Abstract
Context: Primary hyperparathyroidism (PHPT) disproportionately affects older patients, who may face higher thresholds for surgical intervention compared to young patients.
Objective: The aim was to examine for differences in the utilization of parathyroidectomy attributable to age.
Design: We conducted a retrospective cohort study.
Participants: Patients with biochemically diagnosed PHPT during the years 1995–2008 were identified within an integrated health care delivery system in Southern California encompassing approximately 3 million individuals.
Main Outcome Measures: The outcome measures were parathyroidectomy (PTx) and time interval to surgery.
Results: We found 3388 patients with PHPT, 964 (28%) of whom underwent PTx. Patients aged 60+ yr comprised 60% of the study cohort. The likelihood of PTx decreased linearly among patients aged 60+ when compared to patients aged 50–59, an effect that persisted in multivariate analysis: odds ratio 0.68 for ages 60–69 (P < 0.05); 0.41 for ages 70–79 (P < 0.0001), and 0.11 for age 80+ (P < 0.0001). The PTx rate for patients aged 70+ was 14%. Among patients meeting 2002 consensus criteria for surgical treatment, 45% of those aged 60–69 and 24% of those aged 70+ underwent PTx. A Cox proportional hazards model showed that patients aged 60+ experienced significantly longer delays from diagnosis to surgery compared to young patients (P < 0.0001).
Conclusions: PHPT is undertreated in the elderly. We observed a progressive age-related decline in PTx rate that renders patients aged 70+ unlikely to have definitive treatment, irrespective of comorbidity and eligibility for surgery.
Elderly patients with primary hyperparathyroidism are less likely to receive surgical treatment than young patients, after controlling for comorbidity status and satisfaction of the consensus criteria for surgery.
Primary hyperparathyroidism (PHPT) is predominantly a disease of the elderly, affecting an estimated 1.5% of Americans aged 65 and older (1). Established clinical manifestations of PHPT include nephrolithiasis, osteoporosis, and a number of nonspecific musculoskeletal, abdominal, and neuropsychiatric complaints (2). Surgery, which remains the only definitive treatment, brings about resolution or at least partial reversal of many of these problems (3,4,5).
Several single-institution studies have raised the possibility that elderly patients may face barriers to receiving appropriate treatment for PHPT (6,7). Among patients who are referred for parathyroidectomy (PTx), elderly patients possess more severe biochemical abnormalities than their younger counterparts (6), which may reflect higher thresholds for surgical referral or delays in surgical referral (7). A growing body of literature indicates that PTx using modern techniques is safe and effective in elderly patients. Experienced centers report success rates of 98%, complication rates of less than 5%, and mortality rates of less than 1% in large series examining patients aged more than 70 yr (6,7,8,9,10,11,12,13,14,15). These results are comparable to those achieved in young patients (16).
Existing literature on the treatment of PHPT in the elderly is limited by the paucity of data regarding patients who are not treated surgically. We sought to determine PTx rates in the elderly by examining a large population of patients with an established biochemical diagnosis of PHPT.
Subjects and Methods
Subjects
Cases were identified through the (KP-SCAL) Laboratory Management System patient database system from 1995–2008. Patients possessing all of the following laboratory values were defined as having PHPT: serum intact PTH greater than 65 pg/ml (normal range, 15–65); serum calcium greater than 10.5 mg/dl (2.6 mmol/liter) (normal range, 8.5–10.5); and serum creatinine less than 2.5 mg/dl (221.0 mmol/liter). To avoid contamination of the sample with tertiary hyperparathyroidism patients, any patient who had at least two separate blood samples drawn for the measurement of cyclosporine, tacrolimus, or sirolimus was considered a likely kidney transplant recipient and was excluded from the study. A second database, the KP-SCAL Discharge Abstract Database, was cross-referenced to exclude patients with dialysis-dependent chronic kidney disease.
KP-SCAL is an integrated health care delivery system that serves more than 3 million subscribers annually, representing approximately 15% of the insured population of the region. The KP-SCAL membership closely approximates the population of the greater Los Angeles metropolitan area demographically and socioeconomically, except for the extremes of the income distribution (17,18).
Study protocol
Patients were classified into five age strata: 0–49, 50–59, 60–69, 70- 79, and 80 or above. The main outcome variable was PTx after the biochemical diagnosis of PHPT. To control for other potential predictors of PTx, the following additional data were abstracted: patient sex, race, ethnicity, Charlson comorbidity index, and 24-h urinary calcium. Specific International Classification of Diseases, Ninth Edition (ICD-9) codes were used to identify patients with a history of kidney stones or fracture. Chart review was performed on a randomly selected subset of 397 patients to acquire bone mineral density data in the form of total hip dual-energy x-ray absorptiometry T-scores. This subsample size was estimated to achieve a statistical power of 0.90 to detect a 20% difference in PTx rate with α set to 0.05.
To compare the frequency of PTx within KP-SCAL to that observed in other communities, benchmarks for prevalence of this procedure were created. Frequency of PTx procedures in 2002 for New York, Florida, and California were produced through analysis of publicly available databases. These analyses were limited to patients with private health insurance. Population data available from the 2000 U.S. Census were used to estimate prevalence of PTx per 100,000 individuals for each state.
Statistical analysis
For univariate analyses, contingency table analysis was applied to test for an association between age and the proportion of surgically to nonsurgically managed patients. Student’s t test was applied to compare any differences in age or biochemical severity of PHPT between surgically and nonsurgically treated patients. These two statistics were also applied to examine the associations between age and other predictor variables. Likelihood of PTx was estimated by applying multivariable logistic regression models controlling for demographics, comorbidity, biochemical parameters, and the clinical outcome variables listed above. A Cox proportional hazards model with identical controls was used to examine age group differences in time to surgery. A value of P < 0.05 was the criterion for statistical significance. All analyses were performed using SAS version 9.13 (SAS Institute, Cary, NC). The study protocol was reviewed and approved by the institutional review board of KP-SCAL and the University of California, Los Angeles.
Results
Patient characteristics
We identified 3388 nonuremic patients with hypercalcemia and inappropriately elevated PTH levels (Table 1). The cohort included 2682 women (79%) and 706 men (21%), with 2024 of these patients (60%) being 60 or more years old. Fractures and nephrolithiasis were uncommon complications, occurring in 8.7 and 7.8% of the population, respectively; the latter occurred more frequently among patients younger than 60 (P < 0.0001). T-scores were evenly distributed among the normal, osteopenic, and osteoporotic ranges. Patients aged 60+ were more commonly osteoporotic (P < 0.0001) and experienced more fractures (P < 0.0001). Although the comorbidity score did correlate positively with age (r = 0.22; P < 0.0001), the great majority of patients were free of major comorbidities. The following proportions of patients in each age group had a comorbidity score less than or equal to one: 0–49, 95%; 50–59, 94%; 60–69, 90%; 70–79, 81%; and 80+, 67%.
Table 1.
Patient characteristics
| Full cohort | Age (yr)
|
P value | |||||
|---|---|---|---|---|---|---|---|
| <50 | 50–59 | 60–69 | 70–79 | 80+ | |||
| No. of subjects | 3388 | 548 | 816 | 906 | 776 | 342 | |
| Mean age (yr) | 62.2 | 38.7 | 54.7 | 64.4 | 74.3 | 83.9 | <0.0001a |
| % Female | 79 | 64 | 83 | 82 | 81 | 83 | <0.0001 |
| Race (%) | |||||||
| White | 55 | 38 | 48 | 55 | 65 | 72 | |
| Black | 15 | 15 | 16 | 15 | 15 | 16 | |
| Hispanic | 14 | 27 | 18 | 12.0 | 9 | 4 | |
| Asian/Pacific Islander | 4 | 7 | 4 | 4 | 2 | 1 | |
| Other/unknown | 12 | 14 | 14 | 15 | 9 | 7 | <0.0001 |
| Charlson comorbidity score (%) | |||||||
| 0 | 71 | 86 | 82 | 72 | 62 | 43 | |
| 1 | 16 | 9 | 12 | 18 | 20 | 25 | |
| >2 | 13 | 5 | 6 | 10 | 19 | 33 | <0.0001 |
| Nephrolithiasis (%) | 7.8 | 15.3 | 10.3 | 6.2 | 3.9 | 3.2 | <0.0001 |
| History of fracture (%) | 8.7 | 2.7 | 4.9 | 6.5 | 14.2 | 21.4 | <0.0001 |
| T-score (total hip, n = 397) (%) | |||||||
| >−1.00 | 33 | 51 | 51 | 33 | 15 | 0 | |
| −2.49 to −1.00 | 41 | 35 | 34 | 47 | 50 | 17 | |
| <−2.50 | 25 | 14 | 16 | 20 | 35 | 83 | <0.0001 |
| Serum calcium (mg/dl)b,c | 11.2 ± 0.7 | 11.3 ± 0.7 | 11.2 ± 0.8 | 11.1 ± 0.6 | 11.2 ± 0.7 | 11.3 ± 0.8 | <0.005a |
| Intact PTH (pg/ml) | 126 ± 82 | 137 ± 102 | 123 ± 78 | 118 ± 64 | 124 ± 82 | 141 ± 105 | <0.0001a |
| Serum creatinine (mg/dl) | 0.89 ± 0.26 | 0.86 ± 0.26 | 0.84 ± 0.24 | 0.87 ± 0.23 | 0.94 ± 0.28 | 1.01 ± 0.36 | <0.0001a |
Comparisons made using one-way ANOVA. Remaining comparisons were made using contingency table analysis.
To convert calcium to mmol/liter, multiply by 0.25.
Biochemical values are expressed as mean ± sd.
Age and PTx
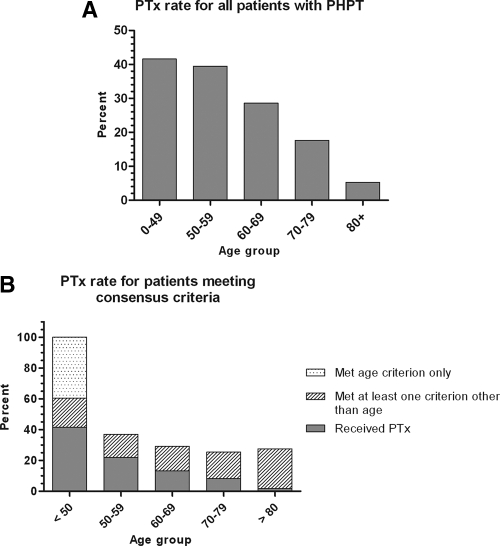
Overall, 964 of the 3388 patients (29%) underwent PTx. Mean serum calcium and PTH levels were higher among surgically treated patients compared with those who were observed (11.4 vs. 11.1 mg/dl, P < 0.0001; 149 vs. 117 pg/ml, P < 0.0001). In univariate analysis, the PTx rate declined significantly with age, with 18% of patients aged 70–79 and 5% of patients age 80+ undergoing PTx (Fig. 1A).
Figure 1.
A, Proportion of all patients with PHPT undergoing PTx according to age group (P < 0.0001). B, Proportion of patients meeting consensus criteria undergoing PTx according to age group (P < 0.0001).
To address the issue of appropriateness of care, a subset analysis was performed on patients satisfying at least one of the following 2002 consensus criteria for surgical treatment (19): nephrolithiasis; calcium greater than 11.5 mg/dl; 24-h urinary calcium excretion greater than 400 mg/d; and osteoporosis. Patients meeting the aforementioned criteria and those meeting only the age less than 50 consensus criterion are shown separately in Fig. 1B. The creatinine clearance criterion was necessarily omitted because serum creatinine was used as an initial screen to exclude patients with renal hyperparathyroidism from the study cohort. The proportion of patients meeting one or more consensus criteria for surgery was 42% overall and 30% for patients aged 50+. Given that the bone mineral density data were incomplete, these figures represent the lower limit of patients meeting consensus criteria. The PTx rate declined with increasing age in this subset of 1405 patients, similar to the pattern seen in the entire cohort of patients with PHPT. The overall PTx rate among patients meeting consensus criteria was 43%, with the highest rate observed being 59% for the age 50–59 group. The PTx rate for patients aged 70+ was significantly lower at 24% (P < 0.0001).
The following predictors entered the final multivariable logistic regression model: gender, race, comorbidity score, nephrolithiasis, serum calcium, PTH, 24-h urinary calcium, and serum creatinine. The age 50–59 group was used as the reference. The odds ratio for PTx declined progressively with increasing age for all patients with PHPT (Table 2). This pattern persisted when the subset of patients meeting at least one of five consensus criteria was analyzed (age less than 50; nephrolithiasis; calcium >11.5 mg/dl; 24-h urinary calcium excretion >400 mg/d; and osteoporosis; Table 3).
Table 2.
Multivariable analysis of PTx likelihood by age in full cohort
| Age group | Full cohort (n = 3388)
|
||
|---|---|---|---|
| Odds ratio | 95% CI | Significance | |
| 0–49 | 0.99 | 0.78–1.26 | 0.94 |
| 50–59 | — | — | — |
| 60–69 | 0.68 | 0.55–0.84 | P < 0.0005 |
| 70–79 | 0.41 | 0.32–0.52 | P < 0.0001 |
| 80+ | 0.11 | 0.07–0.19 | P < 0.0001 |
The reference is age group 50–59 yr. CI, Confidence interval.
Table 3.
Multivariable analysis of PTx likelihood by age in consensus subgroup
| Age group | Consensus subgroup 2 (n = 1405)
|
||
|---|---|---|---|
| Odds ratio | 95% CI | Significance | |
| 0–49a | 0.64 | 0.47–0.89 | 0.01 |
| 0–49b | 0.90 | 0.62–1.30 | 0.57 |
| 50–59 | — | — | — |
| 60–69 | 0.62 | 0.44–0.88 | P < 0.01 |
| 70–79 | 0.43 | 0.28–0.64 | P < 0.0001 |
| 80+ | 0.07 | 0.03–0.16 | P < 0.0001 |
The subgroup consists of patients meeting at least one consensus criterion (nephrolithiasis; calcium >11.5 mg/dl; 24-h urinary calcium excretion >400 mg/d; and osteoporosis). The reference is age group 50–59. CI, Confidence interval.
Includes all patients aged 0–49 yr.
Includes patients aged 0–49 yr who met at least one criterion in addition to age.
By definition, all patients aged 0–49 met at least one consensus criterion for surgery. To account for a potential confounding effect of including this group in multivariable analysis, a separate multivariable analysis for all patients with PHPT excluding the 0–49 age group was performed. This yielded odds ratios and significance values for the remaining age groups that were identical to those shown in Table 2.
State level benchmarks
The PTx prevalence ranged from 5.8 per 100,000 for New York to 3.4 and 3.3 per 100,000 for Florida and California, respectively. Using the current data and number of enrollees for KP-SCAL in 2002, we estimated a PTx prevalence of 4.2 per 100,000 patients in this study sample.
Time interval to surgery
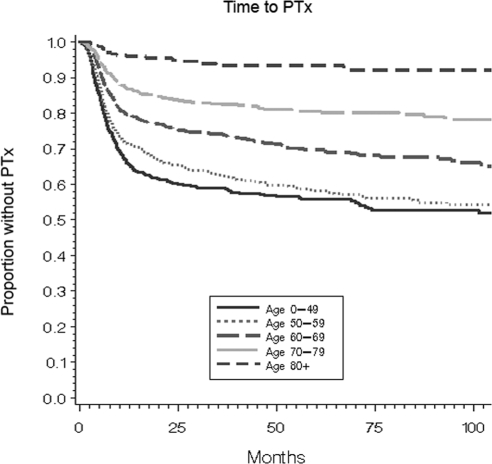
To determine whether older patients experienced delays in surgical treatment, another potential indicator of age bias, we applied a multivariable time-to-event analysis. In a Cox proportional hazards model of time to PTx with identical controls for gender, race, comorbidity score, nephrolithiasis, serum calcium, PTH, 24-h urinary calcium, and serum creatinine, patients in every age group over 59 experienced longer delays to surgery compared with patients aged 0–49 or 50–59 (Fig. 2; P < 0.0001). The mean time interval from diagnosis to surgery was 12.4 months for patients aged less 60 and ranged from 14.8 to 16.2 months for the elderly age groups.
Figure 2.
Cox proportional hazards model of time interval to PTx according to age group. The proportion of patients managed without surgery is shown on the vertical axis (P < 0.0001).
Discussion
Systematic study of practice patterns in the management of PHPT has been limited by the general unavailability of clinical data on patients who are not managed surgically. The existing literature is comprised predominantly of single-institution studies in which most or all subjects have undergone PTx, or prospective studies involving relatively small numbers of patients who, in some instances, have been selected to have mild disease (5,20,21,22,23). Efforts at studying PHPT using population-level administrative data have been possible in Scandinavian countries (24,25,26) but have been hampered in the United States because of coding bias, which again favors identification of patients who undergo surgery (16).
We have leveraged the fact that PHPT can be diagnosed strictly using biochemical data to identify all patients with the condition in a manner that does not depend on either coding or physician recognition of the diagnosis. Our study cohort thus represents the truest denominator in the literature (to our knowledge), from which practice patterns regarding the surgical management of PHPT may be discerned.
Our data demonstrate an inverse relationship between age and likelihood of PTx. This effect is independent of comorbidity, gender, race, clinical and biochemical disease severity, and objective standards regarding appropriateness of surgical care. In other words, older patients, as a group, undergo fewer parathyroid operations than their younger counterparts, apparently based in large part on age alone. We also find that increasing age predicts a longer time interval to PTx, which may reflect judicious consideration of the risks and benefits of surgery in the elderly population. Patients aged 60 and over who did undergo PTx typically carried the diagnosis for more than 15 months before receiving surgical care. The available evidence suggests that disease progression generally does not occur within this time frame but rather develops over longer intervals without treatment (27). In keeping with established reports, out data show that PHPT manifests differently in younger vs. older patients (8,28,29), with nephrolithiasis occurring more commonly in patients aged less than 60 and osteoporosis being more common in patients aged 60 and over. Not surprisingly, both nephrolithiasis and osteoporosis were much more prevalent in our cohort with PHPT compared with normocalcemic age- and gender-matched controls (data not shown).
Health insurance status is a critical determinant of care utilization, particularly with respect to elective surgery (30). The differences in PTx rate that we observed cannot be explained by differences in insurance status, which is uniform within our study population. We acknowledge several limitations of the present study. The subject inclusion and exclusion methods employed, although specific for PHPT, may omit several classes of patients, such as those who did not have the serum calcium tested, those with hypercalcemia in whom the PTH level was not tested, those with nonclassical PHPT presenting with hypercalcemia and an inappropriately normal/nonsuppressed PTH, those with incipient (normocalcemic) PHPT, and a likely small number of patients with PHPT and a serum creatinine greater than 2.5 mg/dl. Another limitation is that the dual-energy x-ray absorptiometry scores available within the database did not cover all sites and all subjects. Importantly, the available data do not allow us to determine the specific reasons underlying the age-related decline in the PTx rate. Possibilities include decreased rates of surgical referral in the elderly, surgeon aversion toward operating on elderly patients, and patient preference/refusal of surgery. Our communications with several surgeons indicated that most patients referred for surgery are evaluated by an endocrinologist first. No additional information regarding referral patterns was available in the database.
The KP-SCAL patient population is ethnically diverse and representative of the larger community. However, the financial structure of the health maintenance organization may potentially limit the external validity of our findings. To address this concern, we compared the per capita PTx prevalence within KP-SCAL against benchmark population-level data for several large states over a 1-yr period. The PTx prevalence of 4.2 per 100,000 found in the study sample fell within the 3.3 to 5.8 per 100,000 range for privately insured patients in California as a whole as well as elsewhere in the country. Thus, we cautiously conclude that practice patterns regarding the surgical management of PHPT within KP-SCAL approximate those in the general community setting.
It has previously been estimated that 10–20% of all patients with PHPT undergo surgery (31,32). We found a comparatively higher PTx rate of 29% for all patients with PHPT. Although the majority of patients with PHPT in our cohort were 60 or more years old, the majority of those treated surgically were younger than age 60. This fact raises the following paradox: although PHPT is predominantly a disease of the elderly, elderly patients are unlikely to receive definitive treatment precisely because of their age.
We have shown that comorbidity alone is insufficient to explain the progressive age-related decline in PTx rate. Similarly, nontreatment of PHPT in the elderly cannot be rationalized based on life expectancy or cost-effectiveness arguments. U.S. Census life tables indicate that the majority of patients aged 70+ in our cohort have a life expectancy of greater than 10 yr (33). A recent long-term follow-up study of patients with PHPT demonstrated significant declines in bone mineral density at the femoral neck and distal radius within 10 yr of diagnosis in patients not managed surgically (27), although some experts have noted limitations to this analysis (34). A decision model analysis examining age at diagnosis showed that surgery is cost-effective in patients with PHPT who have a predicted life expectancy of 5 yr or more (35).
The benefits of PTx in elderly patients are similar to those experienced by young patients with respect to symptom relief (6,7,11,15). Furthermore, the application of minimally invasive techniques to parathyroid surgery has brought about significant reductions in length of hospitalization (12) and, in some centers (including UCLA), eliminated the need for general anesthesia (36), thus reducing the burden of care. In considering these various factors, we conclude that PHPT is undertreated in elderly patients.
A serendipitous finding in our study was that PTx is underutilized not only in the elderly, but indeed across all age groups. The majority of patients with biochemically confirmed PHPT who meet 2002 consensus criteria for surgery did not undergo PTx. At this point, we can only conclude that nonadherence to the 2002 consensus guidelines is occurring in our study population. However, because our benchmarking data describe PTx rates in 25% of the U.S. population, we suspect that nonadherence may be a widespread issue that merits further study. Furthermore, we have identified the largest domestic cohort to date of patients with PHPT not managed surgically, which will enable detailed examination of the natural history of this disease.
Footnotes
M.W.Y. received support from the University of California-Los Angeles Older Americans Independence Center, National Institutes of Health (NIH)/National Institute on Aging (NIA) Grant P30-AG028748. The content does not necessarily represent the official views of the NIA or the NIH.
Disclosure Summary: The authors have no competing interests to declare.
First Published Online July 7, 2010
Abbreviations: PHPT, Primary hyperparathyroidism; PTx, parathyroidectomy.
References
- Coker LH, Rorie K, Cantley L, Kirkland K, Stump D, Burbank N, Tembreull T, Williamson J, Perrier N 2005 Primary hyperparathyroidism, cognition, and health-related quality of life. Ann Surg 242:642–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan AK, Duh QY, Katz MH, Siperstein AE, Clark OH 1995 Clinical manifestations of primary hyperparathyroidism before and after parathyroidectomy. A case-control study. Ann Surg 222:402–412; discussion 412–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eigelberger MS, Cheah WK, Ituarte PH, Streja L, Duh QY, Clark OH 2004 The NIH criteria for parathyroidectomy in asymptomatic primary hyperparathyroidism: are they too limited? Ann Surg 239:528–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollerup CL, Vestergaard P, Frøkjaer VG, Mosekilde L, Christiansen P, Blichert-Toft M 2002 Risk of renal stone events in primary hyperparathyroidism before and after parathyroid surgery: controlled retrospective follow-up study. BMJ 325:807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverberg SJ, Shane E, Jacobs TP, Siris E, Bilezikian JP 1999 A 10-year prospective study of primary hyperparathyroidism with or without parathyroid surgery. N Engl J Med 341:1249–1255 [DOI] [PubMed] [Google Scholar]
- Chen H, Parkerson S, Udelsman R 1998 Parathyroidectomy in the elderly: do the benefits outweigh the risks? World J Surg 22:531–535; discussion 535–536 [DOI] [PubMed] [Google Scholar]
- Kebebew E, Duh QY, Clark OH 2003 Parathyroidectomy for primary hyperparathyroidism in octogenarians and nonagenarians: a plea for early surgical referral. Arch Surg 138:867–871 [DOI] [PubMed] [Google Scholar]
- Bachar G, Gilat H, Mizrachi A, Shimon I, Feinmesser R, Kaizerman I, Shpitzer T 2008 Comparison of perioperative management and outcome of parathyroidectomy between older and younger patients. Head Neck 30:1415–1421 [DOI] [PubMed] [Google Scholar]
- Biertho L, Chu C, Inabnet WB 2003 Image-directed parathyroidectomy under local anaesthesia in the elderly. Br J Surg 90:738–742 [DOI] [PubMed] [Google Scholar]
- Chigot JP, Menegaux F, Achrafi H 1995 Should primary hyperparathyroidism be treated surgically in elderly patients older than 75 years? Surgery 117:397–401 [DOI] [PubMed] [Google Scholar]
- Egan KR, Adler JT, Olson JE, Chen H 2007 Parathyroidectomy for primary hyperparathyroidism in octogenarians and nonagenarians: a risk-benefit analysis. J Surg Res 140:194–198 [DOI] [PubMed] [Google Scholar]
- Irvin 3rd GL, Carneiro DM 2001 “Limited” parathyroidectomy in geriatric patients. Ann Surg 233:612–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Politz D, Norman J 2007 Hyperparathyroidism in patients over 80: clinical characteristics and their ability to undergo outpatient parathyroidectomy. Thyroid 17:333–339 [DOI] [PubMed] [Google Scholar]
- Shin SH, Holmes H, Bao R, Jimenez C, Kee SS, Potylchansky E, Lee JE, Evans DB, Perrier ND 2009 Outpatient minimally invasive parathyroidectomy is safe for elderly patients. J Am Coll Surg 208:1071–1076 [DOI] [PubMed] [Google Scholar]
- Stechman MJ, Weisters M, Gleeson FV, Sadler GP, Mihai R 2009 Parathyroidectomy is safe and improves symptoms in elderly patients with primary hyperparathyroidism (PHPT). Clin Endocrinol (Oxf) 71:787–791 [DOI] [PubMed] [Google Scholar]
- Stavrakis AI, Ituarte PH, Ko CY, Yeh MW 2007 Surgeon volume as a predictor of outcomes in inpatient and outpatient endocrine surgery. Surgery 142:887–899 [DOI] [PubMed] [Google Scholar]
- Dabelea D, Bell RA, D'Agostino Jr RB, Imperatore G, Johansen JM, Linder B, Liu LL, Loots B, Marcovina S, Mayer-Davis EJ, Pettitt DJ, Waitzfelder B 2007 Incidence of diabetes in youth in the United States. JAMA 297:2716–2724 [DOI] [PubMed] [Google Scholar]
- Lawrence JM, Lukacz ES, Nager CW, Hsu JW, Luber KM 2008 Prevalence and co-occurrence of pelvic floor disorders in community-dwelling women. Obstet Gynecol 111:678–685 [DOI] [PubMed] [Google Scholar]
- Bilezikian JP, Potts Jr JT, Fuleihan Gel-H, Kleerekoper M, Neer R, Peacock M, Rastad J, Silverberg SJ, Udelsman R, Wells SA 2002 Summary statement from a workshop on asymptomatic primary hyperparathyroidism: a perspective for the 21st century. J Clin Endocrinol Metab 87:5353–5361 [DOI] [PubMed] [Google Scholar]
- Ambrogini E, Cetani F, Cianferotti L, Vignali E, Banti C, Viccica G, Oppo A, Miccoli P, Berti P, Bilezikian JP, Pinchera A, Marcocci C 2007 Surgery or surveillance for mild asymptomatic primary hyperparathyroidism: a prospective, randomized clinical trial. J Clin Endocrinol Metab 92:3114–3121 [DOI] [PubMed] [Google Scholar]
- Bollerslev J, Jansson S, Mollerup CL, Nordenström J, Lundgren E, Tørring O, Varhaug JE, Baranowski M, Aanderud S, Franco C, Freyschuss B, Isaksen GA, Ueland T, Rosen T 2007 Medical observation, compared with parathyroidectomy, for asymptomatic primary hyperparathyroidism: a prospective, randomized trial. J Clin Endocrinol Metab 92:1687–1692 [DOI] [PubMed] [Google Scholar]
- Mack LA, Pasieka JL 2004 Asymptomatic primary hyperparathyroidism: a surgical perspective. Surg Clin North Am 84:803–816 [DOI] [PubMed] [Google Scholar]
- Rao DS, Phillips ER, Divine GW, Talpos GB 2004 Randomized controlled clinical trial of surgery versus no surgery in patients with mild asymptomatic primary hyperparathyroidism. J Clin Endocrinol Metab 89:5415–5422 [DOI] [PubMed] [Google Scholar]
- Hedbäck GM, Odén AS 2002 Cardiovascular disease, hypertension and renal function in primary hyperparathyroidism. J Intern Med 251:476–483 [DOI] [PubMed] [Google Scholar]
- Nilsson IL, Wadsten C, Brandt L, Rastad J, Ekbom A 2004 Mortality in sporadic primary hyperparathyroidism: nationwide cohort study of multiple parathyroid gland disease. Surgery 136:981–987 [DOI] [PubMed] [Google Scholar]
- Vestergaard P, Mosekilde L 2003 Cohort study on effects of parathyroid surgery on multiple outcomes in primary hyperparathyroidism. BMJ 327:530–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin MR, Bilezikian JP, McMahon DJ, Jacobs T, Shane E, Siris E, Udesky J, Silverberg SJ 2008 The natural history of primary hyperparathyroidism with or without parathyroid surgery after 15 years. J Clin Endocrinol Metab 93:3462–3470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilezikian JP, Khan AA, Potts Jr JT 2009 Guidelines for the management of asymptomatic primary hyperparathyroidism: summary statement from the Third International Workshop. J Clin Endocrinol Metab 94:335–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uden P, Chan A, Duh QY, Siperstein A, Clark OH 1992 Primary hyperparathyroidism in younger and older patients: symptoms and outcome of surgery. World J Surg 16:791–797; discussion 798 [DOI] [PubMed] [Google Scholar]
- Halpern MT, Bian J, Ward EM, Schrag NM, Chen AY 2007 Insurance status and stage of cancer at diagnosis among women with breast cancer. Cancer 110:403–411 [DOI] [PubMed] [Google Scholar]
- Ljunghall S, Hellman P, Rastad J, Akerström G 1991 Primary hyperparathyroidism: epidemiology, diagnosis and clinical picture. World J Surg 15:681–687 [DOI] [PubMed] [Google Scholar]
- Wermers RA, Khosla S, Atkinson EJ, Achenbach SJ, Oberg AL, Grant CS, Melton 3rd LJ 2006 Incidence of primary hyperparathyroidism in Rochester, Minnesota, 1993–2001: an update on the changing epidemiology of the disease. J Bone Miner Res 21:171–177 [DOI] [PubMed] [Google Scholar]
- National Center for Health Statistics 2009 Health, United States, 2008. Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention [Google Scholar]
- El-Hajj Fuleihan G 2008 Hyperparathyroidism: time to reconsider current clinical decision paradigms? J Clin Endocrinol Metab 93:3302–3304 [DOI] [PubMed] [Google Scholar]
- Zanocco K, Sturgeon C 2008 How should age at diagnosis impact treatment strategy in asymptomatic primary hyperparathyroidism? A cost-effectiveness analysis. Surgery 144:290–298 [DOI] [PubMed] [Google Scholar]
- Sosa JA, Udelsman R 2003 Minimally invasive parathyroidectomy. Surg Oncol 12:125–134 [DOI] [PubMed] [Google Scholar]