Abstract
Context: The biochemical hallmark of pseudohypoparathyroidism type 1a (PHP1a) is resistance to PTH, but based on tissue-specific imprinting of GNAS, PTH resistance may be limited to the renal cortex. Some studies have shown that bone is responsive to PTH, suggesting that PHP1a patients with chronically elevated PTH levels may have low bone mineral density (BMD).
Setting: This observational study was conducted at the Institute of Clinical and Translational Research, Johns Hopkins Medical Institutions.
Subjects: Twenty-two children and adults with PHP1a were studied.
Main Outcome Measure: The main outcome measure was BMD Z-score at the lumbar spine (LS), total hip, femoral neck, and total body using dual-energy x-ray absorptiometry, relative to height, weight, and pubertal status.
Results: The mean (±sd) Z-score for height was 0.77 ± 1.66 and 1.85 ± 1.15 for BMI. The BMD Z-score at each of the four sites studied was as follows: LS, 0.29 ± 1.08; total hip, 0.27 ± 1.24; femoral neck, 0.02 ± 1.26; and total body, 0.98 ± 1.50. Only two subjects (9%) had BMD Z-scores less than −2, and each had additional risk factors for low BMD. BMD in total body and LS spine corrected for height-for-age Z-score was significantly greater than normal. There was no correlation between PTH level and BMD Z-score or between body mass index and BMD Z-score.
Conclusions: Despite secondary hyperparathyroidism, region-specific BMD is not reduced in patients with PHP1a, and total body BMD is significantly greater than normal.
Pseudohypoparathyroidism type 1a is characterized by elevated serum levels of intact PTH, but bone density is normal or increased.
Pseudohypoparathyroidism (PHP) type 1a is characterized by endocrine dysfunction and a constellation of unusual somatic features that are collectively termed Albright hereditary osteodystrophy (AHO; Mendelian Inheritance in Man 103580). AHO includes short stature, brachydactyly, heterotopic membranous bone formation, and dental abnormalities. Both hormone resistance and AHO result from heterozygous inactivating mutations in GNAS (formerly GNAS1 Mendelian Inheritance in Man 139320), the gene that encodes the α-chain of the stimulatory guanine nucleotide-binding protein (Gαs) that couples many heptahelical receptors to activation of adenylyl cyclase (1,2).
GNAS is a complex imprinted gene with three alternative first exons, and transcripts encoding Gαs have been shown to be preferentially expressed from the maternal allele in pituitary somatotropes, the gonad, and the thyroid in humans (3,4,5,6) and in the renal proximal tubule and the gonads in mice (7). GNAS mutations on maternally inherited alleles result in the AHO phenotype plus hormone resistance in cells in which Gαs is imprinted [e.g. renal cortex, thyroid, gonad, somatotropes (1,2)]. By contrast, similar GNAS mutations on the paternal allele lead to AHO without hormone resistance, a condition termed pseudopseudohypoparathyroidism (PPHP). Some patients with paternal GNAS mutations develop only severe and extensive heterotopic membranous ossifications and are considered to have the related disorder progressive osseous heteroplasia (1,2,8).
Patients with the genetically distinct disorder PHP1b lack typical features of AHO and have endocrine dysfunction that is limited to PTH resistance in the renal proximal tubule, although recently an overlap between PHP1a and PHP1b has been described in which some patients manifest brachydactyly (9). Subjects with PHP1b have normal Gαs activity in accessible tissues (10). The coding region of GNAS is normal, but an epigenetic defect switches the maternal GNAS allele to a paternal pattern of methylation (i.e. paternal epigenotype). In most familial cases, the epigenetic defect results from microdeletions in the STX16 gene located approximately 220 kb centromeric of GNAS exon 1A (11,12) or deletions removing differentially methylated regions of GNAS exon NESP55 and exons 3 and 4 of the antisense transcript (13). Inheritance of a mutation from a female (or spontaneous mutation of a maternally derived allele) abolishes Gαs expression from both alleles in imprinted tissues and results in PTH resistance (14).
The unusual skeletal features of AHO have invited investigation of Gs-dependent signaling in this tissue. PTH, as well as the related gene product PTHrP, binds and stimulates the type I PTH/PTHrP receptor (PTHR1) with equal affinity. Nevertheless, recent studies now suggest that the two ligands may have very different signaling properties because PTH1-34 stimulates generation of more cAMP than PTHrP1-36 (15). The basis for this unexpected dichotomy appears to be the prolonged association of PTH1-34 with the PTHR1 and Gαs within a persistently active, internalized ternary complex, whereas PTHrP1-36 action is restricted to the cell membrane and is short lived (15).
Activation of this Gs-coupled receptor on osteoblasts and renal tubule cells is responsible for the classical effects of PTH on calcium and phosphate metabolism and, similarly, mediates the paraneoplastic effects of PTHrP. In skeletal growth plates, PTHrP is expressed in the perichondrium and maturing chondrocytes, whereas its receptor is primarily found in the proximal prehypertrophic layer. Recent studies demonstrated that decreased signaling through Gαs-coupled pathways in the growth plate leads to accelerated differentiation of chondrocytes with consequent premature closure of the growth plates (16,17,18). Moreover, loss of PTHrP leads to short-limbed dwarfism in mice (19) and brachydactyly type E in humans (20), whereas loss of the PTHR1 leads to Blomstrand chondrodystrophy (21).
Gαs is biallelically expressed in human bone (22) and mouse chondrocytes (17,18), and thus, haploinsufficiency of Gαs has been implicated as the cause of defective PTHrP signaling in the growth plate. By contrast, skeletal responsiveness to PTH appears intact in patients with PHP1a and PHP1b (23,24,25,26,27,28,29,30). Indeed, hyperparathyroid bone disease has been described in at least some patients with PHP1a (27,30) and PHP1b (23,24,25,29), and patients with PHP1b have been shown to have lower bone mineral density (BMD) than age-, race-, and sex-matched controls (31,32). By contrast, previously reported studies of BMD in PHP1a have been limited by small numbers of subjects, and thus, definitive conclusions about bone density are lacking (30,31,32,33,34).
The primary objective of this study was to determine BMD, relative to height, weight, and pubertal status, in a large cohort of children, adolescents, and adults with PHP1a. Secondary objectives were to determine the relationships between BMD and PTH levels related to PHP1a.
Subjects and Methods
Subjects
BMD measurements at the lumbar spine (LS), total hip (TH), femoral neck (FN), and total body (TB) were obtained in 22 subjects with PHP1a, 18 of 22 with confirmed mutations in the GNAS gene (data not shown). Bone densitometry, laboratory studies, anthropometric data, and bone age were all obtained during the same evaluation. A diagnosis of PHP1a had been made months to years before enrollment in this study with the exception of subject 13, who was newly diagnosed, and treatment with calcium and/or calcitriol was begun between ages 2 and 7 yr. The criteria for PHP1a included the presence of AHO features combined with multihormone resistance, in particular, resistance to PTH (elevated serum intact PTH, sometimes associated with hypocalcemia and hyperphosphatemia), TSH (elevated TSH with low or normal free T4), GHRH (associated with GH deficiency), or clinical symptoms of GnRH resistance (amenorrhea, oligomenorrhea). Features of AHO included short stature, brachydactyly, round face, sc ossifications, and cognitive impairment. Pubertal Tanner staging was performed by clinical examination. Adults were defined by age 18 yr or older and Tanner stage V.
Two subjects were taking glucocorticoids for chronic medical conditions that were unrelated to PHP1a.
All subjects were evaluated at the Institute for Clinical and Translational Research at the Johns Hopkins Hospital (Baltimore, MD), and all studies were approved by the Institutional Review Board of the Johns Hopkins Medical Institutions. Informed consent was obtained from all subjects, or a parent of each child, and assent was obtained from children when appropriate.
Laboratory evaluation
Laboratory evaluation was performed after an overnight fast of at least 8 h. Serum concentrations of intact PTH (Intact PTH immunoradiometric assay; Nichols, San Juan Capistrano, CA), TSH, free T4, calcium, phosphate, and alkaline phosphatase levels were obtained as described previously (16). GH stimulation testing was performed by both arginine-l-dopa and arginine-GHRH testing for each subject with the assessment of either GH deficiency or sufficiency being consistent with the two methods. The GH stimulation testing and diagnosis of GH deficiency has been described in detail previously (16,35). The laboratory data presented were obtained at the time of the dual-energy x-ray absorptiometry (DXA) scans; this was the initial visit for 16 of the 22 patients. The other six patients had been followed up by us long term.
Anthropometric evaluation
Standing height was assessed using a digital stadiometer (Harpendon; Holtain Ltd., Crymych, Dyfed, UK). Weight was measured using a digital electronic scale (Detecto Scale Co., Webb City, MO). Height measurements were also expressed as sd from the mean (adjusted for age and gender). Body mass index (BMI; kilograms per square meter) was calculated from height and weight measurements, and age- and gender-specific Z-scores in children were calculated using applications from Baylor College of Medicine (www.bcm.edu/bodycomplab/) (36).
GNAS mutation analysis
Mutation analysis of the GNAS gene was performed on the 13 coding exons and flanking intronic sequences in the Johns Hopkins DNA Diagnostics Laboratory (Clinical Laboratory Improvement Amendments-approved laboratory) or in our research laboratory at the Johns Hopkins University School of Medicine, as previously described (16,35). Southern blot analysis was performed to assess large deletions, insertions, or imprinting defects (16).
Imaging studies
Study subjects underwent whole-body DXA scan (Hologic QDR 4500, Waltham MA) as well as DXA of the LS (L1–L4), TH, FN, and TB less head in the array mode. In the Institute for Clinical and Translational Research, the coefficient of variation of BMD is less than 1%.
BMD results were expressed as Z-scores, the sd from the mean for age- and gender-matched controls, and were based on chronological age, not bone age. Z-scores were calculated from a Hologic Pediatric Reference Database based on data collected at five centers on white children aged 5–20 yr for the spine (n = 1444) and hip (n = 1047) and children aged 3–20 yr for whole body (n = 1948). For children between the ages of 7 and 17 yr, we also determined BMD Z-scores that were adjusted for height using the height-for-age Z-score (HAZ) (37).
Bone mineral content (BMC) Z-scores for TB were calculated using normative data that are relative to age, gender, and race (www.bcm.edu/bodycomplab/) (36).
Bone age of the left hand and wrist was determined in children and adolescents using the standards of Gruelich and Pyle. The bone age interpretations were conducted by a pediatric radiologist on the faculty at the Johns Hopkins University School of Medicine as well as independently by a pediatric endocrinologist on the faculty (E.L.G.-L.).
Statistics
All values are expressed as means ± sd. Data were analyzed by unpaired two-tailed Student t tests. Differences between group Z-scores and normal means (Z-score = 0) were determined by one-group t tests. P < 0.05 was considered significant. A Pearson coefficient was calculated for analysis of correlations except for analysis of alkaline phosphatase and bone density, for which a Spearman correlation was determined.
Results
Subjects
DXA scans were obtained in 22 subjects with PHP1a, including seven adults and 15 children who ranged in age from 3 to 54 yr. All subjects had features of AHO, including brachydactyly type D and/or E and evidence of PTH and/or TSH resistance at the time of initial diagnosis based on either past records or on our evaluation when diagnosed in our clinic. Biochemical data at the time of the DXA scans are shown in Table 1. Tanner stage information along with BMD Z-scores is provided in Table 2.
Table 1.
Biochemical data
| Patient | Age (yr) | sCalcium (mmol/liter) | sPhosphate (mmol/liter) | PTH (ng/liter) | Alk phos (U/liter) | TSH (mIU/liter) | Free T4 (ng/dl) | GH deficient |
|---|---|---|---|---|---|---|---|---|
| 1 | 3.3 | 2.4 | 1.52 | 97 | 232 | 0.29 | 1.5 | No |
| 2a | 3.6 | 2.47 | 1.74 | 135 | 243 | 8.72 | 1.2 | Yes |
| 3a | 6.3 | 2.62 | 1.71 | 325 | 350 | 3.15 | 0.9 | Yes |
| 4a | 7 | 2.37 | 2.09 | 155 | 328 | 0.63 | 1.3 | Yes |
| 5 | 7.1 | 2.3 | 1.74 | 66 | 228 | 7.51 | 1.4 | Yes |
| 6a | 7.8 | 2.25 | 1.81 | 458 | 206 | 4.13 | 1.2 | Yes |
| 7a | 8.9 | 2.32 | 1.65 | 420 | 216 | 8.6 | 1.2 | Yes |
| 8 | 9.9 | 2.5 | 1.81 | 141 | 286 | 3.4 | 1.3 | Yes |
| 9a | 9.9 | 2.3 | 1.97 | 231 | 230 | 7.36 | 1.3 | Yes |
| 10a | 10.2 | 2.67 | 1.49 | 6 | 154 | 1.93 | 1.7 | No |
| 11a | 10.3 | 2.17 | 2.23 | 169 | 291 | 0.42 | 1.2 | Yes |
| 12 | 10.8 | 2.45 | 1.52 | 70 | 315 | 13.05 | 1.3 | Yes |
| 13 | 11 | 2.22 | 1.74 | 409 | 172 | 5.65 | 1.1 | Yes |
| 14a | 11.3 | 2.3 | 1.81 | 299 | 419 | 4.43 | 0.8 | Yes |
| 15a | 12.5 | 2.22 | 1.78 | 171 | 253 | 4.37 | 0.9 | Yes |
| 16a | 18.3 | 2.25 | 1.29 | 133 | 64 | 2.86 | 1.1 | Yes |
| 17 | 18.5 | 2.35 | 1.07 | 103 | 86 | 1.4 | 1.5 | No |
| 18a | 19.6 | 2.17 | 1.74 | 253 | 77 | 4.29 | 1.1 | Yes |
| 19a | 21.2 | 2.5 | 1.39 | 93 | 62 | 0.12 | 1.9 | Yes |
| 20 | 26.8 | 2.4 | 1.49 | 78 | 71 | 5.24 | 1.3 | No |
| 21 | 54 | 2.5 | 1.45 | 29 | 70 | 3.01 | 1 | Yes |
| 22a | 54.7 | 2.37 | 1.52 | 49 | 71 | 8.08 | 1.2 | No |
| Normal range | 2.18–2.58 | 0.74–1.52 | 10–65 | 30–120 | 0.5–4.5 | 0.7–1.8 | ||
| Adults | Adults | |||||||
| 1.29–2.26 | 100–320 | |||||||
| Children | Children |
sCalcium, Serum calcium; sPhosphate, serum phosphate; Alk phos, alkaline phosphatase.
On treatment with oral calcitriol and/or calcium.
Table 2.
Bone densitometry and clinical features
| Patient | Age (yr) | LS BMD Z-score | LS BMDhaz Z-score | TH Z-score | FN Z-score | TB Z-score | Tanner stage | Bone age (yr) | Height sd | BMI Z-score |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 3.3 | −0.3 | 1 | 6 | −4.53 | 0.14 | ||||
| 2 | 3.6 | −0.1 | 2.8 | 1 | 5 | 2.31 | 4.98 | |||
| 3 | 6.3 | 1.1 | 2.2 | 1.3 | 3.1 | 1 | 11 | 0.75 | 2.9 | |
| 4 | 7 | 2.2 | 2.2 | 2.5 | 1 | 1.5 | 1 | 11 | 1.29 | 2.52 |
| 5 | 7.1 | −0.4 | −0.4 | −1.1 | −2.1 | −1.2 | 1 | 7.83 | −2.11 | 2.06 |
| 6 | 7.8 | −1 | −1 | −0.8 | −1.4 | −1.2 | 1 | 6.33 | −1.77 | 2.06 |
| 7 | 8.9 | 0.9 | 0.9 | 1.6 | 1.2 | 0.1 | 1 | 13.5 | 0.56 | 1.93 |
| 8 | 9.9 | −0.4 | −0.4 | −1.1 | −0.9 | −0.6 | Early 2 | 15.5/11.6–12.6 | −0.58 | 2.14 |
| 9 | 9.9 | 0.9 | 0.9 | 0.5 | 0.3 | 1.8 | Early 2 | 10,11 | −0.2 | 2.7 |
| 10 | 10.2 | 0.3 | 0.3 | −0.5 | −0.8 | 0.5 | Early 2 | 8.67 | −1.17 | −0.73 |
| 11 | 10.3 | 1.2 | 1.2 | 0.6 | 0.3 | 1.6 | 3 | 13 | 0.26 | 1.73 |
| 12 | 10.8 | 1.1 | 1.1 | 1.4 | 1.1 | 1.8 | 3 | 12 | 0.88 | 2.02 |
| 13 | 11 | 1 | 1.0 | −0.4 | −0.3 | 0.4 | Early 2 | 13 | 1.33 | 2.38 |
| 14 | 11.3 | 0.4 | 0.4 | −0.6 | −1.1 | 0.5 | Early 3 | 14 | 0.13 | 1.28 |
| 15 | 12.5 | 1.3 | 1.3 | 0.1 | −0.2 | 1.1 | 4 | 16 | −0.67 | 2.02 |
| 16 | 18.3 | 1.5 | 1.0 | 2.3 | 2.1 | 5 | Adult | −1.9 | 2.42 | |
| 17 | 18.5 | −0.4 | 0.7 | −0.4 | 0.6 | 5 | Adult | −1.46 | 1.45 | |
| 18 | 19.6 | 0.7 | 2.2 | 2.3 | 4.8 | 5 | Adult | −1.79 | 2.26 | |
| 19 | 21.2 | −0.5 | −0.2 | 0.3 | −0.2 | 5 | Adult | −3.08 | 2.24 | |
| 20 | 26.8 | −1.1 | −1.5 | −1.5 | −0.4 | 5 | Adult | −0.59 | 0.05 | |
| 21 | 54 | −2.6 | −1.4 | −1.5 | 5 | Adult | −2.96 | 1.61 | ||
| 22 | 54.7 | 0.6 | 0.2 | 0.5 | 0.4 | 5 | Adult | −1.63 | 0.73 | |
| Mean Z-score (±sd) | 0.29 (±1.08) | 0.63 (±0.89) | 0.27 (±1.24) | 0.02 (±1.26) | 0.98 (±1.50) | 0.77 (±1.66) | 1.85 (±1.15) | |||
| Median Z-score (range) | 0.5 (−2.6–2.2) | 0.9 (0.3–2.2) | 0.15 (−1.5–2.5) | 0.05 (−2.1–2.3) | 0.55 (−1.2–4.8) | −0.63 (−4.5–2.3) | 2.04 (−0.73–4.9) |
BMD Z-scores for each subject for LS (L1–L4), TH, FN, and TB with age, Tanner stage, hand/wrist bone age, height sd, and BMI Z-score at the time of the DXA scan. The mean and median ± sd as well as the range are shown for BMD Z-scores at each DXA site and also for the height sd and BMI Z-score.
Laboratory evaluation
The biochemical characteristics of study subjects at the time of the DXA scans are shown in Table 1. Fourteen of 22 subjects were receiving calcitriol and calcium at the time that bone densitometry was performed (indicated by an asterisk in Table 1). Intact PTH levels ranged from 6 to 458 pg/ml, with elevated levels reflecting inadequate dosing of calcium and calcitriol vs. noncompliance with medication. No subjects had hypocalcemia, whereas one of the 22 subjects (subject 18) had hyperphosphatemia. Alkaline phosphatase levels were normal for age in all subjects except for subject 14, who had a mild elevation. Serum levels of 25-hydroxy-vitamin D were available for five of 22 subjects and were within the normal range for four of the five subjects (data not shown).
All patients were taking levothyroxine with the exception of patient 13, who was newly diagnosed. TSH levels were elevated in eight subjects; only one subject had a TSH concentration above 9 μIU/ml. Free T4 levels were normal in all subjects, consistent with very mild TSH resistance. Seventeen of 22 (77%) subjects were GH deficient at the time that BMD was measured, consistent with our prior data (15). None of the subjects had received GH in the past except patient 16 (18.3 yr old) who had received GH for a short period of time (ages 9.5–12.0 yr).
Anthropometric evaluation
We recently described the height, weight, and BMI characteristics of these subjects in detail (38). For the purposes of this study, we calculated BMI Z-scores to evaluate for a relationship between BMI and BMD Z-scores; Z-scores in adults were calculated using the age of 20 yr as the adult BMI standard (38). BMI Z-scores and height sds are shown in Table 2. The mean BMI Z-score (n = 22) was 1.86 ± 1.15. Ten of 15 children (67%) had a BMI Z-score greater than +2 sds. Three of seven adults (43%) had a BMI Z-score greater than +2 sds. The mean height sd in children (n = 15) was −0.2 ± 1.69, whereas the mean height sd in adults (n = 7) was −1.9 ± 0.87, further supporting our previous finding that children with PHP1a have normal stature until they undergo premature epiphyseal fusion resulting in adult short stature (16,35,38).
GNAS mutation analyses
Eighteen of 22 subjects had a confirmed mutation in one of the 13 coding exons of GNAS [data not shown (38)]. Southern blot analysis was performed in three (subjects 1, 5, and 22) of the four subjects (subjects 1, 5, 13, and 22) who did not have a confirmed GNAS mutation and showed normal maternal methylation of exon 1A, thus excluding an imprinting defect.
Bone measures
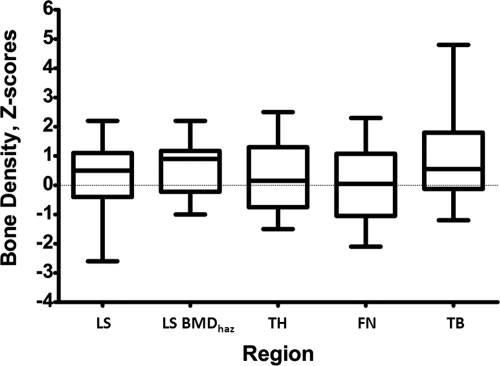
DXA BMD Z-scores for the LS (L1–L4), TH, FN, and TB are shown in Table 2 and Fig. 1. The mean BMD Z-score for the LS (n = 22) was 0.29 ± 1.08 (range −2.6 to 2.2), which is not significantly different from normal. Moreover, 14 subjects (64%) had Z-scores within ±1, similar to expected for the normative population. We also used a recently developed method that utilized a HAZ to adjust for the effect of stature on bone density to analyze LS BMD in study subjects between the ages of 7 and 17 yr (37). This generated a HAZ-adjusted BMD Z-score of 0.63 ± 0.89, which was significantly (P = 0.033) greater than normal. There is no generally accepted method to adjust for the effect of short stature on BMD in adults.
Figure 1.
Distribution of BMD Z-scores for LS (L1–L4), LS BMDhaz, and HAZ-adjusted TH, FN, and TB. The box-and-whisker plots indicate the minimum, 25th percentile, median, 75th percentile, and maximum observation for all BMD Z-scores.
As shown in Fig. 1, the mean BMD Z-score for the total hip (n = 20) was 0.27 ± 1.24 (range −1.5 to 2.5), and the HAZ-adjusted BMD Z-score for children (n = 12) was 0.19 ± 0.91, both not significantly different from normal. Eleven subjects (55%) had Z-scores within ±1: six (30%) between 0 and 1 and five (25%) between 0 and −1. Two subjects (10%) had Z-scores between greater than 1 and 2; four subjects (20%) had Z-scores between less than −1 and −2; three subjects (15%) had Z scores greater than 2; and no subjects had a Z-score of less than − 2.
The mean BMD Z-score for the FN (n = 20) was 0.02 ± 1.26 (range −2.1 to 2.3). Ten subjects (50%) had Z-scores within ±1: five (25%) between 0 and 1; five (25%) between 0 and −1. Three subjects (15%) had Z-scores between greater than 1 and 2; four subjects (20%) between less than −1 and −2; two subjects (10%) had Z-scores greater than 2; and one patient (5%) had a Z-score less than −2.
The mean Z-score for whole-body BMD (n = 20) was 0.98 ± 1.50 (range −1.2 to 4.8), which is significantly (P = 0.006) greater than normal. Nine subjects (45%) had Z-scores within ±1: six (30%) between 0 and 1 and three (15%) between 0 and −1. Five subjects (25%) had Z-scores between greater than 1 and 2; two subjects (10%) had Z-scores between less than −1 and −2; four subjects (20%) had Z-scores greater than 2; and no subjects had Z-scores less than −2.
Overall, only two subjects (9%) had BMD Z-scores less than −2. Five subjects (23%) had BMD-Z scores greater than +2.
Mean total body BMC Z-score was 0.32 ± 0.24 and not significantly different from normal controls.
Correlations
The height Z-score was positively associated with LS Z-score, as expected (r = 0.53, P = 0.01). Age was highly skewed, but the correlation between height Z-score and age showed a Spearman r of −0.5953, P = 0.0035.
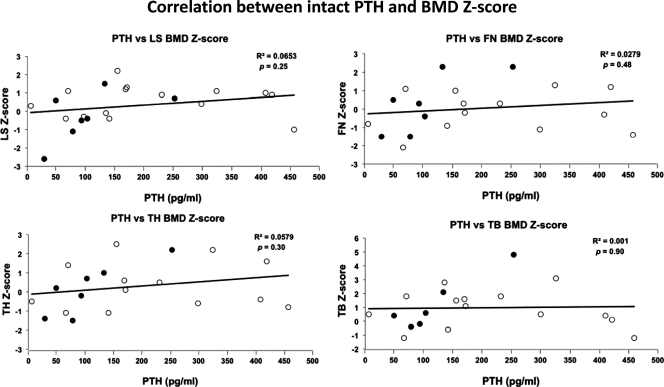
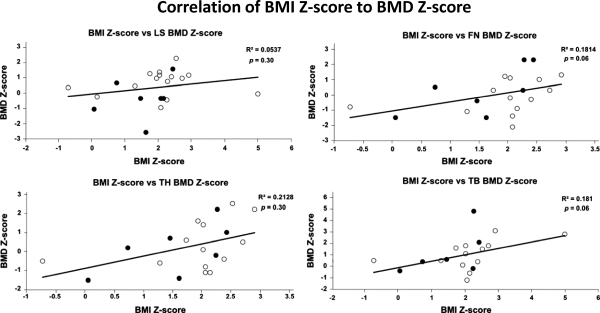
BMD Z-scores and BMC Z-scores were highly correlated (r = 0.78). The serum alkaline phosphatase levels correlated with LS Z-scores (r = 0.44, two tailed P = 0.04) but not with bone density measured at other sites. There were no significant correlations of serum phosphate levels and BMD at any sites. In addition, we found no correlations between intact PTH levels and BMD Z-scores at the LS, TH, FN, or TB sites (Fig. 2) for the patient population as a whole as well as for the children and adults when analyzed separately. There were also no correlations between BMI Z-scores and BMD Z-scores at the LS, TH, FN, and TB (Fig. 3). This was true for the entire group and the children and adults analyzed separately. Finally, we did not find a significant correlation between BMI Z-score and BMC Z-score for the TB (data not shown).
Figure 2.
Correlation between intact PTH levels and BMD Z-scores for LS (L1–L4), TH, FN, and TB. White circles indicate children (<18 yr old); black circles indicate adults (≥18 yr old). R2, Correlation coefficient, p, P value.
Figure 3.
Correlation of BMI Z-score and BMD Z-scores for LS (L1–L4), TH, FN, and TB. White circles indicate children (<18 yr old); black circles indicate adults (≥18 yr old). R2, Correlation coefficient, p, P value.
Total fat mass correlated with TB BMC (r = 0.77) but not TB BMC Z-score or TB BMD Z-score (data not shown). Similarly, lean tissue mass was highly correlated with TB BMC (r = 0.92) but not correlated with TB BMC Z-score or TB BMD Z-score.
Seventeen of 22 subjects (77%) were GH deficient at the time that subjects underwent bone densitometry, but there was no significant difference in mean BMD Z-scores at any site between the GH-deficient and GH-sufficient group (data not shown).
Sex steroids have been shown to play a critical role in the pubertal increase in bone mass (39). The adolescent and adult female subjects included in our study all entered puberty at an appropriate time. However, three of the four adult women had a history of secondary amenorrhea, and the fourth had experienced a premature menopause at age 40 yr. Despite evidence of gonadotropin resistance, two of the three women with secondary amenorrhea had BMD measurements greater than +2 sds.
Discussion
PTH can have both anabolic and catabolic effects on the skeleton. Constant elevation of PTH, as occurs in primary hyperparathyroidism, causes bone loss, with greater effects on cortical than cancellous bone (40). By contrast, intermittent elevations of PTH can increase bone mass, primarily at skeletal sites that are enriched for cancellous bone (40,41). Finally, a lack of PTH, as occurs in hypoparathyroidism, is associated with both increased bone density and reduced bone remodeling, which can result in either beneficial or potentially adverse effects, respectively (33,42,43,44).
Despite significant secondary hyperparathyroidism, we found that the subjects with PHP1a in our study had significantly increased whole-body bone density when compared with healthy control subjects of the same age and gender. Moreover, BMD Z-scores were normal in the LS, TH, and FN. Total body BMC was also not reduced.
Our results contrast with previous studies that suggest that BMD is decreased in PHP1a (20,27,28). However, these previous studies were limited by small sample size (30,31,32,34,43), did not discriminate between PHP1a and PHP1b, and were confounded by widely variable BMD Z-scores (33,34). For example, the largest series, reported by Breslau et al. (31), had only three PHP1a subjects. Our analysis of BMD in 22 subjects with PHP1a demonstrates that bone mass is either normal or increased at the four sites studied (LS, TH, FN, TB).
This study has several technical limitations. First, bone density is greatly influenced by pubertal status. Although pubertal maturation is often delayed in patients with PHP1a, we felt it would be inappropriate to apply an adjustment for bone age in our subjects because PHP1a children experience premature fusion of epiphyses and have markedly advanced bone ages from an early age (16). In addition, they exhibit inconsistencies between bone age in the hand/wrist and that in the lower extremity (16,35).
Second, although areal BMD (grams per square centimeter) as determined by DXA is the preferred method to assess and monitor bone disease in adults, this technique has important shortcomings when used in growing children. In particular, DXA is greatly influenced by bone size (45,46) because areal bone density is determined by only two rather than three dimensions. Thus, smaller patients, with smaller bones, have lower BMD measurements when DXA is used to assess bone density. The International Society of Clinical Densitometry has therefore recommended that spinal and total body (less head) BMC and areal BMD results for children with linear growth or maturational delay be adulated for absolute height or height age, or compared with pediatric reference data that provide Z-scores that are specific for age, sex, and height (47).
Accordingly, several corrections have been proposed to adjust BMD Z-scores in children who are short for age or who have a delayed puberty because mineral accrual in childhood is linked more closely to pubertal and skeletal maturation than to chronologic age (39). Although there are no accepted guidelines on how to adjust areal BMD for absolute height, we used a recently developed technique for adjustment of LS BMD by HAZ that provides significant advantages over the height-age correction that is commonly used (37). Although the application of this correction methodology to our LS data may be of limited validity because we did not use the same normative reference database that was used to develop the technique, this analysis showed that HAZ-adjusted BMD for the LS spine (LS BMDhazZ) was significantly greater in PHP1a subjects aged 7 through 17 yr than in normal subjects, thus confirming the results of the total body bone densitometry.
The World Health Organization criteria for assessing BMD is limited to postmenopausal women and relies on T-scores (sds from mean peak bone mass) to establish a diagnosis of osteopenia or osteoporosis. Thus, T-scores, and these criteria, cannot be used when studying children, adolescents, and young adults who have not yet reached peak bone mass (39). We therefore followed recommendations of the International Society for Clinical Densitometry and used Z scores to interpret DXA measurements and defined low BMD on the basis of age-adjusted Z-scores less than −2.0 (39). Using this standard, only two subjects (9%) met the criteria for low BMD; the FN BMD Z-score in patient 5 (child) and the LS BMD Z-score in patient 21 (adult). Both subjects had additional risk factors for low BMD; patient 5 had limited mobility due to joint problems in addition to frequently requiring oral glucocorticoid steroids for reactive airway disease; patient 21 had undergone early menopause at age 40 yr without subsequent hormone replacement in addition to requiring long-term glucocorticoid therapy for chronic lung disease.
PHP1a patients who are undertreated or noncompliant with calcium and calcitriol therapy typically have elevated PTH levels. We measured intact PTH at the same time that we measured bone density, and in 18 patients (82%), the PTH values at the time of the DXA scan correlated with their chronic parathyroid homeostasis over the prior years (many of the patients who were referred had received inadequate treatment). We expected that high PTH levels would be associated with low BMD Z-scores, especially at sites with a greater proportion of cortical vs. trabecular bone. As depicted in Fig. 2, no correlation was found between BMD Z-scores and PTH levels at any of the BMD sites studied. Both subjects with BMD Z-scores of less than −2 (subjects 5 and 21) had normal PTH levels (66 and 29 pg/ml, respectively). Furthermore, patient 18 had the highest total body Z-score of 4.8 with a PTH level of 253 pg/ml.
There are several potential explanations for increased bone density in PHP1a. First, haploinsufficiency of Gαs in bone cells may reduce PTH-dependent bone remodeling similar to that which occurs in rodents (48,49) and humans with hypoparathyroidism (44,50,51). Although the presence of brachydactyly in patients with PHP1a implies defective PTHr1 signaling in the growth plate (19,20), other studies have shown apparently normal PTH responsiveness in vitro of bone cells from patients with PHP1a (30) as well as the development of osteitis fibrosa cystica in some patients with PHP1a (27,30). Thus, it is conceivable that increased bone density in PHP1a results from the short half-life of calcitriol, which can cause fluctuations in serum calcium levels and thereby induce changes in serum PTH that resemble those achieved by intermittent administration of PTH.
Recent clinical studies indicated that daily administration of recombinant human PTH 1-34 (40,41) and recombinant human PTH1-84 (40,41,52,53,54) significantly increases BMD, particularly at skeletal sites that are enriched for cancellous bone and reduces fracture risk. However, sites enriched for cortical bone show smaller increases (e.g. total hip) or actual decreases (distal radius) in BMD (54). By contrast, we found that whole-body BMD, which represents predominately cortical bone, was significantly increased in patients with PHP1a and cancellous BMD was only modestly increased. These observations are inconsistent with the typical anabolic effects of PTH on the skeleton (55).
Alternatively, increased total-body BMD in patients with PHP1a may reflect the effect of altered Gαs signaling in the brain. Recent work suggests the existence of a neural control mechanism for bone mass that uses both positive and negative mediators of bone formation and resorption. One control pathway involves leptin signaling, and one of the mediators expressed in hypothalamic neurons that leptin uses to inhibit osteoclast differentiation and thereby bone resorption is cocaine- and amphetamine-regulated transcript. Cocaine- and amphetamine-regulated transcript expression is also increased in both humans (56) and mice (57) lacking only one allele of the melanocortin 4 receptor (MC4R), a Gs-coupled receptor that is highly expressed in hypothalamic neurons and that controls energy intake and energy expenditure. Remarkably, deficiency of MC4R leads to not only obesity but also decreased bone resorption and high bone mass (57). The recent observation (58) that mice with mutations in maternal Gnas (i.e. replicating PHP1a) that are limited to the central nervous system have some of the same features as those of MC4R mutations (obesity, reduced sympathetic nervous system activity and energy expenditure, and impaired glucose metabolism) suggests that increased bone density in PHP1a may derive from disordered hypothalamic signaling rather than defective PTH action on the skeleton. In this situation one would expect that patients with paternal GNAS mutations (i.e. PPHP) would not develop increased bone density similar to their lack of abnormal weight gain (38).
Because low BMD is associated with an increased fracture risk in both adults (59) and children (60,61), our findings may have important clinical implications. Specifically, because increased whole-body BMD represents an increase predominately in cortical bone, which contributes to overall mechanical strength of the skeleton, PHP1a patients may have a reduced risk for fracture. Future studies of BMD in patients with PPHP will be needed to assess the relative effects of elevated levels of PTH and Gαs haploinsufficiency on the skeleton.
Acknowledgments
We acknowledge the support of all the patients and their families who made this research possible. The authors also are grateful to Dr. Mary Leonard for critical review of the manuscript.
Footnotes
This work was supported by U.S. Food and Drug Administration Orphan Products Development Grant R01 FD-R-002568 (to E.L.G.-L.), Thrasher Research Foundation Grant 02818-8 (to E.L.G.-L.), National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases Grant T32 DK007751 (to the Johns Hopkins University School of Medicine Endocrinology Fellowship Training Program, D.N.L.), and National Institutes of Health/National Center for Research Resources Grant UL1 RR 025005 (a component of the National Institutes of Health Roadmap for Medical Research; the contents of this paper are solely the responsibility of the authors and do not necessarily represent the official view of National Center for Research Resources/National Institutes of Health.) This research was also supported by the generosity of the Bosworth and Friedman families.
Disclosure Statement: The authors have nothing to disclose.
First Published Online July 7, 2010
Abbreviations: AHO, Albright hereditary osteodystrophy; BMC, bone mineral content; BMD, bone mineral density; BMI, body mass index; DXA, dual-energy x-ray absorptiometry; FN, femoral neck; Gαs, α-chain of the stimulatory guanine nucleotide-binding protein; HAZ, height-for-age Z-score; LS, lumbar spine; LS BMDhazZ, HAZ-adjusted BMD for the LS spine; MC4R, melanocortin 4 receptor; PHP, pseudohypoparathyroidism; PPHP, pseudopseudohypoparathyroidism; PTHR1, PTHrP receptor; TB, total body; TH, total hip.
References
- Levine MA, Germain-Lee E, Jan de Beur SM 2003 Genetic basis for resistance to parathyroid hormone. Horm Res 60(Suppl 3):87–95 [DOI] [PubMed] [Google Scholar]
- Plagge A, Kelsey G, Germain-Lee EL 2008 Physiological functions of the imprinted Gnas locus and its protein variants Gαs and XLαs in human and mouse. J Endocrinol 196:193–214 [DOI] [PubMed] [Google Scholar]
- Germain-Lee EL, Ding CL, Deng Z, Crane JL, Saji M, Ringel MD, Levine MA 2002 Paternal imprinting of Gαs in the human thyroid as the basis of TSH resistance in pseudohypoparathyroidism type 1a. Biochem Biophys Res Commun 296:67–72 [DOI] [PubMed] [Google Scholar]
- Mantovani G, Ballare E, Giammona E, Beck-Peccoz P, Spada A 2002 The Gαs gene: predominant maternal origin of transcription in human thyroid gland and gonads. J Clin Endocrinol Metab 87:4736–4740 [DOI] [PubMed] [Google Scholar]
- Liu J, Erlichman B, Weinstein LS 2003 The stimulatory G protein α-subunit Gsα is imprinted in human thyroid glands: implications for thyroid function in pseudohypoparathyroidism types 1A and 1B. J Clin Endocrinol Metab 88:4336–4341 [DOI] [PubMed] [Google Scholar]
- Hayward BE, Barlier A, Korbonits M, Grossman AB, Jacquet P, Enjalbert A, Bonthron DT 2001 Imprinting of the G(s)α gene GNAS1 in the pathogenesis of acromegaly. J Clin Invest 107:R31–R36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain-Lee EL, Schwindinger W, Crane JL, Zewdu R, Zweifel LS, Wand G, Huso DL, Saji M, Ringel MD, Levine MA 2005 A mouse model of Albright hereditary osteodystrophy generated by targeted disruption of exon 1 of the Gnas gene. Endocrinology 146:4697–4709 [DOI] [PubMed] [Google Scholar]
- Shore EM, Ahn J, Jan de Beur S, Li M, Xu M, Gardner RJ, Zasloff MA, Whyte MP, Levine MA, Kaplan FS 2002 Paternally inherited inactivating mutations of the GNAS1 gene in progressive osseous heteroplasia. N Engl J Med 346:99–106 [DOI] [PubMed] [Google Scholar]
- de Nanclares GP, Fernández-Rebollo E, Santin I, Garcia-Cuartero B, Gaztambide S, Menéndez E, Morales MJ, Pombo M, Bilbao JR, Barros F, Zazo N, Ahrens W, Jüppner H, Hiort O, Castaño L, Bastepe M 2007 Epigenetic defects of GNAS in patients with pseudohypoparathyroidism and mild features of Albright’s hereditary osteodystrophy. J Clin Endocrinol Metab 92:2370–2373 [DOI] [PubMed] [Google Scholar]
- Levine MA, Zapalowski C, Kappy MS 2005 Disorders of calcium, phosphate, parathyroid hormone and vitamin D. In: Kappy MS, Allen DB, Geffner ME, eds. Principles and practice of pediatric endocrinology. Springfield, IL: Charles C. Thomas; 695–814 [Google Scholar]
- Linglart A, Gensure RC, Olney RC, Jüppner H, Bastepe M 2005 A novel STX16 deletion in autosomal dominant pseudohypoparathyroidism type Ib redefines the boundaries of a cis-acting imprinting control element of GNAS. Am J Hum Genet 76:804–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastepe M, Fröhlich LF, Hendy GN, Indridason OS, Josse RG, Koshiyama H, Körkkö J, Nakamoto JM, Rosenbloom AL, Slyper AH, Sugimoto T, Tsatsoulis A, Crawford JD, Jüppner H 2003 Autosomal dominant pseudohypoparathyroidism type Ib is associated with a heterozygous microdeletion that likely disrupts a putative imprinting control element of GNAS. J Clin Invest 112:1255–1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastepe M, Fröhlich LF, Linglart A, Abu-Zahra HS, Tojo K, Ward LM, Jüppner H 2005 Deletion of the NESP55 differentially methylated region causes loss of maternal GNAS imprints and pseudohypoparathyroidism type Ib. Nat Genet 37:25–27 [DOI] [PubMed] [Google Scholar]
- Jüppner H, Linglart A, Fröhlich LF, Bastepe M 2006 Autosomal-dominant pseudohypoparathyroidism type Ib is caused by different microdeletions within or upstream of the GNAS locus. Ann NY Acad Sci 1068:250–255 [DOI] [PubMed] [Google Scholar]
- Ferrandon S, Feinstein TN, Castro M, Wang B, Bouley R, Potts JT, Gardella TJ, Vilardaga JP 2009 Sustained cyclic AMP production by parathyroid hormone receptor endocytosis. Nat Chem Biol 5:734–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain-Lee EL, Groman J, Crane JL, Jan de Beur SM, Levine MA 2003 Growth hormone deficiency in pseudohypoparathyroidism type 1a: Another manifestation of multi-hormone resistance. J Clin Endocrinol Metab 88:4059–4069 [DOI] [PubMed] [Google Scholar]
- Bastepe M, Weinstein LS, Ogata N, Kawaguchi H, Jüppner H, Kronenberg HM, Chung UI 2004 Stimulatory G protein directly regulates hypertrophic differentiation of growth plate cartilage in vivo. Proc Natl Acad Sci USA 101:14794–14799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto A, Chen M, Kobayashi T, Kronenberg HM, Weinstein LS 2005 Chondrocyte-specific knockout of the G protein G(s)α leads to epiphyseal and growth plate abnormalities and ectopic chondrocyte formation. J Bone Miner Res 20:663–671 [DOI] [PubMed] [Google Scholar]
- Karaplis AC, Luz A, Glowacki J, Bronson RT, Tybulewicz VL, Kronenberg HM, Mulligan RC 1994 Lethal skeletal dysplasia from targeted disruption of the parathyroid hormone-related peptide gene. Genes Dev 8:277–289 [DOI] [PubMed] [Google Scholar]
- Klopocki E, Hennig BP, Dathe K, Koll R, de Ravel T, Baten E, Blom E, Gillerot Y, Weigel JF, Kruger G, Hiort O, Seemann P, Mundlos S 2010 Deletion and point mutations of PTHLH cause brachydactyly type E. Am J Hum Genet 86:434–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobert AS, Zhang P, Couvineau A, Bonaventure J, Roume J, Le Merrer M, Silve C 1998 Absence of functional receptors for parathyroid hormone and parathyroid hormone-related peptide in Blomstrand chondrodysplasia. J Clin Invest 102:34–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani G, Bondioni S, Locatelli M, Pedroni C, Lania AG, Ferrante E, Filopanti M, Beck-Peccoz P, Spada A 2004 Biallelic expression of the Gsα gene in human bone and adipose tissue. J Clin Endocrinol Metab 89:6316–6319 [DOI] [PubMed] [Google Scholar]
- Kidd GS, Schaaf M, Adler RA, Lassman MN, Wray HL 1980 Skeletal responsiveness in pseudohypoparathyroidism: a spectrum of clinical disease. Am J Med 68:772–781 [DOI] [PubMed] [Google Scholar]
- Murray TM, Rao LG, Wong MM, Waddell JP, McBroom R, Tam CS, Rosen F, Levine MA 1993 Pseudohypoparathyroidism with osteitis fibrosa cystica: direct demonstration of skeletal responsiveness to parathyroid hormone in cells cultured from bone. J Bone Miner Res 8:83–91 [DOI] [PubMed] [Google Scholar]
- Eubanks PJ, Stabile BE 1998 Osteitis fibrosa cystica with renal parathyroid hormone resistance: a review of pseudohypoparathyroidism with insight into calcium homeostasis. Arch Surg 133:673–676 [DOI] [PubMed] [Google Scholar]
- Cohen RD, Vince FP 1969 Pseudohypoparathyroidism with raised plasma alkaline phosphatase. Arch Dis Child 44:96–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb FO, Steinbach HL 1962 Pseudohypoparathyroidism with secondary hyperparathyroidism and osteitis fibrosa. J Clin Endocrinol Metab 22:59–70 [DOI] [PubMed] [Google Scholar]
- Giraud P, Audran M, Rohmer V, Jallet P, Basle MF, Bregeon C, Bigorgne JC 1995 Direct effect of calcitriol on the regulation of parathyroid hormone secretion in a case of pseudo-hypoparathyroidism (a 24-month follow-up study). Clin Rheumatol 14:287–291 [DOI] [PubMed] [Google Scholar]
- Tollin SR, Perlmutter S, Aloia JF 2000 Serial changes in bone mineral density and bone turnover after correction of secondary hyperparathyroidism in a patient with pseudohypoparathyroidism type Ib. J Bone Miner Res 15:1412–1416 [DOI] [PubMed] [Google Scholar]
- Ish-Shalom S, Rao LG, Levine MA, Fraser D, Kooh SW, Josse RG, McBroom, Wong MM, Murray TM 1996 Normal parathyroid hormone responsiveness of bone-derived cells from a patient with pseudohypoparathyroidism. J Bone Miner Res 11:8–14 [DOI] [PubMed] [Google Scholar]
- Breslau NA, Moses AM, Pak CYC 1983 Evidence for bone remodeling but lack of calcium mobilization response to parathyroid hormone in pseudohypoparathyroidism. J Clin Endocrinol Metab 57:638–644 [DOI] [PubMed] [Google Scholar]
- Kanatani M, Sugimoto T, Kaji H, Ikeda K, Chihara K 2001 Skeletal responsiveness to parathyroid hormone in pseudohypoparathyroidism. Eur J Endocrinol 144:263–269 [DOI] [PubMed] [Google Scholar]
- Touliatos JS, Sebes JI, Hinton A, McCommon D, Karas JG, Palmieri GM 1995 Hypoparathyroidism counteracts risk factors for osteoporosis. Am J Med Sci 310:56–60 [DOI] [PubMed] [Google Scholar]
- Ahmed SF, Russell S, Rashid R, Beattie TJ, Murphy AV, Ramage IJ, Maxwell H 2005 Bone mineral content, corrected for height or bone area, measured by DXA is not reduced in children with chronic renal disease or in hypoparathyroidism. Pediatr Nephrol 20:1466–1472 [DOI] [PubMed] [Google Scholar]
- Germain-Lee EL 2006 Short stature, obesity, and growth hormone deficiency in pseudohypoparathyroidism type 1a. Pediatr Endocrinol Rev 3(Suppl 2):318–327 [PubMed] [Google Scholar]
- Ellis KJ, Shypailo RJ 1998 Bone mineral and body composition measurements: cross-calibration of pencil-beam and fan-beam dual-energy X-ray absorptiometers. J Bone Miner Res 13:1613–1618 [DOI] [PubMed] [Google Scholar]
- Zemel BS, Leonard MB, Kelly A, Lappe JM, Gilsanz V, Oberfield S, Mahboubi S, Shepherd JA, Hangartner TN, Frederick MM, Winer KK, Kalkwarf HJ 2010 Height adjustment in assessing dual energy X-ray absorptiometry measurements of bone mass and density in children. J Clin Endocrinol Metab 95:1265–1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long DN, McGuire S, Levine MA, Weinstein LS, Germain-Lee EL 2007 Body mass index differences in pseudohypoparathyroidism type 1a versus pseudopseudohypoparathyroidism may implicate paternal imprinting of Gαs in the development of human obesity. J Clin Endocrinol Metab 92:1073–1079 [DOI] [PubMed] [Google Scholar]
- Bachrach LK 2005 Osteoporosis and measurement of bone mass in children and adolescents. Endocrinol Metab Clin North Am 34:521–535, vii [DOI] [PubMed] [Google Scholar]
- Rubin MR, Cosman F, Lindsay R, Bilezikian JP 2002 The anabolic effects of parathyroid hormone. Osteoporos Int 13:267–277 [DOI] [PubMed] [Google Scholar]
- Thomas T 2006 Intermittent parathyroid hormone therapy to increase bone formation. Joint Bone Spine 73:262–269 [DOI] [PubMed] [Google Scholar]
- Laway BA, Goswami R, Singh N, Gupta N, Seith A 2006 Pattern of bone mineral density in patients with sporadic idiopathic hypoparathyroidism. Clin Endocrinol (Oxf) 64:405–409 [DOI] [PubMed] [Google Scholar]
- Chan FK, Tiu SC, Choi KL, Choi CH, Kong AP, Shek CC 2003 Increased bone mineral density in patients with chronic hypoparathyroidism. J Clin Endocrinol Metab 88:3155–3159 [DOI] [PubMed] [Google Scholar]
- Rubin MR, Dempster DW, Zhou H, Shane E, Nickolas T, Sliney J, Silverberg SJ, Bilezikian JP 2008 Dynamic and structural properties of the skeleton in hypoparathyroidism. J Bone Miner Res 23:2018–2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaney RP 2003 Bone mineral content, not bone mineral density, is the correct bone measure for growth studies. Am J Clin Nutr 78:350–351; author reply 351–352 [DOI] [PubMed] [Google Scholar]
- Nevill AM, Holder RL, Maffulli N, Cheng JC, Leung SS, Lee WT, Lau JT 2002 Adjusting bone mass for differences in projected bone area and other confounding variables: an allometric perspective. J Bone Miner Res 17:703–708 [DOI] [PubMed] [Google Scholar]
- Gordon CM, Bachrach LK, Carpenter TO, Crabtree N, El-Hajj Fuleihan G, Kutilek S, Lorenc RS, Tosi LL, Ward KA, Ward LM, Kalkwarf HJ 2008 Dual energy X-ray absorptiometry interpretation and reporting in children and adolescents: the 2007 ISCD Pediatric Official Positions. J Clin Densitom 11:43–58 [DOI] [PubMed] [Google Scholar]
- Ueno Y, Shinki T, Nagai Y, Murayama H, Fujii K, Suda T 2003 In vivo administration of 1,25-dihydroxyvitamin D3 suppresses the expression of RANKL mRNA in bone of thyroparathyroidectomized rats constantly infused with PTH. J Cell Biochem 90:267–277 [DOI] [PubMed] [Google Scholar]
- Miao D, He B, Lanske B, Bai XY, Tong XK, Hendy GN, Goltzman D, Karaplis AC 2004 Skeletal abnormalities in Pth-null mice are influenced by dietary calcium. Endocrinology 145:2046–2053 [DOI] [PubMed] [Google Scholar]
- Langdahl BL, Mortensen L, Vesterby A, Eriksen EF, Charles P 1996 Bone histomorphometry in hypoparathyroid patients treated with vitamin D. Bone 18:103–108 [DOI] [PubMed] [Google Scholar]
- Rubin MR, Dempster DW, Kohler T, Stauber M, Zhou H, Shane E, Nickolas T, Stein E, Sliney Jr J, Silverberg SJ, Bilezikian JP, Müller R 2010 Three dimensional cancellous bone structure in hypoparathyroidism. Bone 46:190–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A, Dempster DW, Müller R, Guo XE, Nickolas TL, Liu XS, Zhang XH, Wirth AJ, van Lenthe GH, Kohler T, McMahon DJ, Zhou H, Rubin MR, Bilezikian JP, Lappe JM, Recker RR, Shane E 2010 Assessment of trabecular and cortical architecture and mechanical competence of bone by high-resolution peripheral computed tomography: comparison with transiliac bone biopsy. Osteoporos Int 21:263–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin MR, Sliney Jr J, McMahon DJ, Silverberg SJ, Bilezikian JP 22 January 2010 Therapy of hypoparathyroidism with intact parathyroid hormone. Osteoporos Int 10.1007/s00198-009-1149x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenspan SL, Bone HG, Ettinger MP, Hanley DA, Lindsay R, Zanchetta JR, Blosch CM, Mathisen AL, Morris SA, Marriott TB 2007 Effect of recombinant human parathyroid hormone (1-84) on vertebral fracture and bone mineral density in postmenopausal women with osteoporosis: a randomized trial. Ann Intern Med 146:326–339 [DOI] [PubMed] [Google Scholar]
- Hodsman AB, Hanley DA, Ettinger MP, Bolognese MA, Fox J, Metcalfe AJ, Lindsay R 2003 Efficacy and safety of human parathyroid hormone-(1-84) in increasing bone mineral density in postmenopausal osteoporosis. J Clin Endocrinol Metab 88:5212–5220 [DOI] [PubMed] [Google Scholar]
- Yeo GS, Lank EJ, Farooqi IS, Keogh J, Challis BG, O'Rahilly S 2003 Mutations in the human melanocortin-4 receptor gene associated with severe familial obesity disrupts receptor function through multiple molecular mechanisms. Hum Mol Genet 12:561–574 [DOI] [PubMed] [Google Scholar]
- Ahn JD, Dubern B, Lubrano-Berthelier C, Clement K, Karsenty G 2006 Cart overexpression is the only identifiable cause of high bone mass in melanocortin 4 receptor deficiency. Endocrinology 147:3196–3202 [DOI] [PubMed] [Google Scholar]
- Chen M, Wang J, Dickerson KE, Kelleher J, Xie T, Gupta D, Lai EW, Pacak K, Gavrilova O, Weinstein LS 2009 Central nervous system imprinting of the G protein G(s)α and its role in metabolic regulation. Cell Metab 9:548–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siris ES, Miller PD, Barrett-Connor E, Faulkner KG, Wehren LE, Abbott TA, Berger ML, Santora AC, Sherwood LM 2001 Identification and fracture outcomes of undiagnosed low bone mineral density in postmenopausal women: results from the National Osteoporosis Risk Assessment. JAMA 286:2815–2822 [DOI] [PubMed] [Google Scholar]
- Clark EM, Tobias JH, Ness AR 2006 Association between bone density and fractures in children: a systematic review and meta-analysis. Pediatrics 117:e291–e297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark EM, Ness AR, Bishop NJ, Tobias JH 2006 Association between bone mass and fractures in children: a prospective cohort study. J Bone Miner Res 21:1489–1495 [DOI] [PMC free article] [PubMed] [Google Scholar]