Abstract
Objective: The role of testosterone in the regulation of metabolic and physiological function in men is well defined, but its role in women remains enigmatic. Thus, the present study sought to assess the contribution of endogenous circulating androgens to the regulation of metabolic function, body morphometry, and physical function in normal naturally postmenopausal women.
Methods: Using a cross-sectional design, we measured serum androgens in a cohort of 29 naturally postmenopausal women and correlated the results with metabolic, morphometric, and functional outcome parameters. These included insulin sensitivity, whole-body fat and lean body mass, visceral/abdominal fat areasm and aerobic capacity.
Results: Higher serum testosterone levels were related to greater maximal aerobic capacity and reduced adiposity. Additionally, higher serum dihydrotestosterone, dehydroepiandrosterone sulfate, androstenedione, and androstenetriol glucuronidate levels were correlated to greater insulin sensitivity.
Conclusion: In naturally postmenopausal women, endogenous androgens may play a role in the maintenance of beneficial patterns of metabolic, morphometric, and functional parameters.
In naturally postmenopausal women, endogenous androgens are correlated with beneficial metabolic, morphometric, and functional patterns.
In men and women, sex steroids regulate metabolic function, body composition, and fat distribution. In men, the model of hypogonadism and hormone replacement provides convincing evidence for the physiological relevance of androgens (1,2), but in women, the situation is less clear. There are clinical situations of female sex steroid hormone deficiency and excess that are associated with metabolic dysfunction and altered body morphometry. The clearest example of this would be polycystic ovary syndrome (PCOS), a condition characterized by androgen and estrogen excess and associated with obesity, insulin resistance, and increased cardiovascular morbidity. Another prominent example is menopause, a situation characterized by estrogen deficiency, where adverse changes in metabolic function and body morphometry are observed. Despite these clinical examples of sex hormone deficiency and excess in women, there are still questions regarding the role of these steroids in the regulation of physiological and metabolic function in women.
In menopause, although much attention has been directed to the potential role of estrogen replacement in the maintenance and regulation of metabolic function and body composition in women, interventions with various estrogen replacement regimens have not demonstrated reproducible attenuation or reversal of menopause-associated adverse anthropomorphic changes, insulin resistance, and declines in functional capacity (3). We have demonstrated that a state of pharmacologically induced ovarian estrogen deprivation is not associated with changes in insulin sensitivity or body composition (4,5). Thus, the possibility exists that sex steroids other than estradiol are important for maintenance of physiological and metabolic function in postreproductive women.
Although an order of magnitude lower than in men, testosterone (T) circulates in appreciable amounts in women, and androgen receptors are prevalent in a variety of nonreproductive tissues (6), particularly skeletal muscle and fat (7,8). In contrast to men, where T is widely regarded as beneficial, in women, largely based on observations in PCOS-associated hyperandrogenemia, conventional wisdom suggests the effect of T on these parameters is adverse. Several lines of evidence suggest, however, that androgen levels in women within the normal physiological range may have beneficial effects on metabolic function, body morphometry, and functional capacity (9,10,11,12,13,14,15,16,17,18). On balance, these studies raise the possibility that androgens influence metabolic and physiological function in menopausal women under some circumstances, although the nature of this effect is poorly defined.
Thirty percent of women enter menopause surgically (19), and oophorectomy after the age of 40 is standard gynecological procedure for ovarian cancer prophylaxis (20), the prevalence of the procedure having doubled in the last 30 yr (21). By virtue of the loss of LH-driven stromal and thecal T production, serum androgen levels decline 30–50% in oophorectomized women (9,15,22), creating a potential iatrogenic female androgen deficiency state. Despite this fact, the physiological relevance of endogenous circulating levels of androgens in postmenopausal women has not been clearly defined. Indeed, recent data imply an excess mortality in women who have undergone prophylactic oophorectomy, even after the natural age of menopause (23,24). The situation is further confused by problems related to measuring low circulating androgen levels in postreproductive women (25). Thus, the primary goal of the current study was to evaluate the relationship of circulating androgens, androgen precursors, and androgen metabolites as potential determinants of body morphometry, insulin sensitivity, muscle strength, and aerobic capacity in naturally menopausal women. We hypothesized that endogenous circulating androgens would exhibit relationships to these variables suggesting a beneficial effect on body composition/fat distribution, insulin sensitivity, and functional capacity. To test this hypothesis, we designed a cross-sectional study in healthy naturally postmenopausal women free from the confounding effect of obesity, insulin resistance, and previous PCOS and using validated techniques to measure the low circulating androgen levels and their metabolites in this population. These hormonal assays were coupled with criterion techniques for estimating body composition, fat distribution, metabolic function, and functional capacity.
Subjects and Methods
Subject characteristics
Women who were normal weight to nonobese (i.e. nonobese; body mass index ≤ 30 kg/m2) and between 50 and 70 yr of age were recruited. We excluded anyone with a past reproductive history of PCOS (Rotterdam criteria, 2004) or infertility. Women with a past or present history of androgen blockade use or a history of hyperandrogenemia or hyperinsulinemia were also excluded. We studied women that underwent natural menopause, defined as at least 1 yr since the last menses. In all cases, menopause was confirmed by FSH higher than 50 mIU/ml and estradiol less than 30 pg/ml. Subjects were determined to be euthyroid based on a TSH level between 0.5 and 5.0 mIU/ml, and patients with diabetes (hemoglobin A1C < 6% and fasting glucose > 112 mg/dl) were excluded. Finally, subjects were excluded if they had used any form of estrogen, androgen, or phytoestrogen preparations in the last year. Written consent was obtained by all participants, and the protocol was approved by the Committee on Human Research of the University of Vermont.
Experimental protocol
Subjects were recruited from the Reproductive Endocrine service of Fletcher Allen Health Care and through local advertising. After a brief telephone interview, subjects that met initial eligibility requirements were invited to an outpatient screening visit, where a medical history, physical exam, and blood work was performed to assure they met inclusion criteria. Volunteers who were eligible after screening were invited to one outpatient and one inpatient visit. Strength and aerobic capacity measurements were performed on the outpatient visit. After an interval of at least 1 wk, and after a 2-d standardized, weight-maintenance diet that provided at least 200 g carbohydrate/d, subjects were admitted for an inpatient visit. On the evening of admission, an abdominal computed tomography (CT) scan was performed, and the volunteer fasted from 1900 h until the completion of testing the following day. On the following morning, a hyperinsulinemic-euglycemic clamp was performed, and body composition was measured by dual-energy x-ray absorptiometry (DEXA) scan.
Skeletal muscle strength
Knee extensor torque production was measured under isometric and isokinetic conditions using a multijoint dynamometer (HUMAC/NORM; Computer Sports Medicine Inc., Stoughton, MA). The right leg was tested in all volunteers. After instructions, volunteers were allowed to perform two practice trials for each condition at moderate intensity (∼25%) to ensure familiarity with the procedure. For isometric measurements, the lever arm was fixed at 55°. Volunteers performed three brief (5 sec) maximal voluntary contractions each separated by 1 min of rest. The highest torque (Newton · meter) value for each contraction was recorded, and the average from the three trials calculated. Isokinetic measurements were performed at 90°/sec. The range of motion was set from 0–90° flexion relative to full knee extension, and five consecutive contractions were performed. The average of the three highest torque values was calculated.
Exercise capacity
Maximal aerobic capacity [maximal oxygen consumption (VO2 max)] was assessed by a progressive and continuous test to exhaustion on a treadmill, as described (26). VO2 max was defined as the highest 30-sec average VO2 reached during the test.
CT
Abdominal fat, thigh fat, and muscle cross-sectional areas were assessed by CT scan, as described previously (5). Cross-sectional scans of 10 mm thickness were obtained centered between L4–L5 vertebral disc spaces and at the midpoint between the anterior superior iliac crest and the patella. Tissue areas were delineated using attenuation values of −190 to −30 Hounsfield units (HU) for adipose tissue, 0–100 HU for muscle, and more than 200 HU for bone. The boundary for sc and visceral abdominal adipose tissue depots was defined manually as the muscle wall surrounding the abdominal cavity. The abdominal sc adipose tissue area was calculated by subtracting the abdominal visceral adipose tissue area from the total abdominal adipose tissue area.
Hyperinsulinemic-euglycemic clamp
A euglycemic-hyperinsulinemic clamp was performed according to the method of DeFronzo et al. (27) to measure insulin sensitivity. After an overnight fast, catheters were placed in an antecubital vein for infusion and in a dorsal hand vein for blood sampling. The subject’s hand was warmed in a heated hand box (air temperature 50–55 C) for at least 15 min before blood draws to obtain arterialized venous blood (28). After placement of the iv lines, a 1-h baseline period was observed at which samples for fasting plasma glucose and insulin were drawn. After this baseline period, a continuous infusion of insulin (40 mU/m2 · min) was started and maintained for 2 h to produce an elevation of plasma insulin similar to postprandial conditions. Euglycemia was maintained during hyperinsulinemia by a variable-rate infusion of 20% dextrose. Plasma glucose concentration was measured every 5 min during insulin infusion, and the 20% dextrose infusion rate was adjusted to maintain euglycemia. The average rate of exogenous glucose infusion was used as a proxy of insulin sensitivity, assuming near full suppression of endogenous glucose production. This assumption is reasonable because in similarly aged women, we have previously shown that suppression of glucose production is near maximal at this insulin dose (3,29). Throughout the manuscript, insulin sensitivity is presented as the value adjusted for lean body mass.
DEXA
DEXA scans were used to measure total body fat mass and lean body mass using a GE Lunar Prodigy fan beam densitometer (Madison, WI), as described previously (30). In addition to whole-body composition measurements, appendicular and skeletal muscle mass was also assessed (31).
Hormone and substrate assays
Insulin and glucose measurements collected during the hyperinsulinemic-euglycemic clamp were measured in the General Clinical Research Center biochemistry core, using a validated ELISA and the glucose oxidase method, respectively. For steroid assays, the serum was stored at −80 C and batch assayed for androstenedione (Δ4A), dehydroepiandrosterone (DHEA), total T, estrone (E1), and estradiol (E2) using RIA methods, validated for the lower levels of these hormones seen in this population (32,33). Appropriate tritiated internal standards were added to each sample to follow procedural losses. A single aliquot of sample was taken to extract T, Δ4A, DHEA, E1, and E2 using ethyl acetate/hexane (3:2). This was followed by separation of the four steroids by Celite column partition chromatography, using ethylene glycol as the stationary phase. Δ4A was eluted with isooctane alone, and DHEA and T were eluted with 15 and 25% toluene in isooctane, respectively, whereas E1 and E2 were eluted with 15 and 40% ethyl acetate in isooctane, respectively. After drying each fraction, the residue was reconstituted in assay buffer. An aliquot of each was taken to determine procedural loss, and duplicate aliquots were taken for the RIA of each hormone. Each RIA used an iodinated radioligand in conjunction with a specific antiserum. A seven-point standard was included in each assay, encompassing the expected range for postmenopausal women. After an overnight incubation, antibody-bound steroid was separated from unbound steroid by precipitation of the first antibody with a second antibody, and subsequent centrifugation. DHEA sulfate (DHEAS) and SHBG were quantified by direct chemiluminescent immunoassay using the Immulite analyzer (Siemens Medical Solutions Diagnostics, Los Angeles, CA). The SHBG concentration and an average albumin concentration were then used in a validated algorithm with total T to calculate free T (34,35,36). Quality control samples containing low, medium, and high levels of the androgens, estrogens, and SHBG were used in each assay. The intraassay coefficients of variation of these assays range between 4.0 and 7.5% for all the compounds, whereas the interassay coefficients of variation range between 8.0 and 13%. The assay sensitivities for Δ4A, DHEA, T, E1, E2, DHEAS, and SHBG are 0.03 ng/ml, 0.2 ng/ml, 1.5 ng/dl, 5 pg/ml, 3 mg/ml, 3 μg/dl, and 1 nm, respectively. Dihydrotestosterone (DHT) and androsterone glucoronide (ADT-G) were measured by HPLC mass spectrometry with inter- and intraassay coefficients of variation that did not exceed 6.4%. The limit of detection of the DHT and ADT-G were 10 pg/ml and 2 ng/ml, respectively.
Statistical analysis
Before statistical analysis, the normality of the distribution of variables was assessed using Shapiro-Wilks test. Any variables that were not normally distributed (e.g. hormone levels) were log10 transformed, and normality was reassessed. All variables were normally distributed after log10 transformation. VO2 max data were adjusted statistically for lean body mass using analysis of covariance. Relationships between variables were assessed by Pearson correlation coefficients. All data are presented as means ± se.
Results
Physical characteristics and hormone levels
Twenty-nine subjects participated, all naturally menopausal. The baseline physical characteristics and steroid hormone levels of the subjects are outlined in Tables 1 and 2. Data are missing for DHT and ADT-G measurements because levels of these hormones were below the detection limit of the assay in some volunteers.
Table 1.
Baseline demographic, metabolic, and functional characteristics
| Variable | Mean ± se | Range |
|---|---|---|
| Age (yr) | 60.7 ± 1.0 | 52–70 |
| Years from menopause | 10.5 ± 1.3 | 3–27 |
| Body mass index (kg/m2) | 24.8 ± 0.5 | 20.3–30.2 |
| Lean body mass (kg) | 42.3 ± 0.71 | 34.8–48.4 |
| Fat mass (kg) | 26.3 ± 1.3 | 13.2–39.7 |
| Body fat (%) | 37.9 ± 1.2 | 23.1–51.3 |
| Total abdominal fat (cm2) | 406.0 ± 20.3 | 197–613 |
| Abdominal visceral fat (cm2) | 114.9 ± 7.6 | 65–224 |
| Abdominal subcutaneous fat (cm2) | 291.1 ± 15.2 | 124–427 |
| Visceral/sc ratio | 0.409 ± 0.023 | 0.22–0.63 |
| Insulin sensitivity (mg/kg lean body mass · min) | 12.00 ± 0.55 | 6.8–17.5 |
| VO2 max (ml/kg lean body mass · min) | 47.0 ± 1.2 | 33.4–56.4 |
| Knee extensor isokinetic torque (N · m) | 126.2 ± 6.0 | 55.0–176.5 |
| Knee extensor isometric torque (N · m) | 189.9 ± 9.4 | 73.4–313.7 |
Table 2.
Baseline hormonal characteristics
| Variable | Mean ± se | Range |
|---|---|---|
| Δ4A (ng/dl) | 473.8 ± 33.2 | 199–922 |
| DHEA (ng/ml) | 1.9 ± 0.2 | 0.50–4.35 |
| DHEAS (μg/dl) | 45.9 ± 5.5 | 15–123 |
| T (ng/dl) | 22.6 ± 1.5 | 10.6–482 |
| Free T (pg/ml) | 4.3 ± 0.3 | 2.1–10.1 |
| E2 (pg/ml) | 6.9 ± 0.4 | 4–13.9 |
| E1 (pg/ml) | 26.6 ± 2.0 | 15–67 |
| SHBG (nmol/liter) | 49.3 ± 3.2 | 26–99 |
| DHT (ng/dl) | 24.4 ± 3.4 (n = 24) | 5.1–61.8 |
| ADT-G (ng/ml) | 10.0 ± 2.0 (n = 26) | 2.4–45.5 |
Note that data for all hormones are provided as raw values, although correlation analysis was performed on log10-transformed values. Data represent n = 29, except for DHT and ADT-G measurements, which have lower sample sizes because levels were below the detection limit of the assay in several volunteers (sample size in parentheses).
Correlations between steroid levels and outcome parameters
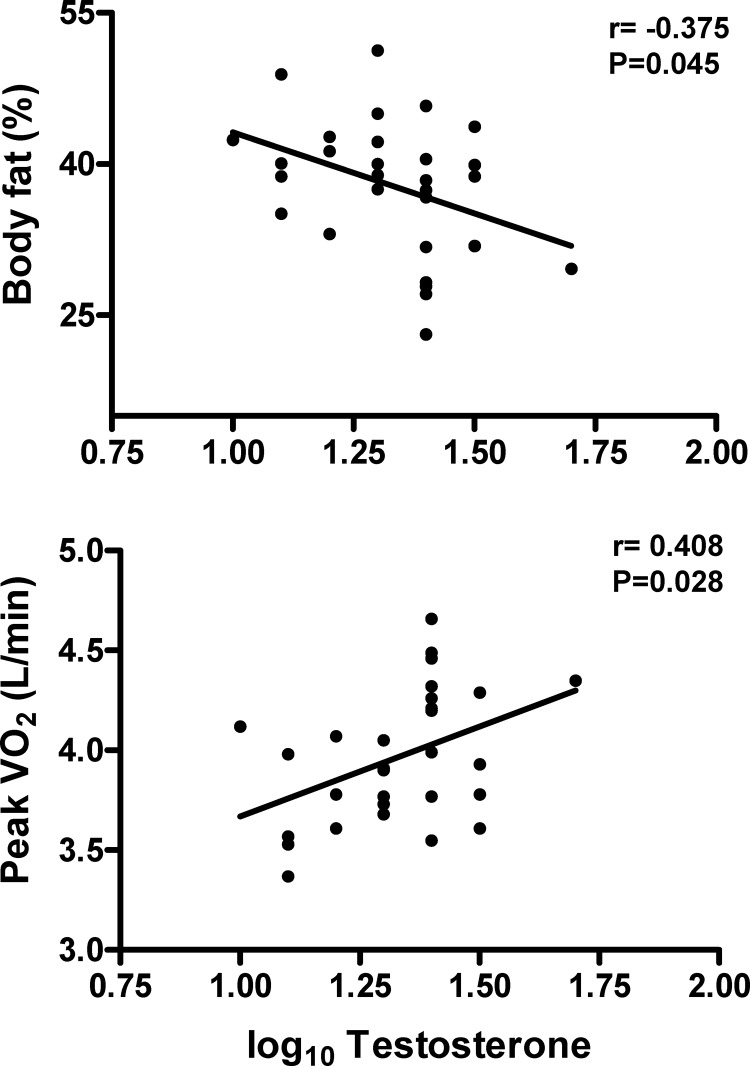
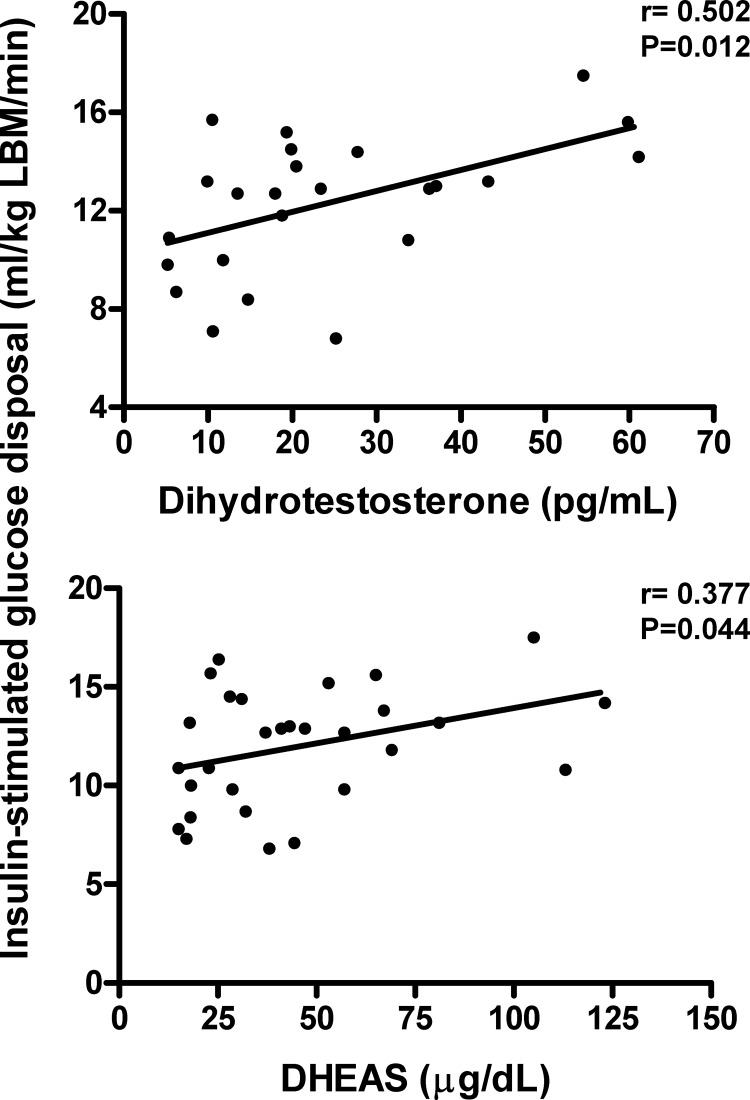
In these women, several associations were seen (Table 3 and Figs. 1 and 2). Most notably, of the major androgens, total T levels were negatively related to body fat percentage (P < 0.05) and fat mass (P < 0.10) and were positively related to aerobic capacity (uncorrected for lean body mass, P < 0.05, shown in Fig. 1; P < 0.10, corrected for lean body mass, correlation coefficient shown in Table 3). Additionally, DHT was positively related to insulin sensitivity (P < 0.05). As expected, SHBG was negatively correlated with body fat indices (P < 0.01) and positively related to insulin sensitivity (P < 0.01). Also of interest, the androgenic precursors DHEAS and Δ4A showed positive associations with insulin sensitivity (P < 0.05), and there was a negative relationship between DHEAS and thigh muscle area. Finally, the androgen metabolite ADT-G was positively related to insulin sensitivity (P < 0.05).
Table 3.
Correlations between log10-transformed steroid data and morphological, physiological, and metabolic variables
| Pearson correlation coefficients |
|||||||
|---|---|---|---|---|---|---|---|
| DHT | T | Free T | DHEAS | SHBG | Δ4A | ADT-G | |
| Body fat percentage | −0.175 | −0.375 | −0.084 | 0.081 | −0.536 | −0.202 | 0.033 |
| Fat mass | −0.293 | −0.332 | −0.028 | 0.037 | −0.580 | −0.146 | −0.012 |
| Lean body mass | 0.224 | 0.203 | 0.206 | −0.081 | −0.063 | 0.225 | −0.081 |
| Insulin sensitivity | 0.502 | 0.282 | 0.033 | 0.377 | 0.470 | 0.367 | 0.442 |
| VO2 max | 0.331 | 0.336 | 0.226 | 0.085 | 0.171 | 0.121 | 0.243 |
| Total abdominal fat | 0.152 | −0.090 | −0.084 | 0.188 | −0.007 | 0.027 | −0.027 |
| Visceral abdominal fat | 0.340 | −0.110 | −0.155 | 0.003 | 0.128 | −0.190 | −0.111 |
| Subcutaneous abdominal fat | 0.040 | −0.065 | −0.034 | 0.251 | −0.074 | 0.132 | 0.023 |
| Visceral/sc ratio | 0.224 | 0.044 | −0.030 | −0.246 | 0.181 | −0.311 | −0.134 |
| Thigh muscle area | 0.029 | 0.163 | 0.007 | −0.441 | 0.316 | −0.157 | −0.210 |
All data are n = 29, with the exception of correlations for DHT (n = 24), ADT-G (n = 26), and thigh muscle area (n = 28). Data are missing for hormone levels because levels were below the limit of detection of the assay and for thigh muscle area because of technical problems with completing the thigh scan on one patient.
P < 0.05.
P < 0.01.
P < 0.10.
Figure 1.
Correlations between serum T levels and morphometric and functional measures (n = 29). Note that the data shown for the relationship to peak VO2 are for data expressed as liters per minute, whereas data shown in Table 3 show correlations for peak VO2 expressed per kilogram of lean body mass (LBM).
Figure 2.
Positive correlations between insulin sensitivity and serum DHT (n = 24) and DHEAS (n = 29).
Discussion
In this study, we selected naturally postmenopausal women who had no history of hyperandrogenemia, hyperinsulinemia, or polycystic ovarian disease to isolate the associations between endogenous androgen levels and metabolic, morphometric, and functional parameters. These relationships generally mirror those present in men and suggest that increasing androgen levels within the physiological range (i.e. not hyperandrogenemia) in nonobese, postmenopausal women may be beneficial in maintaining more favorable metabolic function, body composition, and physical function.
In these women, increasing androgen levels were associated with lower total body fat and higher aerobic capacity. These results suggest that in the setting of the natural menopause transition, it appears that postmenopausal androgen levels show beneficial relationships to indices of total and abdominal adiposity. This view runs counter to the conventional wisdom drawn from the clinical syndrome of PCOS, where increasing androgenicity is thought to potentiate adiposity and further suggests that the relationship of endogenous androgens in women is far more complex.
Another interesting finding was that androgen precursors and metabolites all showed positive relationships to insulin sensitivity in this group of naturally menopausal women. Based on the clinical syndrome of PCOS, it has historically been postulated that increased androgenicity promotes insulin resistance in women (although we acknowledge more recent findings suggesting that insulin resistance and the resulting hyperinsulinemia may, in fact, potentiate hyperandrogenemia). However, our results would suggest that in postmenopausal women that have undergone natural menopause, androgens may have a sustaining effect on tissue insulin sensitivity. Our results agree with recent studies showing that DHEA replacement improves insulin sensitivity in hypoadrenal women (37). The nature of this effect is unclear. It may reflect a direct action of androgens on skeletal muscle and other insulin-sensitive tissues to increase glucose metabolism and/or the size of the tissue (i.e. muscle mass) or may be a result of the association of androgens to lower body fat levels, as mentioned above. The former interpretation, where androgens affect tissue glucose metabolism, appears more likely considering that the effects of androgen precursors on insulin sensitivity appear over a relatively short period of time, where body fat and lean body mass do not change (37). Further reinforcing this conclusion, we found that circulating DHT and ADT-G are both related to insulin sensitivity. Considering that both are formed by the interaction of androgens with enzymes at target tissues that contain steroid-metabolizing enzymes, these relationships provide further circumstantial evidence supporting a direct effect on insulin-sensitive tissues.
The correlations between SHBG and parameters of obesity and insulin resistance in both cohorts are not surprising given that circulating levels of this globulin are down-regulated with increasing body fat and insulinemia (38,39). These relationships are likely partially the result of our inclusion of women with a range of adiposity levels, from normal weight to overweight. Because of this effect of adiposity and insulin on SHBG levels, our results on SHBG are likely associative rather than causative. In this regard, the relationship between increased free T and adiposity in the cohort likely reflect the corresponding regulation of SHBG by body fat and insulinemia, although studies that evaluate changes in these variables over time are needed to clarify these relationships.
Circulating DHT is considered a marker of androgen effect on tissues endowed with the enzyme 5α-reductase type II. Thus, the relationship between DHT and morphometric and metabolic variables may be a better indicator of the physiological effects of androgens on target tissues, although we are careful to acknowledge that circulating levels of DHT may not reflect tissue levels. In this study, DHT levels were positively associated with insulin sensitivity. Similarly, ADT-G, considered a marker of target tissue androgen effect, was also associated with insulin sensitivity.
The adrenal androgens Δ4A and DHEAS were also associated with greater insulin sensitivity in these naturally menopausal women, even after correction for fat-free mass. Our data support the insulin-sensitizing effect of DHEA replacement observed by some (18,37) but not all (40). The reason for these disparate findings is not clear, although we note that the present study and others (21) that have found relationships between DHEA and insulin sensitivity have used the criterion technique for estimating tissue insulin sensitivity, the euglycemic-hyperinsulinemic clamp. Moreover, we cannot discount the possibility that there are differences between the physiological effects of endogenous vs. exogenous orally administered androgens/androgen precursors.
Our data support the contention that endogenous androgens are, in general, associated with beneficial patterns of morphometric, metabolic, and functional parameters in naturally postmenopausal women. It must be acknowledged that an alternate interpretation of the data is that circulating androgens are simply a biomarker of better health in this group. Thus, firm conclusions regarding the effect of androgens on metabolic function and body composition and physical function will require studies that manipulate androgen levels and/or action and the resulting alterations in these variables.
Another interesting finding is that different androgens have differential relationships with outcome variables. DHT, Δ4A, and DHEAS were related to greater insulin sensitivity, whereas endogenous T was more related to beneficial morphometric indices and functional capacity. The former androgens are notable for their relation to end-organ androgen metabolism. Certainly DHEA and DHEAS have been related to improved insulin sensitivity in a number of studies. Thus, some of the observed correlations between androgen precursors and outcome variables may reflect intracrine effects, a contention supported by the close association seen between insulin sensitivity and the target tissue metabolites of adrenal androgens DHT and ADT-G.
In naturally menopausal, nonobese women, without evidence of current or previous hyperinsulinemia or hyperandrogenemia, endogenous androgens are associated with a more beneficial body morphometry, insulin sensitivity, and functional capacity profile. An important strength of our study is the methodology used to measure androgens and their precursors and metabolites, specifically, to obtain reliable estimates of androgen levels at the low circulating concentrations usually present in postmenopausal women. Our novel findings, although correlative in nature and needing confirmation in larger cohorts and more rigorous prospective and mechanistic protocols, suggest a potential beneficial role for physiological levels of endogenous androgens in metabolic and physiological function and morphometry in postmenopausal women.
Footnotes
This work was supported by National Institutes of Health University of Vermont General Clinical Research Center Award M01 RR00109 (CReFF Award to P.R.C.).
Disclosure Summary: The authors have nothing to disclose.
First Published Online June 21, 2010
Abbreviations: Δ4A, Androstenedione; ADT-G, androsterone glucoronide; CT, computed tomography; DEXA, dual-energy x-ray absorptiometry; DHEA, dehydroepiandrosterone; DHEAS, DHEA sulfate; DHT, dihydrotestosterone; E1, estrone; E2, estradiol; HU, Hounsfield units; PCOS, polycystic ovary syndrome; T, testosterone; VO2 max, maximal oxygen consumption.
References
- Ferrando AA, Sheffield-Moore M, Yeckel CW, Gilkison C, Jiang J, Achacosa A, Lieberman SA, Tipton K, Wolfe RR, Urban RJ 2002 Testosterone administration to older men improves muscle function: molecular and physiological mechanisms. Am J Physiol Endocrinol Metab 282:E601–E607 [DOI] [PubMed] [Google Scholar]
- Bhasin S 2003 Effects of testosterone administration on fat distribution, insulin sensitivity, and atherosclerosis progression. Clin Infect Dis 37(Suppl 2):S142–S149 [DOI] [PubMed] [Google Scholar]
- Sites CK, L'Hommedieu GD, Toth MJ, Brochu M, Cooper BC, Fairhurst PA 2005 The effect of hormone replacement therapy on body composition, body fat distribution, and insulin sensitivity in menopausal women: a randomized, double-blind, placebo-controlled trial. J Clin Endocrinol Metab 90:2701–2707 [DOI] [PubMed] [Google Scholar]
- Cooper BC, Sites CK, Casson PR, Toth MJ 2007 Ovarian suppression with gonadotropin-releasing hormone agonist does not alter insulin-stimulated glucose disposal. Fertil Steril 87:1131–1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth MJ, Cooper BC, Pratley RE, Mari A, Matthews DE, Casson PR 2008 Role of ovarian hormones in the regulation of glucose homeostasis: effect of ovarian suppression with gonadotropin-releasing hormone agonist on glucose disposal and insulin secretion. Am J Physiol Endocrinol Metab 294:E1035–E1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CM, McPhaul MJ 1996 A and B forms of the androgen receptor are expressed in a variety of human tissues. Mol Cell Endocrinol 120:51–57 [DOI] [PubMed] [Google Scholar]
- Copas P, Bukovsky A, Asbury B, Elder RF, Caudle MR 2001 Estrogen, progesterone, and androgen receptor expression in levator ani muscle and fascia. J Womens Health Gend Based Med 10:785–795 [DOI] [PubMed] [Google Scholar]
- Anderson LA, McTernan PG, Harte AL, Barnett AH, Kumar S 2002 The regulation of HSL and LPL expression by DHT and flutamide in human subcutaneous adipose tissue. Diabetes Obes Metab 4:209–213 [DOI] [PubMed] [Google Scholar]
- Sowers MF, Beebe JL, McConnell D, Randolph J, Jannausch M 2001 Testosterone concentrations in women aged 25–50 Years: associations with lifestyle, body composition, and ovarian status. Am J Epidemiol 153:256–264 [DOI] [PubMed] [Google Scholar]
- Dobs AS, Nguyen T, Pace C, Roberts CP 2002 Differential effects of oral estrogen versus oral estrogen-androgen replacement therapy on body composition in postmenopausal women. J Clin Endocrinol Metab 87:1509–1516 [DOI] [PubMed] [Google Scholar]
- Gruber DM, Sator MO, Kirchengast S, Joura EA, Huber JC 1998 Effect of percutaneous androgen replacement therapy on body composition and body weight in postmenopausal women. Maturitas 29:253–259 [DOI] [PubMed] [Google Scholar]
- Davis SR, McCloud P, Strauss BJ, Burger H 1995 Testosterone enhances estradiol’s effects on postmenopausal bone density and sexuality. Maturitas 21:227–236 [DOI] [PubMed] [Google Scholar]
- Diamond P, Cusan L, Gomez JL, Belanger A, Labrie F 1996 Metabolic effects of 12-month percutaneous dehydroepiandrosterone replacement therapy in postmenopausal women. J Endocrinol 150(Suppl):S43–S50 [PubMed] [Google Scholar]
- Villareal DT, Holloszy JO, Kohrt WM 2000 Effects of DHEA replacement on bone mineral density and body composition in elderly women and men. Clin Endocrinol (Oxf) 53:561–568 [DOI] [PubMed] [Google Scholar]
- Kritz-Silverstein D, Barrett-Connor E, Wingard DL 1997 Hysterectomy, oophorectomy, and heart disease risk factors in older women. Am J Public Health 57:676–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casson PR, Faquin LC, Stentz FB, Straughn AB, Andersen RN, Abraham GE, Buster JE 1995 Replacement of dehydroepiandrosterone (DHEA) enhances T-lymphocyte insulin binding in postmenopausal women. Fertil Steril 63:1027–1031 [PubMed] [Google Scholar]
- Bates Jr GW, Egerman RS, Umstot ES, Buster JE, Casson PR 1995 Dehydroepiandrosterone (DHEA) attenuates study-induced declines in insulin sensitivity in postmenopausal women. Ann NY Acad Sci 774:291–293 [DOI] [PubMed] [Google Scholar]
- Villareal DT, Holloszy JO 2004 Effect of DHEA on abdominal fat and insulin action in elderly women and men: a randomized controlled trial. JAMA 292:2243–2248 [DOI] [PubMed] [Google Scholar]
- Dawson DA, Thompson GB 1987 Breast cancer risk factors and screening: United States, 1987. National Center for Health Statistics. Vital Health Stat Series 10, No. 172. Hyattsville, MD: Department of Health and Human Services [PubMed] [Google Scholar]
- American College of Obstetricians and Gynecologists 1999 Prophylactic oophorectomy. ACOG practice bulletin No. 7. Washington, DC: American College of Obstetricians and Gynecologists [Google Scholar]
- Keshavarz H, Hillis SD, Kieke BA, Marchbanks PA 2002 Hysterectomy surveillance: United States, 1994–1999. MMWR Morb Mortal Wkly Rep 51 (SS05):1–8 [Google Scholar]
- Judd HL, Judd GE, Lucas WE, Yen SS 1974 Endocrine function of the postmenopausal ovary: concentration of androgens and estrogens in ovarian and peripheral vein blood. J Clin Endocrinol Metab 39:1020–1024 [DOI] [PubMed] [Google Scholar]
- Parker WH, Broder MS, Liu Z, Shoupe D, Farquhar C, Berek JS 2005 Ovarian conservation at the time of hysterectomy for benign disease. Obstet Gynecol 106:219–226 [DOI] [PubMed] [Google Scholar]
- Parker WH, Broder MS, Chang E, Feskanich D, Farquhar C, Liu Z, Shoupe D, Berek JS, Hankinson S, Manson JE 2009 Ovarian conservation at the time of hysterectomy and long-term health outcomes in the Nurses’ Health Study. Obstet Gynecol 113:1027–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosner W, Auchus RJ, Azziz R, Sluss PM, Raff H 2007 Utility, limitations, and pitfalls in measuring testosterone: an endocrine society position statement. J Clin Endocrinol Metab 92:405–413 [DOI] [PubMed] [Google Scholar]
- Toth MJ, Goran MI, Ades PA, Howard DB, Poehlman ET 1993 Examination of data normalization procedures for expressing peak VO2 data. J Appl Physiol 75:2288–2292 [DOI] [PubMed] [Google Scholar]
- DeFronzo RA, Tobin JD, Andres R 1979 Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 237:E214–E223 [DOI] [PubMed] [Google Scholar]
- Abumrad NN, Rabin D, Diamond MP, Lacy WW 1981 Use of a heated superficial hand vein as an alternative site for the measurement of amino acid concentrations and for the study of glucose and alanine kinetics in man. Metabolism 30:936–940 [DOI] [PubMed] [Google Scholar]
- Toth MJ, Poehlman ET, Matthews DE, Tchernof A, MacCoss MJ 2001 Effect of estradiol and progesterone on body composition, protein synthesis and lipoprotein lipase in rats. American Journal of Physiology 280:E496–E501 [DOI] [PubMed] [Google Scholar]
- Toth MJ, Poehlman ET, Matthews DE, Tchernof A, MacCoss MJ 2000 Effect of menopausal status on body composition and abdominal fat distribution. Int J Obes Relat Metab Disord 24:226–231 [DOI] [PubMed] [Google Scholar]
- Heymsfield SB, Smith R, Aulet M, Bensen B, Lichtman S, Wang J, Pierson Jr RN 1990 Appendicular skeletal muscle mass: measurement by dual-photon absorptiometry. Am J Clin Nutr 52:214–218 [DOI] [PubMed] [Google Scholar]
- Goebelsmann U, Arce JJ, Thorneycroft IH, Mishell Jr DR 1974 Serum testosterone levels in women throughout the menstrual cycle and following hCG administration. Am J Obstet Gynecol 119:445–452 [DOI] [PubMed] [Google Scholar]
- Goebelsmann U, Bernstein GS, Gale JA, Kletzky OA, Nakamura RM, Coulson AH, Korelitz JJ 1979 Serum gonadotropin, testosterone, estradiol and estrone levels prior to and following bilateral vasectomy. New York: Academic Press [Google Scholar]
- Sodergard R, Backstrom T, Shanbhag V, Carstensen H 1982 Calculation of free and bound fractions of testosterone and estradiol-17β to human plasma protein at body temperature. J Steroid Biochem 26:801–810 [DOI] [PubMed] [Google Scholar]
- Vermeulen A, Verdonck L, Kaufman JM 1999 A critical evaluation of simple methods for estimation of free testosterone in serum. J Clin Endocrinol Metab 84:3666–3672 [DOI] [PubMed] [Google Scholar]
- Rinaldi S, Geay A, Déchaud H, Biessy C, Zeleniuch-Jacquotte A, Akhmedkhanov A, Shore RE, Riboli E, Toniolo P, Kaaks R 2002 Validity of free testosterone and free estradiol determinations in serum samples from postmenopausal women by theoretical calculations. Cancer Epidemiol Biomarkers Prev 11:1065–1071 [PubMed] [Google Scholar]
- Dhatariya K, Bigelow ML, Nair KS 2005 Effect of dehydroepiandrosterone replacement on insulin sensitivity and lipids in hypoadrenal women. Diabetes 54:765–769 [DOI] [PubMed] [Google Scholar]
- Sternfeld B, Liu K, Quesenberry Jr CP, Wang H, Jiang SF, Daviglus M, Fornage M, Lewis CE, Mahan J, Schreiner PJ, Schwartz SM, Sidney S, Williams OD, Siscovick DS 2008 Changes over 14 years in androgenicity and body mass index in a biracial cohort of reproductive-age women. J Clin Endocrinol Metab 93:2158–2165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plymate SR, Matej LA, Jones RE, Friedl KE 1988 Inhibition of sex hormone binding globulin production in the human hepatoma (Hep G2) cell line by insulin and prolactin. J Clin Endocrinol Metab 67:460–464 [DOI] [PubMed] [Google Scholar]
- Nair KS, Rizza RA, O'Brien P, Dhatariya K, Short KR, Nehra A, Vittone JL, Klee GG, Basu A, Basu R, Cobelli C, Toffolo G, Dalla Man C, Tindall DJ, Melton LJ 3rd, Smith GE, Khosla S, Jensen MD 2006 DHEA in elderly women and DHEA or testosterone in elderly men. N Engl J Med 355:1647–1659 [DOI] [PubMed] [Google Scholar]