Abstract
Objectives: We tested whether African-American (AA) women are different from Caucasian women in regard to lipotoxicity, adipokines, and gene expression in adipose tissue and muscle.
Design: Insulin sensitivity (SI), plasma adipocytokine levels, intramyocellular lipid (IMCL), and the expression of candidate genes in adipose tissue and muscle were measured in AA and Caucasian women.
Setting: This study was performed in an ambulatory general clinical research center.
Subjects: Subjects were healthy, nondiabetic AA and Caucasian women.
Interventions: There were no interventions.
Main Outcome Measures: Comparison of SI, IMCL, plasma adiponectin, and the expression of candidate genes regulating adipogenesis, lipogenesis, and inflammation in adipose tissue and muscle.
Results: AA had lower plasma adiponectin and IMCL when compared with Caucasian women with similar SI. In sc adipose tissue (SAT), the expression of genes involved in adipogenesis including peroxisomal proliferator-activated receptor-γ (PPARγ) and lipin-1β were also reduced in SAT of AA subjects (19%, P = 0.06, and 25%, P = 0.05, respectively). Similarly, 1-acylglycerol-3-phosphate acyltransferase 2 (AGPAT 2), stearoyl-coenzyme A desaturase-1 (SCD1), and CD36 mRNA expression was significantly reduced in SAT by 19, 54, and 28% respectively (P < 0.01 for all) in AA compared with Caucasian women. Yet the expression of CD68 in SAT was similar in both ethnic groups. Gene expression studies in muscle revealed a 31% reduction in expression of AGPAT 2 and a 72% reduction in SCD1 genes in AA.
Conclusion: AA women demonstrated lower expression of several PPARγ-responsive genes in adipose tissue, lower plasma adiponectin, and decreased IMCL levels as compared with Caucasians, which suggests that African-Americans may be protected from lipotoxicity. Together these data suggest significant ethnic differences in the pathophysiology of insulin resistance.
Data indicate significant ethnic differences in the pathophysiology of insulin resistance between African-American and Caucasian women.
The rising incidence of diabetes (1) has paralleled that of obesity (2), particularly among minority populations. The recently reported prevalence of obesity among African-Americans (AAs) has been 51% higher than non-Hispanic Caucasians (2). In addition, AAs have a higher prevalence of type 2 diabetes when compared with Caucasians (3,4,5). Therefore, it is plausible that the increased prevalence of obesity in this ethnic group predisposes them to develop diabetes. However, a cross-sectional study by Haffner et al. (6) failed to fully explain the excess insulin resistance and risk for diabetes in AAs by the increased prevalence of obesity (6).
Whereas the scientific basis of obesity-induced insulin resistance and diabetes continues to evolve, several mechanistic pathways are thought to be crucial, including the pattern of adipose tissue distribution, rate of ectopic fat accumulation, and dysregulation of adipokines. However, as far as adipose tissue distribution is concerned, AA subjects have less visceral (7,8) and intrahepatic fat independent of age and total adiposity when compared with their Caucasian counterparts (8,9). The data on the effect of ethnicity on intramyocellular lipid content are limited and conflicting as well (9,10). In addition to the aforementioned differences in the distribution of fat between ethnic groups, AAs are known to have lower plasma adiponectin compared with Caucasians (11,12). Therefore, the increased risk of diabetes among AAs cannot be solely explained by higher prevalence of obesity or other commonly known contributors such as the pattern of fat distribution.
Lipodystrophy is associated with insulin resistance (13), and it has been proposed that insulin resistance can be triggered by a failure in adipogenesis; peroxisomal proliferator-activated receptor-γ (PPARγ) is one of the key transcriptional factors regulating adipogenesis (14). Dubois et al. (15) showed PPARγ expression is decreased in adipose tissue of obese subjects with type 2 diabetes. There are several downstream genes under the transcriptional control of PPARγ, including fatty acid translocase (CD36), lipin-β, and stearoyl-coenzyme A desaturase-1 (SCD1) (16,17). Yet it is not known whether the expression of PPARγ and other downstream genes are different in AAs when compared with Caucasians.
We hypothesized that there would be differences between AA and Caucasian individuals in the expression of genes regulating adipogenesis, lipogenesis, and inflammation in adipose tissue, resulting in differences in the amount of intramyocellular fat between ethnic groups. Hence, we examined adipose tissue lipid distribution, intramyocellular lipid, and gene expression in adipose tissue and muscle in AA and Caucasian women.
Subjects and Methods
Study population
All subjects provided written, informed consent under a protocol that was approved by the Institutional Review Board of the University of Arkansas for Medical Sciences. Caucasian and AA nondiabetic subjects in good health, between 21 and 65 yr of age and with body mass index (BMI) between 18 and 40 kg/m2, were recruited by local advertisement. Because of the gender differences in the expression of genes regulating insulin sensitivity such as adiponectin, only the data from women were used in this study. A total of 83 Caucasian and 34 AA women were included in this study (Table 1); however, the gene expression was studied in 29 subjects in each ethnic group (Table 1) due to limited biopsy specimens and an attempt to minimize differences in the baseline characteristics.
Table 1.
Baseline characteristics of subjects
| AA (n = 34) | Caucasian (n = 83) | P | AA (n = 29)a | Caucasian (n = 29)a | Pa | |
|---|---|---|---|---|---|---|
| Age (yr) | 39 ± 1.9 | 44 ± 0.9 | 0.01 | 38 ± 2.0 | 41 ± 0.7 | 0.16 |
| BMI (kg/m2) | 31.4 ± 0.8 | 32.0 ± 0.5 | 0.51 | 32 ± 0.8 | 32 ± 0.9 | 0.84 |
| Body fat (%) | 39 ± 1.2 | 43 ± 0.5 | 0.002 | 40 ± 1.1 | 42 ± 0.9 | 0.08 |
| Waist (cm) | 98.0 ± 2.7 | 98.0 ± 1.7 | 0.63 | 98 ± 2.8 | 97 ± 2.7 | 0.80 |
| Hip (cm) | 113.0 ± 3.0 | 112.3 ± 1.3 | 0.80 | 115 ± 3.2 | 113 ± 2.4 | 0.59 |
| Waist to hip ratio | 0.86 ± 0.02 | 0.87 ± 0.01 | 0.52 | 0.86 ± 0.02 | 0.86 ± 0.02 | 0.99 |
| SI [×10−4 min−1/(μU/ml)] | 2.88 ± 0.38 | 2.83 ± 0.24 | 0.91 | 2.97 ± 0.44 | 2.82 ± 0.42 | 0.81 |
| AIRg (μU/ml) | 567 ± 75 | 422 ± 36 | 0.05 | 634 ± 82 | 435 ± 57 | 0.05 |
| Fasting insulin (μU/ml) | 9.7 ± 1.3 | 8.3 ± 1.0 | 0.44 | 10.2 ± 1.6 | 9.9 ± 2.7 | 0.95 |
| Fasting glucose (mg/dl) | 86 ± 2 | 87 ± 1 | 0.71 | 85.8 ± 2.2 | 86.7 ± 1.3 | 0.71 |
The reduced population has been used for gene expression studies.
Study design
All studies were performed in the General Clinical Research Center of the University of Arkansas for Medical Sciences. Body composition (percent body fat) was measured by dual x-ray absorptiometry. A standard 75-g oral glucose tolerance test was performed, and subjects with fasting glucose greater than 126 mg/dl or 2-h glucose greater than 200 mg/dl were excluded. An insulin modified (0.04 U/kg) frequently sampled iv glucose tolerance test was performed as described previously (18). Visits for menstruating women were timed such that the frequently sampled iv glucose tolerance test visit coincided with the follicular phase of the menstrual cycle. All subjects underwent an incisional sc adipose tissue (SAT) biopsy from the lower abdominal wall and a muscle biopsy from vastus lateralis as previously described (19). Single-slice computed tomography images were acquired at the level of the umbilicus and at the midpoint between the inguinal crease and the patella for subsequent analysis. All scans were performed using a General Electric Lightspeed computed tomography (GE Medical Systems, Milwaukee, WI) scanner and saved as DICOM images for analysis using the Slice-O-Matic version 4.3 software (Tomovision, Montréal, Canada).
Laboratory procedures
Insulin was measured using an immunochemiluminescent assay (MLT Assay, Wales, UK) in the General Clinical Research Center Core Laboratory of the University of Arkansas for Medical Sciences. This assay has sensitivity of 0.25 mU/liter for insulin, with 1% cross-reactivity with proinsulin and 4–8% coefficient of variation. Plasma glucose was measured in duplicate by a glucose oxidase assay. Insulin sensitivity was calculated from the insulin and glucose data using the MinMod Millennium program (20,21). Plasma adiponectin, IL-6, and TNFα were measured using ELISA method (R&D Systems, Minneapolis, MN) following the manufacturer’s instruction.
The lipid content of muscle fibers was measured using the oil red O Sudan-type dye staining technique, which predominantly stains triglycerides with an orange-red tint (19,22). Oil red O-stained muscle sections were magnified with an Olympus Provis (Tokyo, Japan) light microscope, and images were digitally captured by using a connected charge-coupled device camera (Sony, Tokyo, Japan). Fiber-typed and oil red O-stained fibers were matched. The oil red O staining intensity of either type 1 or 2 muscle fibers was quantified using National Institutes of Health Image program (http://rsb.info.nih.gov/nih-image/). By adjusting a density threshold, the software was set to recognize the presence of one fat droplet only if its highlighted surface was exceeding 0.40 μm2 or larger. Muscle lipid content was calculated by total area of lipid droplets in a given muscle fiber divided by the total area of the same fiber. The mean number of fibers analyzed per sample was 40 for type 1 and 2 muscle fibers.
Muscle fiber oxidative capacity was determined in each muscle fiber type by quantifying the activity of the mitochondria enzyme succinate dehydrogenase (SDH) (23). Briefly, slides labeled activity, about 6 μm thick, were placed in incubation medium containing a large quantity of succinate (60 μm). Control slides (tissue blanks) in which succinate was absent from the incubation medium, were also completed. Calculations were based on the concentration of 4-nitro blue tetrazolium chloride diformazan deposited within a muscle fiber. The reaction was stopped at 5 min by immediately rinsing sections (both tissue blanks and those with succinate) with distilled water. Sections were dried, mounted, and kept in the dark until images were scanned and digitalized (∼2–3 h).
Total RNA from adipose tissue and muscle were isolated using an RNAeasy minikit (QIAGEN, Valencia, CA) per the manufacturer’s instruction. The quantity and quality of the isolated RNA were determined by an Agilent 2100 bioanalyzer (Palo Alto, CA). Real-time RT-PCR was conducted as described previously (24). All data were expressed in relation to 18S RNA, in which the standard curves were generated using pooled RNA from the samples assayed. Therefore, the data represent arbitrary units that accurately compare each set of samples with each other but do not necessarily accurately compare samples between different assays. Primer sequences of 18S, CD68, leptin, lipin-β, SCD1, PPARγ2, CD36, and 1-acylglycerol-3-phosphate acyltransferase 2 (AGPAT 2) were as published previously (16,25). The other primer sequences were as follows: adipose tissue triglyceride lipase (ATGL), ACCAGCATCCAGTTCAACCT (forward), ATCCCTGCTTGCACATCTCT (reverse); ceramide kinase (CERK) 1, GAGAAGCTGACGTCCAGACC (forward), TATATCCGCTTGCCTTGTCC (reverse); serine palmitoyl transferase (SPT)-1, TGGAAGAGAGCACTGGGTCT (forward), GGGAGGAGGGAGACACTTCT (reverse).
Data analysis
The cross-sectional data from this study were analyzed using t tests to compare outcomes between Caucasians and AAs. Analysis of covariance was used to compare group means adjusted for potential confounders found to be imbalanced between the ethnic groups (Table 1). Results of comparisons between ethnic groups are presented in tabular format with mean ± se for each group, difference of the means, and its 95% confidence interval (CI) (estimated from 1000 bootstrap samples). The P values for unadjusted analyses from the t test and the adjusted P value from the analysis of covariance model are also included.
The P values from tests comparing group means were not adjusted for multiple comparisons because this is a study to identify putative factors involved in the development of insulin resistance and diabetes in Caucasians and AAs. Analyses were done using the R software package (http://www.r-project.org, version 2.7.2).
Results
A total 117 nondiabetic women (34 AAs) were recruited for this study. The majority of subjects in this cohort were overweight or obese defined by BMI greater than 25 kg/m2 (88% of AAs and 92% of Caucasians, P = 0.73). The proportion of postmenopausal women were similar between ethnic groups [18% in AAs and 24% of Caucasian (P = 0.68)]. AA women were younger but had similar BMI and waist and hip circumferences when compared with Caucasians (Table 1). Insulin sensitivity index (SI) were similar between the two groups (AAs: 2.88 ± 0.38 × 10−4 min−1/μU/ml and Caucasians: 2.83 ± 0.24 × 10−4 min−1); however, acute insulin response to glucose (AIRg) was increased in AA compared with Caucasian women (567 ± 75 vs. 422 ± 36 μU/ml, P = 0.05, respectively) (Table 1). The fasting insulin and glucose levels were similar between the two groups (Table 1); 4 AA and 5 Caucasian subjects (P = 0.44) had impaired fasting glucose.
Despite similar BMI, AA women had decreased total body fat with a different pattern of distribution when compared with Caucasian women. The area of SAT was higher in AA women, whereas visceral adipose tissue (VAT) was lower when compared with Caucasian women (Table 2), and AA women had a more favorable lipid profile compared with Caucasians (Table 2). At the same level of insulin sensitivity, AA had decreased plasma adiponectin (7.3 ± 1.3 vs. 10.5 ± 0.6 ng/ml, P = 0.01) but similar plasma TNFα and IL-6 levels when compared with Caucasian women (Table 2). In multivariate regression analysis adjusting for age, body fat, and AIRg, the differences between ethnic groups remained significant except for low-density lipoprotein cholesterol (Table 2).
Table 2.
Unadjusted and adjusted ethnic group metabolic differences
| Outcome | n (AA/Caucasian) | AA | Caucasian | AA-Caucasian (95% CI) | P value unadjusted | P value adjusted |
|---|---|---|---|---|---|---|
| Lipids | ||||||
| LDL C (mg/dl) | 29/69 | 100.7 ± 6.4 | 115.3 ± 3.9 | −14.6 (−29.0, −0.01) | 0.05 | 0.06 |
| HDL C (mg/dl) | 29/71 | 59.5 ± 2.5 | 53.0 ± 1.7 | 6.5 (+0.6, +12.4) | 0.04 | 0.05 |
| TG (mg/dl) | 33/74 | 80.4 ± 6.1 | 143.4 ± 10.0 | −63.0 (−88.0, −38.9) | <0.001 | 0.001 |
| Abdominal fat distribution | ||||||
| SAT (cm2) | 16/36 | 562.0 ± 36.7 | 471.2 ± 17.5 | 89.8 (+12.4, +168.21) | 0.02 | 0.001 |
| VAT (cm2) | 16/36 | 119.6 ± 11.8 | 181.8 ± 9.2 | −62.3 (−91.4, −32.7) | <0.001 | 0.002 |
| Adipocytokines | ||||||
| Adiponectin (ng/ml) | 16/54 | 7.3 ± 0.8 | 10.4 ± 0.6 | −3.1 (−4.8, −1.0) | 0.01 | 0.01 |
| IL-6 (pg/ml) | 15/54 | 2.4 ± 0.4 | 2.5 ± 0.2 | −0.1 (−1.0, +0.9) | 0.87 | 0.96 |
| TNFα (pg/ml) | 15/55 | 1.6 ± 0.3 | 2.1 ± 0.2 | −0.4 (−1.1, +0.3) | 0.34 | 0.30 |
| Intramyocellular fat as a marker of lipotoxicity | ||||||
| IMCL (type 1 fiber) | 24/62 | 3.2 ± 0.3 | 4.1 ± 0.2 | −1.0 (−1.7, −0.3) | 0.02 | 0.09 |
| IMCL (type 2 fiber) | 24/63 | 1.4 ± 0.2 | 1.8 ± 0.2 | −0.4 (−0.8, −0.03) | 0.09 | 0.18 |
| SDHT (type 1 fiber) | 22/62 | 124.0 ± 13.9 | 122.0 ± 5.5 | 2.0 (−26.9, +32.3) | 0.88 | 0.84 |
| SDHT (type 2 fiber) | 22/62 | 49.3 ± 7.0 | 54.5 ± 3.0 | −5.2 (−19.2, 10.6) | 0.43 | 0.39 |
All outcomes were adjusted for age, body fat, and AIRg. HDL, High-density lipoprotein; LDL, low-density lipoprotein; C, cholesterol; TG, triglyceride.
Because adiponectin is expressed only in adipose tissue, we examined the expression of other adipose genes, including genes involved in the regulation of adipogenesis and lipogenesis in 29 women in each ethnic group who were similar in age, BMI, and SI (Table 1). The expression of PPARγ2 and lipin-1β, both of which are regulators of adipocyte differentiation, were decreased in AA compared with Caucasian women by 19% (P = 0.06) and 25% (P = 0.05), respectively (Table 3). In addition, several genes regulating triglyceride synthesis, AGPAT 2, SCD1 and CD36 were down-regulated in SAT of AA (by 19, 54, and 28%, respectively, P < 0.01 for all) when compared with Caucasian women (Table 3). ATGL, an enzyme regulating lipolysis in adipose tissue, was decreased in AAs by 22% (P = 0.01) compared with Caucasian women as well (Table 3). In multivariate regression analysis adjusting for age, body fat, and AIRg, the differences between ethnic groups remained significant (Table 3).
Table 3.
Unadjusted and adjusted ethnic group metabolic differences
| Genes | AA | Caucasian | (AA-Caucasian)/Caucasian × 100 (95% CI) | Unadjusted P value | Adjusted P value |
|---|---|---|---|---|---|
| SAT | |||||
| PPARγ2.18s | 0.98 ± 0.06 | 1.20 ± 0.11 | −18.76 (−33.79, +0.35) | 0.06 | 0.25 |
| Lipin-1β.18S | 2.42 ± 0.44 | 1.23 ± 0.14 | −25.45 (−44.18, −1.65) | 0.05 | 0.02 |
| AGPAT 2.18s | 0.95 ± 0.04 | 1.18 ± 0.07 | −19.47 (−29.56, −7.23) | 0.004 | 0.014 |
| SCD1.18S | 0.64 ± 0.12 | 1.38 ± 0.18 | −53.52 (−69.58, −27.89) | 0.001 | <0.001 |
| CD36.18S | 0.92 ± 0.08 | 1.38 ± 0.08 | −27.65 (−41.16, −11.88) | 0.001 | 0.009 |
| ATGL.18s | 0.89 ± 0.05 | 1.15 ± 0.08 | −22.37 (−33.93, −5.19) | 0.009 | 0.04 |
| Leptin.18S | 1.12 ± 0.08 | 0.98 ± 0.08 | +14.39 (−7.71, +42.89) | 0.24 | 0.19 |
| CD68.18S | 0.52 ± 0.07 | 0.67 ± 0.09 | −21.92 (−46.35, +16.28) | 0.22 | 0.56 |
| RBP4.18S | 1.05 ± 0.07 | 0.94 ± 0.06 | +11.54 (−6.22, +32.53) | 0.23 | 0.21 |
| Muscle tissue | |||||
| SCD1.18S.1 | 0.38 ± 0.19 | 1.33 ± 0.40 | −71.61 (−91.77, −21.78) | 0.04 | 0.02 |
| AGPAT 2.18s.1 | 0.70 ± 0.05 | 1.02 ± 0.06 | −31.34 (−42.71, −17.17) | <0.001 | <0.001 |
| ATGL.18S | 0.78 ± 0.06 | 0.96 ± 0.05 | −18.49 (−32.38, −3.42) | 0.03 | 0.07 |
| SPT1.18s | 1.18 ± 0.08 | 1.32 ± 0.08 | −10.34 (−25.49, +6.28) | 0.24 | 0.21 |
| CERK.18S | 0.84 ± 0.05 | 1.04 ± 0.06 | −18.72 (−30.64, −4.25) | 0.02 | 0.03 |
All outcomes were adjusted for age, body fat, and AIRg.
Altogether these data are suggestive of differences in the expression of genes regulating adipogenesis and lipogenesis in SAT of AA when compared with Caucasian women. In contrast, the gene expressions of markers of inflammation such as CD68 as well as the expression of leptin and RBP4 genes were similar between ethnic groups (Table 3).
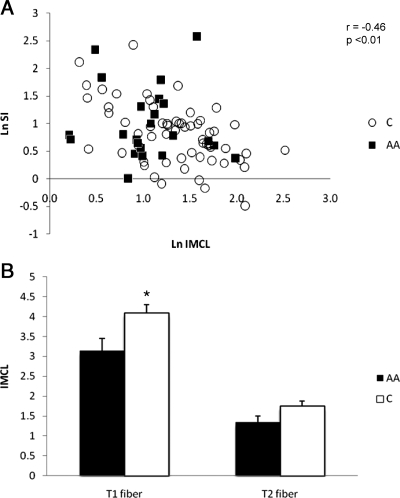
From the muscle biopsies (larger cohort), intramyocellular lipid (IMCL) was measured, as described previously (19), using oil red O staining. As shown in Fig. 1A, the logarithm of IMCL correlated strongly with the logarithm of SI in all subjects (r = −0.46, P < 0.001, Fig. 1A). However, when each group was analyzed separately, the correlation between SI and IMCL became stronger in Caucasians (r = −0.50, P < 0.01) but not significant in AAs (r = −0.31, P = 0.1). We tested whether the association between SI and IMCL was different between ethnic groups using a regression model that included ethnicity and its interaction with ln(SI) as covariates. The slope was not different between ethnic groups (P = 0.27), suggesting that the lack of association of SI and IMCL in AA women was most likely due to smaller sample size in this group. Despite similar SI levels, AA women had decreased IMCL when compared with Caucasian women in type 1 fibers (3.2 ± 0.3 vs. 4.1 ± 0.2%, respectively, P = 0.02), and a trend toward lower IMCL in type 2 fibers (1.4 ± 0.1 vs. 1.8 ± 0.1%, respectively, P = 0.09) (Fig. 1B). When adjusted for age, percent body fat, and AIRg, the difference in type 2 IMCL remained nonsignificant (P = 0.18) and the difference in type 1 IMCL was no longer statistically significant (P = 0.09; Table 2). We also examined these muscle samples for their overall oxidative capacity by measuring SDH activity. SDH activity was similar between ethnic groups (120.4 ± 5.2 vs. 124.0 ± 13.9, P = NS for type 1 fiber and 53.6 ± 2.8 vs. 49.4 ± 7.0 for type 2 fiber in Caucasian vs. AAs, respectively) (Table 2), suggesting no difference in lipid oxidization.
Figure 1.
A, Insulin sensitivity correlated significantly with IMCL when both AA and Caucasian (C) data were combined (r = −0.46, P < 0.01). The data for IMCL and SI have been natural log transformed in this graph. B, AA women had decreased IMCL (percent) in type 1 fiber when compared with Caucasian women. The differences in type 2 fiber IMCL were not significant between ethnic groups. *, P < 0.05.
To better understand the differences in IMCL, the expression of a number of different genes in muscle were measured. The expression of SCD1 and AGPAT 2 in muscle was reduced in AAs by 72% (P = 0.04) and 31% (P = 0.01), respectively, when compared with Caucasian women, suggestive of decreased triglyceride synthesis (Table 3). ATGL expression in muscle was also reduced by 18% (P = 0.03), suggesting a concurrent decrease in muscle lipid lipolysis (Table 3). Ceramide plays an important role in muscle insulin resistance (26), and we therefore studied the expression of SPT-1, which is the rate-limiting enzyme for de novo ceramide synthesis. AA women had similar levels of SPT-1 gene expression when compared with Caucasians (1.18 ± 0.08 vs. 1.32 ± 0.08, respectively, P = NS); however, the expression of CERK1 (ceramide kinase) gene in muscle was 19% lower in AA when compared with Caucasian women (0.84 ± 0.05 vs. 1.03 ± 0.06, respectively, P = 0.02) (Table 3).
Discussion
It is well established that the prevalence of diabetes varies by ethnicity. Compared with Caucasians, AAs have a higher risk of developing type 2 diabetes, are more insulin resistant, and have a different body fat distribution (3,4,5). Previous studies examined body fat distribution according to ethnicity, but their study populations were different in insulin sensitivity (7,8). Our study is unique in that we examined body fat distribution, adipokines, IMCL, and the expression of candidate genes in adipose tissue and muscle in a cohort of AA and Caucasian women who had similar BMI and SI levels, thus minimizing the effects of obesity and insulin resistance on the outcomes. As shown previously (6,27), we found higher AIRg in AA compared with Caucasian women with similar SI. Increased AIRg in AAs has been attributed to reduced hepatic insulin extraction in this ethnic group (28,29). We confirmed the previously reported (7,8) decreased VAT in AA as well as increased SAT when compared with Caucasian women with similar BMI and SI.
In this study, AA women had 30% lower adiponectin levels when compared with Caucasian women. Previous studies reported decreased adiponectin levels in AAs compared with Caucasians in younger age groups (30,31,32), yet such data on adults were quite limited. For example, Hulver et al. (11) found decreased adiponectin levels in nonobese AA compared with Caucasian women, but they did not find similar results among obese adults (11). It has been proposed that hyperinsulinemia in AAs might be responsible for decreased adiponectin levels in this ethnic group (32). Bush et al. (32) reported that the decreased adiponectin in AAs could be statistically explained by higher AIRg in this ethnic group. In our study, the difference in adiponectin between the ethnic groups remained significant after adjusting for age, body fat, and AIRg, suggesting that increased circulatory insulin did not contribute to decreased adiponectin levels in AA.
To elucidate the underlying mechanisms mediating fat distribution and adipokine secretion among ethnic groups, we studied the expression of genes regulating adipogenesis and lipogenesis in SAT. We did not find any differences in leptin gene expression in SAT of AA compared with Caucasian women despite previous reports of decreased circulatory leptin levels in AAs compared with Caucasians (33). However, the expression of a number of genes involved in adipogenesis and lipogenesis in SAT were decreased in AA when compared with Caucasian women, and these genes included PPARγ, lipin-1β, AGPAT 2, SCD1, and ATGL. Whether alterations in the expression of the above-mentioned genes between ethnic groups occur as several independent pathways or are part of an interdependent synchronized response remains unknown. However, a common element to the above genes is PPARγ responsiveness.
Adiponectin is a highly PPARγ-responsive gene, and numerous studies have demonstrated increased plasma adiponectin after treatment with thiazolidinediones (34). We have previously shown that treatment of impaired glucose-tolerant subjects with pioglitazone increased the expression of SCD1, AGPAT2, adiponectin R2, and lipin-β and the inhibition of PPARγ in vitro reduced the expression of SCD1, lipin-1β, and AGPAT 2, suggesting that these genes are all PPARγ responsive (16). Therefore, AAs have decreased adiponectin levels and decreased expression of these PPARγ-responsive genes, together suggesting that AAs may generally demonstrate decreased responsiveness to endogenous PPARγ ligands.
To our knowledge, no study has specifically studied the responsiveness of diabetic AAs vs. diabetic Caucasians to treatment with PPARγ agonists. Finally, adipose tissue inflammatory markers, as measured by expression of CD68 gene as well as plasma IL-6 and TNFα, were not different between ethnic groups. Adipose tissue macrophage number is also responsive to PPARγ agonists (35), but it is possible that this is more a pharmacological effect of a thiazolidinedione and not a function of endogenous PPARγ ligands.
Given the observed differences in the expression of genes regulating adipogenesis and lipogenesis in adipose tissue, we speculated that AAs would have increased ectopic fat accumulation. We assessed IMCL by measuring triglyceride levels in muscle fibers, and this index of lipotoxicity correlated strongly with SI. AAs had decreased IMCL (in type 1 fibers) compared with Caucasian women with similar SI; however, when adjusted for age, AIRg, and body fat, the difference in type 1 IMCL was no longer significant. The difference in IMCL (type 1 fiber) is explained by the difference in the association between IMCL and age in the ethnic groups. In our data there is a strong positive correlation between IMCL levels and age in Caucasians and not in AAs.
It was recently reported that AAs had decreased liver fat with obesity when compared with Caucasian individuals (8), which can potentially explain the favorable lipid profile in AAs reported in this study and others (36). Whether there are differences in lipotoxicity in muscle between ethnic groups is not clear from our studies; however, these data suggest significant differences in lipid metabolism between ethnic groups.
It has been suggested that the intramyocellular triglyceride per se is not the precise cause of insulin resistance but rather denotes increased accumulation of different types of lipids and other proposed lipotoxic substances such as ceramides, diacylglycerol (DAG), or long-chain fatty acids (37). We were not able to measure all these components in muscle due to limited muscle biopsy specimens. However, other changes in gene expression were noted that could affect lipotoxicity. It has been proposed that the saturation of fatty acid in lipid molecules such as DAG plays a role in insulin signaling (37). The decreased expression of SCD1 in AA muscle could be responsible for differences in fatty acid saturation and insulin signaling. We did not detect any differences in SPT-1 in AAs, which is the rate-limiting enzyme in ceramide synthesis, but we noted decreased CERK expression in AA when compared with Caucasian women. CERK increases ceramide phosphate, which has been proposed to have proinflammatory effects by increasing eicosanoid synthesis in vascular smooth muscle (38). It is not known whether ceramide phosphate plays a role in the pathogenesis of insulin resistance.
There are a number of limitations to this study. Only women were studied to avoid the gender differences in fat distribution and adipokine expression, and further studies are needed to elaborate ethnic difference among men. As mentioned above, due to limitation in muscle biopsy sample size, we did not measure lipotoxic substances such as ceramides, DAG, or long-chain fatty acids.
In summary, AAs demonstrated lower expression of several PPARγ-responsive genes in adipose tissue and lower plasma adiponectin, yet AAs might be protected from lipotoxicity, as reflected by decreased intramyocellular triglyceride levels. Together, these data suggest significant ethnic differences in the pathophysiology of insulin resistance.
Acknowledgments
We acknowledge the technical assistance of Bounleut Phanavanh in measuring gene expression. We also thank Regina Dennis for assisting with subject recruitment, S. Ranganathan for insulin measurement, the nursing and laboratory staff of the General Clinical Research Center of the University of Arkansas for Medical Sciences for their invaluable assistance, and Richard Harris for assistance with database design and data management.
Footnotes
This work was supported by a Merit Review Grant from the Veterans Administration (to N.R.), Grants DK080327 and DK071349 (to P.A.K.), and Grant UL1RR029884 from the National Center for Research Resources.
Disclosure Summary: The authors have nothing to disclose.
First Published Online June 30, 2010
Abbreviations: AA, African-American; AGPAT 2, 1-acylglycerol-3-phosphate acyltransferase 2; AIRg, acute insulin response to glucose; ATGL, adipose tissue triglyceride lipase; BMI, body mass index; CERK, ceramide kinase; CI, confidence interval; DAG, diacylglycerol; IMCL, intramyocellular lipid; PPARγ, peroxisomal proliferator-activated receptor-γ; SAT, sc adipose tissue; SCD1, stearoyl-coenzyme A desaturase-1; SDH, succinate dehydrogenase; SI, insulin sensitivity index; SPT, serine palmitoyl transferase; VAT, visceral adipose tissue.
References
- Danaei G, Friedman AB, Oza S, Murray CJ, Ezzati M 2009 Diabetes prevalence and diagnosis in U.S. states: analysis of health surveys. Popul Health Metr 7:16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2009 Differences in prevalence of obesity among black, white, and Hispanic adults—United States, 2006–2008. MMWR Morb Mortal Wkly Rep 58:740–744 [PubMed] [Google Scholar]
- Harris MI, Flegal KM, Cowie CC, Eberhardt MS, Goldstein DE, Little RR, Wiedmeyer HM, Byrd-Holt DD 1998 Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in U.S. adults. The Third National Health and Nutrition Examination Survey, 1988–1994. Diabetes Care 21:518–524 [DOI] [PubMed] [Google Scholar]
- Shai I, Jiang R, Manson JE, Stampfer MJ, Willett WC, Colditz GA, Hu FB 2006 Ethnicity, obesity, and risk of type 2 diabetes in women: a 20-year follow-up study. Diabetes Care 29:1585–1590 [DOI] [PubMed] [Google Scholar]
- Torréns JI, Skurnick J, Davidow AL, Korenman SG, Santoro N, Soto-Greene M, Lasser N, Weiss G 2004 Ethnic differences in insulin sensitivity and {beta}-cell function in premenopausal or early perimenopausal women without diabetes: the Study of Women’s Health Across the Nation (SWAN). Diabetes Care 27:354–361 [DOI] [PubMed] [Google Scholar]
- Haffner SM, D'Agostino R, Saad MF, Rewers M, Mykkänen L, Selby J, Howard G, Savage PJ, Hamman RF, Wagenknecht LE 1996 Increased insulin resistance and insulin secretion in nondiabetic African-Americans and Hispanics compared with non-Hispanic whites. The Insulin Resistance Atherosclerosis Study. Diabetes 45:742–748 [DOI] [PubMed] [Google Scholar]
- Lovejoy JC, de la Bretonne JA, Klemperer M, Tulley R 1996 Abdominal fat distribution and metabolic risk factors: effects of race. Metabolism 45:1119–1124 [DOI] [PubMed] [Google Scholar]
- Guerrero R, Vega GL, Grundy SM, Browning JD 2009 Ethnic differences in hepatic steatosis: an insulin resistance paradox? Hepatology 49:791–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liska D, Dufour S, Zern TL, Taksali S, Cali AM, Dziura J, Shulman GI, Pierpont BM, Caprio S 2007 Interethnic differences in muscle, liver and abdominal fat partitioning in obese adolescents. PLoS One 2:e569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan AS, Nicklas BJ, Berman DM 2002 Racial differences in insulin resistance and mid-thigh fat deposition in postmenopausal women. Obes Res 10:336–344 [DOI] [PubMed] [Google Scholar]
- Hulver MW, Saleh O, MacDonald KG, Pories WJ, Barakat HA 2004 Ethnic differences in adiponectin levels. Metabolism 53:1–3 [DOI] [PubMed] [Google Scholar]
- Araneta MR, Barrett-Connor E 2007 Adiponectin and ghrelin levels and body size in normoglycemic Filipino, African-American, and white women. Obesity 15:2454–2462 [DOI] [PubMed] [Google Scholar]
- Garg A, Misra A 2004 Lipodystrophies: rare disorders causing metabolic syndrome. Endocrinol Metab Clin North Am 33:305–331 [DOI] [PubMed] [Google Scholar]
- Spiegelman BM, Hu E, Kim JB, Brun R 1997 PPARγ and the control of adipogenesis. Biochimie 79:111–112 [DOI] [PubMed] [Google Scholar]
- Dubois SG, Heilbronn LK, Smith SR, Albu JB, Kelley DE, Ravussin E 2006 Decreased expression of adipogenic genes in obese subjects with type 2 diabetes. Obesity 14:1543–1552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao-Borengasser A, Rassouli N, Varma V, Bodles AM, Rasouli N, Unal R, Phanavanh B, Ranganathan G, McGehee Jr RE, Kern PA 2008 Stearoyl-coenzyme A desaturase 1 gene expression increases after pioglitazone treatment and is associated with peroxisomal proliferator-activated receptor-γ responsiveness. J Clin Endocrinol Metab 93:4431–4439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desvergne B, Wahli W 1999 Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocr Rev 20:649–688 [DOI] [PubMed] [Google Scholar]
- Bergman RN, Prager R, Volund A, Olefsky JM 1987 Equivalence of the insulin sensitivity index in man derived by the minimal model method and the euglycemic glucose clamp. J Clin Invest 79:790–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasouli N, Raue U, Miles LM, Lu T, Di Gregorio GB, Elbein SC, Kern PA 2005 Pioglitazone improves insulin sensitivity through reduction in muscle lipid and redistribution of lipid into adipose tissue. Am J Physiol Endocrinol Metab 288:E930–E934 [DOI] [PubMed] [Google Scholar]
- Bergman RN, Ider YZ, Bowden CR, Cobelli C 1979 Quantitative estimation of insulin sensitivity. Am J Physiol Endocrinol Metab 236:E667–E677 [DOI] [PubMed] [Google Scholar]
- Boston RC, Stefanovski D, Moate PJ, Sumner AE, Watanabe RM, Bergman RN 2003 MINMOD Millennium: a computer program to calculate glucose effectiveness and insulin sensitivity from the frequently sampled intravenous glucose tolerance test. Diabetes Technol Ther 5:1003–1015 [DOI] [PubMed] [Google Scholar]
- Malenfant P, Joanisse DR, Thériault R, Goodpaster BH, Kelley DE, Simoneau JA 2001 Fat content in individual muscle fibers of lean and obese subjects. Int J Obes Relat Metab Disord 25:1316–1321 [DOI] [PubMed] [Google Scholar]
- Kern PA, Simsolo RB, Fournier M 1999 Effect of weight loss on muscle fiber type, fiber size, capillarity, and succinate dehydrogenase activity in humans. J Clin Endocrinol Metab 84:4185–4190 [DOI] [PubMed] [Google Scholar]
- Di Gregorio GB, Yao-Borengasser A, Rasouli N, Varma V, Lu T, Miles LM, Ranganathan G, Peterson CA, McGehee RE, Kern PA 2005 Expression of CD68 and macrophage chemoattractant protein-1 genes in human adipose and muscle tissues: association with cytokine expression, insulin resistance, and reduction by pioglitazone. Diabetes 54:2305–2313 [DOI] [PubMed] [Google Scholar]
- Rasouli N, Yao-Borengasser A, Varma V, Spencer HJ, McGehee Jr RE, Peterson CA, Mehta JL, Kern PA 2009 Association of scavenger receptors in adipose tissue with insulin resistance in nondiabetic humans. Arterioscler Thromb Vasc Biol 29:1328–1335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickersgill L, Litherland GJ, Greenberg AS, Walker M, Yeaman SJ 2007 Key role for ceramides in mediating insulin resistance in human muscle cells. J Biol Chem 282:12583–12589 [DOI] [PubMed] [Google Scholar]
- Rasouli N, Spencer HJ, Rashidi AA, Elbein SC 2007 Impact of family history of diabetes and ethnicity on β-cell function in obese, glucose-tolerant individuals. J Clin Endocrinol Metab 92:4656–4663 [DOI] [PubMed] [Google Scholar]
- Gower BA, Granger WM, Franklin F, Shewchuk RM, Goran MI 2002 Contribution of insulin secretion and clearance to glucose-induced insulin concentration in African-American and Caucasian children. J Clin Endocrinol Metab 87:2218–2224 [DOI] [PubMed] [Google Scholar]
- Osei K, Schuster DP 1994 Ethnic differences in secretion, sensitivity, and hepatic extraction of insulin in black and white Americans. Diabet Med 11:755–762 [DOI] [PubMed] [Google Scholar]
- Degawa-Yamauchi M, Dilts JR, Bovenkerk JE, Saha C, Pratt JH, Considine RV 2003 Lower serum adiponectin levels in African-American boys. Obes Res 11:1384–1390 [DOI] [PubMed] [Google Scholar]
- Steffes MW, Gross MD, Schreiner PJ, Yu X, Hilner JE, Gingerich R, Jacobs Jr DR 2004 Serum adiponectin in young adults—interactions with central adiposity, circulating levels of glucose, and insulin resistance: the CARDIA study. Ann Epidemiol 14:492–498 [DOI] [PubMed] [Google Scholar]
- Bush NC, Darnell BE, Oster RA, Goran MI, Gower BA 2005 Adiponectin is lower among African Americans and is independently related to insulin sensitivity in children and adolescents. Diabetes 54:2772–2778 [DOI] [PubMed] [Google Scholar]
- Considine RV, Premkumar A, Reynolds JC, Sebring NG, Ricks M, Sumner AE 2008 Adiponectin and leptin in African Americans. Obesity (Silver Spring) 16:428–434 [DOI] [PubMed] [Google Scholar]
- Riera-Guardia N, Rothenbacher D 2008 The effect of thiazolidinediones on adiponectin serum level: a meta-analysis. Diabetes Obes Metab 10:367–375 [DOI] [PubMed] [Google Scholar]
- Bodles AM, Varma V, Yao-Borengasser A, Phanavanh B, Peterson CA, McGehee Jr RE, Rasouli N, Wabitsch M, Kern PA 2006 Pioglitazone induces apoptosis of macrophages in human adipose tissue. J Lipid Res 47:2080–2088 [DOI] [PubMed] [Google Scholar]
- Sumner AE 2009 Ethnic differences in triglyceride levels and high-density lipoprotein lead to underdiagnosis of the metabolic syndrome in black children and adults. J Pediatr 155:S7.e7–S7.e11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman BC, Perreault L, Hunerdosse DM, Koehler MC, Samek AM, Eckel RH 2009 Intramuscular lipid metabolism in the insulin resistance of smoking. Diabetes 58:2220–2227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houck KL, Fox T, Kester M 2007 Toll-like receptor 4 activated ceramide kinase mediates inflammatory responses in vascular smooth muscle cells. FASEB J 21:A804 [Google Scholar]