ± 1.19 |
| PCE | 8.75 ± 1.07 | 8.49 ± 1.02 | 8.59 ± 1.21 | 8.61 ± 1.25 |
Data are presented as mean ± se. No significant changes were observed in these parameters. LDL, Low-density lipoprotein; HDL, high-density lipoprotein.
Effect of PCE on ROS generation and P47phox expression by MNCs
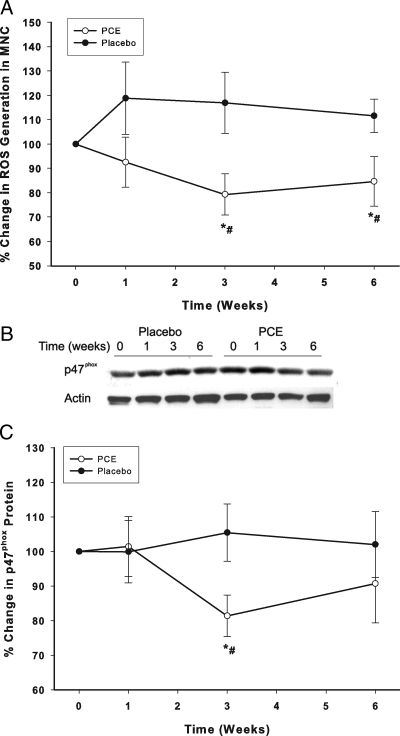
ROS generation by MNCs fell significantly after the intake of the extract by 19 ± 10% (P < 0.05, Fig. 1A) at wk 3 and remained suppressed for the 6-wk treatment period, whereas ROS generation did not change in the placebo group. PCE also suppressed p47phox (nicotinamide adenine dinucleotide phosphate oxidase subunit) protein in MNCs by 15 ± 8% below the baseline at 3 wk (P < 0.05, Fig. 1, B and C) and remained below baseline levels at wk 6, whereas it did not change in the placebo group. The changes in ROS generation and p47phox were significantly different between the PCE and placebo groups (two-way RMANOVA).
Figure 1.
Change from baseline (%) in ROS generation by MNC (A) and p47 subunit protein (B, C) in MNC following PCE (200 mg/d) containing resveratrol (40 mg/d) or placebo treatment for 6 wk in 10 normal healthy subjects per group. *, P < 0.05 comparing changes from baseline by RMANOVA; #, P < 0.05 comparing treatments between the groups by two-way RMANOVA.
Effects of PCE on NFκB DNA binding and proinflammatory mediators
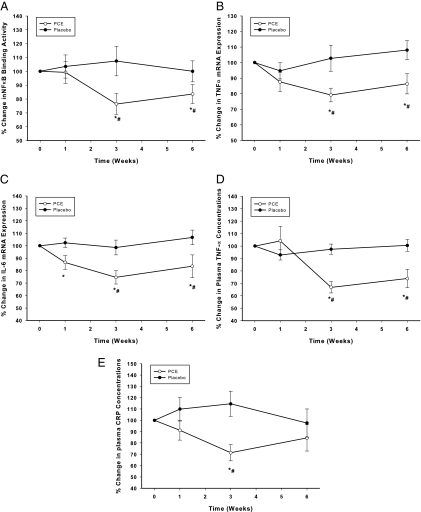
Intranuclear NFκB DNA binding in MNCs fell significantly by 25 ± 7% below the baseline (P < 0.05, Fig. 2A) at 3 wk of treatment with PCE and remained suppressed thereafter, whereas it did not change significantly in the placebo group. TNF-α and IL-6 mRNA expression in MNCs also fell significantly by 20 ± 7 and 22 ± 9% below the baseline, respectively, at wk 3 (P < 0.05, Fig. 2, B and C) after PCE, whereas IL-1β expression did not alter. There was no significant change in any of these indices in the placebo group. The changes in NFκB DNA binding, TNF-α, and IL-6 mRNA expression were significantly different between the PCE and placebo groups (two-way RMANOVA).
Figure 2.
Change from baseline (%) in NFκB DNA binding by EMSA in MNC (A), TNF-α (B), IL-6 (C) mRNA expression in MNC, TNF-α plasma concentrations (D), and CRP concentrations (E) following PCE (200 mg/d) containing resveratrol (40 mg/d) or placebo treatment for 6 wk in 10 normal healthy subjects per group. *, P < 0.05 comparing changes from baseline by RMANOVA; #, P < 0.05 comparing treatments between the groups by two-way RMANOVA.
Effects of PCE on plasma levels of TNF-α and CRP concentrations
PCE intake also resulted in a significant fall by 33 ± 5% below the baseline (from 0.72 ± 0.2 to 0.46 ± 0.2 pg/ml at wk 3, P < 0.05, Fig. 2D) in plasma TNF-α concentrations and by 29 ± 11% below the baseline (from 0.77 ± 0.18 to 0.46 ± 0.1 mg/liter by wk 3, P < 0.05, Fig. 2E) in CRP concentrations, whereas there was no change in these mediators in the placebo group. The fall in TNF-α and CRP concentrations was significantly different between the PCE and placebo groups (two-way RMANOVA).
Effects of PCE on JNK-1, IKKβ, PTP-1B, SOCS-3, IRS-1, and TLR-4 expression in MNCs
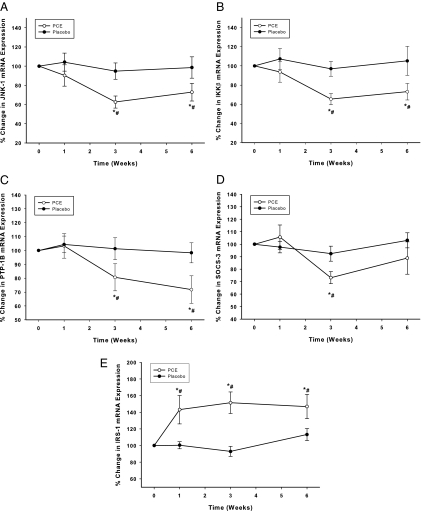
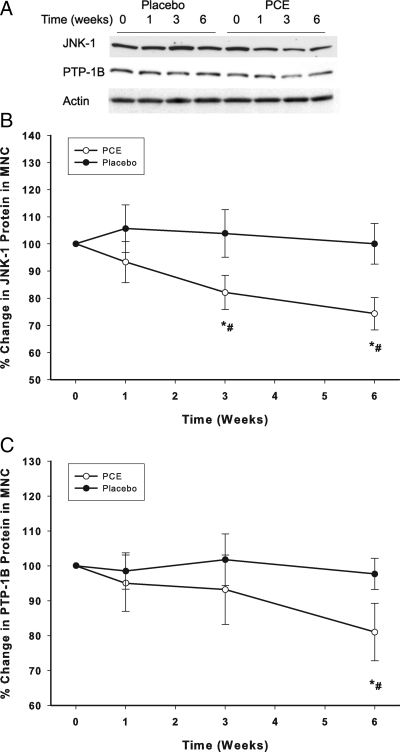
The intake of the extract significantly suppressed JNK-1, IKKβ, PTP-1B, and SOCS-3 mRNA expression by 33 ± 9, 29 ± 10, 28 ± 11, and 24 ± 8%, respectively (P < 0.05, Fig. 3, A–D). This was associated with a significant fall in JNK-1 and PTP-1B proteins by 33 ± 9 and 29 ± 10%, respectively (P < 0.05, Fig. 4, A–C) but not in IKKβ and SOCS-3. On the other hand, PCE-containing resveratrol intake induced a significant increase in the expression of IRS-1 starting at wk 1, reaching 43 ± 8% above the baseline at 3 wk and remaining elevated for the entire treatment period of 6 wk (P < 0.05, Fig. 3E). IRS-1 protein was not detectible in MNCs despite the increase in mRNA levels. There was no change in TLR-4 expression mRNA or protein expression in both groups (data not shown). There was no significant change in any of these genes in the placebo group. Changes in JNK-1, IKKß, PTP-1B, SOCS-3, and IRS-1 expression after PCE were significantly different when compared with placebo group (two-way RMANOVA).
Figure 3.
Change from baseline (%) in mRNA expression of JNK-1 (A), IKKβ (B), PTP-1B (C), SOCS-3 (D), and IRS-1 (E) in MNC following PCE (200 mg/d) containing resveratrol (40 mg/d) or placebo treatment for 6 wk in 10 normal healthy subjects per group. *, P < 0.05 comparing changes from baseline by RMANOVA; #, P < 0.05 comparing treatments between the groups by two-way RMANOVA.
Figure 4.
Change from baseline (%) in protein levels of JNK-1 (A, B) and PTP-1B (A, C) in MNC following PCE (200 mg/d) containing resveratrol (40 mg/d) or placebo treatment for 6 wk in 10 normal healthy subjects per group. *, P < 0.05 comparing changes from baseline by RMANOVA; #, P < 0.05 comparing treatments between the groups by two-way RMANOVA.
Effects of PCE on SIRT-1 expression in MNCs
There was no significant change in SIRT-1 protein expression in MNCs during this period in both groups.
Discussion
Our data show clearly that an extract of P. cuspidatum-containing resveratrol suppressed ROS generation by MNCs and the expression of p47phox, the cardinal subunit of nicotinamide adenine dinucleotide phosphate oxidase, the enzyme that converts molecular O2 into the O2− radical. In addition, the intranuclear binding of NFκB was also suppressed. NFκB is the major proinflammatory transcription factor. Thus, this extract exerts ROS suppressive (antioxidant) and antiinflammatory effects. These actions, demonstrated for the first time, in vivo, are consistent with potential antiatherogenic and antiaging effects. Longer-term studies are required to determine whether these effects are durable and whether higher doses will produce a greater effect.
In addition, there was a significant reduction in the expression of TNF-α and IL-6 in MNCs and the plasma concentration of TNF-α. TNF-α and IL-6 are two major proinflammatory cytokines regulated by NFκB. Plasma CRP concentrations also fell significantly, consistent with an antiinflammatory effect. The magnitude of these effects was similar to that described by us previously for rosiglitazone (22,23).
In parallel with these effects, the expression of two major proinflammatory kinases, JNK-1 and IKKβ, was also suppressed significantly over the 6-wk period of PCE intake. JNK-1 phosphorylates c-Jun and activates the proinflammatory transcription factor activator protein-1. IKKβ phosphorylates NFκB inhibitor α and thus causes its ubiquitination and proteasomal lysis and the translocation of NFκB into the nucleus and the initiation of proinflammatory transcription. The suppression of these kinases is thus antiinflammatory. In addition, there was also a reduction in the expression of SOCS-3, a protein whose expression is modulated by the proinflammatory cytokines TNF-α, IL-1β, and IL-6 (24,25) and has been shown to be up-regulated in obesity (26). PCE-containing resveratrol also caused suppression in the expression of PTP-1B that, in addition to its known negative effects on insulin action, has been shown to be involved in the inflammatory response (17). These observations further support a potent antiinflammatory effect of PCE extract in vivo. It is also noteworthy that the suppression of PTP-1B was not associated with a change in the expression of SIRT-1 because previous work in animal models showed that resveratrol-induced suppression of PTP-1B was mediated by SIRT-1 (15).
On the other hand, TLR4 expression was not altered significantly. TLR4 is the specific receptor for endotoxin or lipopolysaccharide, which triggers the downstream responses leading activation of NFκB.
It is of interest that several of the proinflammatory genes suppressed by the resveratrol-containing extract also interfere with insulin signal transduction and may play a role in the development of insulin resistance. The two kinases, IKKβ and JNK-1, cause serine phosphorylation of IRS-1 and thus interfere with insulin signal transduction (27). SOCS-3, which has been previously shown to be related to body mass index and HOMA-IR and inversely to insulin receptor phosphorylation in obese humans (26), also interferes with insulin signal transduction by causing the ubiquitination and proteasomal degradation of IRS-1 and IRS-2 (28). PTP-1B is the phosphatase that removes phosphate residues from phosphotyrosine in the β-subunit of the insulin receptor and thus limits the magnitude and the duration of the insulin signal at the insulin receptor level (18). In addition, the specific individual deletions of SOCS-3, JNK-1, IKKβ, TLR-4, and PTP-1B protect against the development of diet-induced, obesity-related insulin resistance (3,27,29,30,31). In view of the above observations, it is possible that PCE with resveratrol may be a potential insulin sensitizer because it suppressed SOCS-3, JNK-1, IKKβ, and PTP-1B. Consistent with this effect, there was also an increase in the expression of IRS-1. Clearly this hypothesis needs to be tested in an insulin-resistant population.
The major weakness of our study is that it does not identify which component of the extract is responsible for the effects observed. The PCE used has only 20% resveratrol and the effects that we have described may be due to products other than resveratrol contained in that preparation. This was the purest preparation available more than 2 yr ago when these experiments were conducted. Purer preparations are available now and would need to be tested. On the other hand, our observations also open the way for the investigation of other constituents of P. cuspidatum, which may be responsible for these very potent and interesting biological and clinically relevant effects. There are data showing the antiinflammatory effects of polyphenols, in vitro, but there are no data demonstrating this, in vivo, in the human (32). The other limitation of this study is that it has been conducted in a relatively small number of patients and for a short period of 6 wk.
In conclusion, an extract of P. cuspidatum suppresses ROS generation by MNCs; the expression of p47phox; the intranuclear binding of NFκB; the expression of TNF-α, IL-6, SOCS-3; and plasma concentrations of TNF-α and CRP. It also suppresses the expression of JNK-1 and IKKβ, both of which are mediators of inflammation. In addition, it suppresses the expression of SOCS-3 and PTP-1B, both of which are known to interfere with insulin signal transduction. This would potentially prolong insulin action and its antiinflammatory effects. These comprehensive suppressive effects on ROS generation and inflammation are consistent with an antiaging action of resveratrol.
Footnotes
P.D. is supported by National Institutes of Health Grants R01DK069805-02 and R01DK075877-01-A2 and American Diabetes Association Grant 08-CR-13.
Disclosure Summary: The authors have nothing to disclose.
First Published Online June 9, 2010
Abbreviations: CRP, C-reactive protein; CV, coefficient of variation; FFA, free fatty acid; HOMA-IR, homeostatic model on insulin resistance index; IKK, inhibitor of κB-kinase; IRS, insulin receptor substrate; JNK, jun-N-terminal kinase; MNC, mononuclear cell; NFκB, nuclear factor-κB; PCE, Polygonum cuspidatum extract; PTP, phosphotyrosine phosphatase; RMANOVA, repeated-measures ANOVA; ROS, reactive oxygen species; SIRT, sirtuin; SOCS, suppressor of cytokine signaling; TLR, Toll-like receptor.
References
- Migliaccio E, Giorgio M, Mele S, Pelicci G, Reboldi P, Pandolfi PP, Lanfrancone L, Pelicci PG 1999 The p66shc adaptor protein controls oxidative stress response and life span in mammals. Nature 402:309–313 [DOI] [PubMed] [Google Scholar]
- Napoli C, Martin-Padura I, de Nigris F, Giorgio M, Mansueto G, Somma P, Condorelli M, Sica G, De Rosa G, Pelicci P 2003 Deletion of the p66Shc longevity gene reduces systemic and tissue oxidative stress, vascular cell apoptosis, and early atherogenesis in mice fed a high-fat diet. Proc Natl Acad Sci USA 100:2112–2116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoelson SE, Lee J, Yuan M 2003 Inflammation and the IKKβ/IκB/NF-κB axis in obesity- and diet-induced insulin resistance. Int J Obes Relat Metab Disord 27(Suppl 3):S49–S52 [DOI] [PubMed] [Google Scholar]
- Orr WC, Sohal RS 1994 Extension of life-span by overexpression of superoxide dismutase and catalase in Drosophila melanogaster. Science 263:1128–1130 [DOI] [PubMed] [Google Scholar]
- Hu D, Cao P, Thiels E, Chu CT, Wu GY, Oury TD, Klann E 2007 Hippocampal long-term potentiation, memory, and longevity in mice that overexpress mitochondrial superoxide dismutase. Neurobiol Learn Mem 87:372–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal RS, Weindruch R 1996 Oxidative stress, caloric restriction, and aging. Science 273:59–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DW, Yu BP 1990 Modulation of free radicals and superoxide dismutases by age and dietary restriction. Aging (Milano) 2:357–362 [DOI] [PubMed] [Google Scholar]
- Dandona P, Mohanty P, Hamouda W, Ghanim H, Aljada A, Garg R, Kumar V 2001 Inhibitory effect of a two day fast on reactive oxygen species (ROS) generation by leucocytes and plasma ortho-tyrosine and meta-tyrosine concentrations. J Clin Endocrinol Metab 86:2899–2902 [DOI] [PubMed] [Google Scholar]
- Dandona P, Mohanty P, Ghanim H, Aljada A, Browne R, Hamouda W, Prabhala A, Afzal A, Garg R 2001 The suppressive effect of dietary restriction and weight loss in the obese on the generation of reactive oxygen species by leukocytes, lipid peroxidation, and protein carbonylation. J Clin Endocrinol Metab 86:355–362 [DOI] [PubMed] [Google Scholar]
- Dandona P, Weinstock R, Thusu K, Abdel-Rahman E, Aljada A, Wadden T 1998 Tumor necrosis factor-alpha in sera of obese patients: fall with weight loss. J Clin Endocrinol Metab 83:2907–2910 [DOI] [PubMed] [Google Scholar]
- Birrell MA, McCluskie K, Wong S, Donnelly LE, Barnes PJ, Belvisi MG 2005 Resveratrol, an extract of red wine, inhibits lipopolysaccharide induced airway neutrophilia and inflammatory mediators through an NF-κB-independent mechanism. FASEB J 19:840–841 [DOI] [PubMed] [Google Scholar]
- Rahman I, Biswas SK, Kirkham PA 2006 Regulation of inflammation and redox signaling by dietary polyphenols. Biochem Pharmacol 72:1439–1452 [DOI] [PubMed] [Google Scholar]
- Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA 2003 Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 425:191–196 [DOI] [PubMed] [Google Scholar]
- Barger JL, Kayo T, Vann JM, Arias EB, Wang J, Hacker TA, Wang Y, Raederstorff D, Morrow JD, Leeuwenburgh C, Allison DB, Saupe KW, Cartee GD, Weindruch R, Prolla TA 2008 A low dose of dietary resveratrol partially mimics caloric restriction and retards aging parameters in mice. PLoS One 3:e2264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, Auwerx J 2006 Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1α. Cell 127:1109–1122 [DOI] [PubMed] [Google Scholar]
- Sun C, Zhang F, Ge X, Yan T, Chen X, Shi X, Zhai Q 2007 SIRT1 improves insulin sensitivity under insulin-resistant conditions by repressing PTP1B. Cell Metab 6:307–319 [DOI] [PubMed] [Google Scholar]
- Zabolotny JM, Kim YB, Welsh LA, Kershaw EE, Neel BG, Kahn BB 2008 Protein-tyrosine phosphatase 1B expression is induced by inflammation in vivo. J Biol Chem 283:14230–14241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller DE 2001 New drug targets for type 2 diabetes and the metabolic syndrome. Nature 414:821–827 [DOI] [PubMed] [Google Scholar]
- Hsu CY, Chan YP, Chang J 2007 Antioxidant activity of extract from Polygonum cuspidatum. Biol Res 40:13–21 [DOI] [PubMed] [Google Scholar]
- Aljada A, Mohanty P, Ghanim H, Abdo T, Tripathy D, Chaudhuri A, Dandona P 2004 Increase in intranuclear nuclear factor κB and decrease in inhibitor κB in mononuclear cells after a mixed meal: evidence for a proinflammatory effect. Am J Clin Nutr 79:682–690 [DOI] [PubMed] [Google Scholar]
- Ghanim H, Garg R, Aljada A, Mohanty P, Kumbkarni Y, Assian E, Hamouda W, Dandona P 2001 Suppression of nuclear factor-κB and stimulation of inhibitor κB by troglitazone: evidence for an anti-inflammatory effect and a potential antiatherosclerotic effect in the obese. J Clin Endocrinol Metab 86:1306–1312 [DOI] [PubMed] [Google Scholar]
- Mohanty P, Aljada A, Ghanim H, Hofmeyer D, Tripathy D, Syed T, Al-Haddad W, Dhindsa S, Dandona P 2004 Evidence for a potent antiinflammatory effect of rosiglitazone. J Clin Endocrinol Metab 89:2728–2735 [DOI] [PubMed] [Google Scholar]
- Ghanim H, Dhindsa S, Aljada A, Chaudhuri A, Viswanathan P, Dandona P 2006 Low-dose rosiglitazone exerts an antiinflammatory effect with an increase in adiponectin independently of free fatty acid fall and insulin sensitization in obese type 2 diabetics. J Clin Endocrinol Metab 91:3553–3558 [DOI] [PubMed] [Google Scholar]
- Emanuelli B, Peraldi P, Filloux C, Chavey C, Freidinger K, Hilton DJ, Hotamisligil GS, Van Obberghen E 2001 SOCS-3 inhibits insulin signaling and is up-regulated in response to tumor necrosis factor-α in the adipose tissue of obese mice. J Biol Chem 276:47944–47949 [DOI] [PubMed] [Google Scholar]
- Senn JJ, Klover PJ, Nowak IA, Zimmers TA, Koniaris LG, Furlanetto RW, Mooney RA 2003 Suppressor of cytokine signaling-3 (SOCS-3), a potential mediator of interleukin-6-dependent insulin resistance in hepatocytes. J Biol Chem 278:13740–13746 [DOI] [PubMed] [Google Scholar]
- Ghanim H, Aljada A, Daoud N, Deopurkar R, Chaudhuri A, Dandona P 2007 Role of inflammatory mediators in the suppression of insulin receptor phosphorylation in circulating mononuclear cells of obese subjects. Diabetologia 50:278–285 [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS 2006 Inflammation and metabolic disorders. Nature 444:860–867 [DOI] [PubMed] [Google Scholar]
- Rui L, Yuan M, Frantz D, Shoelson S, White MF 2002 SOCS-1 and SOCS-3 block insulin signaling by ubiquitin-mediated degradation of IRS1 and IRS2. J Biol Chem 277:42394–42398 [DOI] [PubMed] [Google Scholar]
- Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS 2006 TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest 116:3015–3025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali MI, Ketsawatsomkron P, Belin de Chantemele EJ, Mintz JD, Muta K, Salet C, Black SM, Tremblay ML, Fulton DJ, Marrero MB, Stepp DW 2009 Deletion of protein tyrosine phosphatase 1b improves peripheral insulin resistance and vascular function in obese, leptin-resistant mice via reduced oxidant tone. Circ Res 105:1013–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elchebly M, Payette P, Michaliszyn E, Cromlish W, Collins S, Loy AL, Normandin D, Cheng A, Himms-Hagen J, Chan CC, Ramachandran C, Gresser MJ, Tremblay ML, Kennedy BP 1999 Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1B gene. Science 283:1544–1548 [DOI] [PubMed] [Google Scholar]
- Biesalski HK 2007 Polyphenols and inflammation: basic interactions. Curr Opin Clin Nutr Metab Care 10:724–728 [DOI] [PubMed] [Google Scholar]