Abstract
Context: Antimitogenic effects of estradiol on vascular smooth muscle cells (VSMCs) may be cardioprotective, and these effects are mediated by estrogen receptor-α-dependent and -independent mechanisms, with the latter involving the conversion of estradiol to 2-hydroxyestradiol/2-methoxyestradiol by CYP450. Because resveratrol inhibits CYP450 and is an estrogen-receptor-α antagonist, resveratrol may abrogate the antimitogenic effects of estradiol.
Objective: The objective of the study was to examine the interaction of pharmacologically relevant concentrations of resveratrol with estradiol, 2-hydroxyestradiol, and 2-methoxyestradiol in human female coronary artery VSMCs.
Methods and Results: In human female coronary VSMCs, resveratrol (0.1–10 μm) alone did not influence serum-induced DNA or collagen synthesis or cell proliferation or migration; however, resveratrol abrogated the inhibitory effects of estradiol, but not 2-hydroxyestradiol or 2-methoxyestradiol, on these responses. Resveratrol also abrogated the inhibitory effects of estradiol on positive growth regulators (cyclin A, cyclin D, MAPK phosphorylation) and the stimulatory effects of estradiol on negative growth regulators (p21, p27). In microsomes and cells, dietarily relevant levels of resveratrol (0.001–1 μm) inhibited the metabolism of estradiol to 2-hydroxestradiol/2-methoxyestradiol. Propylpyrazoletriol (estrogen receptor-α agonist, 100 nmol/liter), but not diarylpropionitrile (estrogen receptor-β agonist, 10 nmol/liter), inhibited VSMC mitogenesis, and this effect was blocked by resveratrol (5 μmol/liter). Higher concentrations (>25–50 μm) of resveratrol, never attainable in vivo, inhibited VSMC growth, an effect blocked by GW9662 (peroxisomal proliferator-activated receptor-γ antagonist).
Conclusion: In conclusion, dietarily relevant levels of resveratrol abrogate the antimitogenic effects of estradiol by inhibiting CYP450-mediated estradiol metabolism and blocking estrogen receptor-α.
Circulating-concentrations of resveratrol (red-wine constituent) block the growth-inhibitory actions of estradiol on vascular smooth muscle cells and may abrogate its vasoprotective actions in women.
Resveratrol is a polyphenol that at high concentrations (5–100 μmol/liter) decreases platelet aggregation, promotes vasorelaxation, reduces lipid peroxidation, scavenges oxygen radicals in vitro, protects against ischemic-reperfusion injury in isolated organs, and suppresses atherosclerosis and lowers serum cholesterol and triglyceride concentrations in vivo (1,2). Because red wine contains resveratrol (1), some propose that resveratrol in red wine explains the French paradox, i.e. the low incidence of coronary disease in the French despite diet high in saturated fats (1).
Estradiol mediates vasculoprotective actions via multiple mechanisms including inhibition of vascular smooth muscle cell (VSMC) proliferation (3,4). Although the role of estrogen receptors (ERs) in the vasculoprotective actions of estradiol are controversial (3), most likely estradiol induces vascular protection via both ER-α-dependent (4) and ER-independent (3) mechanisms. With regard to ER-independent mechanisms, studies using pharmacological agents that block or stimulate the sequential conversion of estradiol to hydroxyestradiols by CYP450s and to methoxyestradiols by catechol-O-methyltransferase (COMT) and studies in COMT knockout mice that cannot form methoxyestradiols provide evidence that methoxyestradiols (little affinity for ERs) mediate the ER-independent inhibitory effects of estradiol on VSMC proliferation and migration (5,6). Also, evidence supports the conclusion that methoxyestradiols mediate the inhibitory effects of estradiol on injury-induced neointima formation (7) as well as the inhibitory effects of estradiol on cardiac fibroblasts and glomerular mesangial cells (8).
Resveratrol is a weak ER ligand with mixed agonist/antagonist activity (9,10,11,12); resveratrol is both an ER-β agonist and ER-α antagonist (9,10,11,12). Moreover, resveratrol inhibits the CYP450 isozymes responsible for the metabolism of estradiol to hydroxyestradiol (2). Because both sequential conversion of estradiol by CYP450 and COMT to hydroxyestradiols and methoxyestradiols and ER-α mediate the vasculoprotective actions of estradiol (4,5), we hypothesize that resveratrol may abrogate the vasoprotective effects of estradiol by blocking mechanisms by which estradiol inhibits VSMC growth.
The goal of the present study was to test the hypothesis that resveratrol, at concentrations obtainable by moderate wine consumption, abrogates the antigrowth effects of estradiol on VSMCs via mechanisms that involve the competitive inhibition of hydroxyestradiol formation by CYP450 and blockade of ER-α.
Materials and Methods
Cell culture
Female human coronary artery VSMCs were obtained from Cascade Biologics (Portland, OR) and cultured as described previously (13). VSMCs in third to fifth passage were grown under standard tissue culture conditions in phenol red-free DMEM/F12 medium supplemented with 10% steroid-free fetal calf serum (FCS) and antibiotics (for details see supplemental material, published on The Endocrine Society’s Journals Online web site at http://jcem.endojournals.org).
VSMC growth studies
[3H]thymidine incorporation (index of DNA synthesis), [3H]proline incorporation (index of collagen synthesis), and cell-proliferation were conducted as previously described (5,13) (see supplemental material for details).
Migration studies
Modified Boydens chamber (Neuro Probe Inc., Cabin John, MD) was used to assess whether resveratrol abrogates the inhibitory actions of estradiol on platelet-derived growth factor (PDGF)-BB-induced VSMC migration and as described before (13) (see supplemental material for details).
Treatments
To assess whether resveratrol blocks the growth-inhibitory effects of estradiol, VSMCs were plated in 24-well plates in DMEM/F12 medium containing 10% FCS. When subconfluent, VSMCs were growth arrested for 48 h by feeding DMEM containing 0.4% albumin and subsequently stimulated with 2.5% FCS in the presence and absence of 1–1000 nmol/liter estradiol containing or lacking resveratrol (0.1–10 nmol/liter). Cell growth was assessed by counting cells on d 6 (treatments changed every 48 h) or DNA synthesis after 24 h or collagen synthesis after 36 h of treatment, as described before (13).
To assess whether the inhibitory effects of estradiol are blocked by the CYP450 inhibitor, the effects of estradiol (0.1 μmol/liter) on 2.5% FCS-induced VSMC growth were assayed in the presence or absence of CYP450 inhibitor 1-aminobenzotriazole (ABT; 5 μmol/liter) and as described above. To assess whether resveratrol blocks the antimitogenic effects of the endogenous estradiol metabolites, VSMC growth in response to 2.5% FCS was assessed in cells treated with 0.1 μmol/liter of either 2-hydroxyestradiol or 2-methoxyxestradiol in the presence or absence of 5 μmol/liter resveratrol and as described above. To elucidate the role of ERs in mediating the modulatory actions of resveratrol, growth-arrested VSMCs were treated with ER-α agonist propylpyrazoletriol (PPT; 100 nmol/liter) or ER-β agonist diarylpropionitrile (100 nmol/liter) in the presence or absence of resveratrol (5 μmol/liter) and the stimulatory effects of 2.5% FCS on DNA synthesis assessed after 24 h.
To assess the role of peroxisomal proliferator-activated receptor (PPAR)-γ and ERs in mediating the growth effects of resveratrol at high concentrations, growth-arrested VSMCs were treated with 10, 25, and 50 μmol/liter resveratrol in the presence or absence of PPARγ antagonist GW9662 (GW; 5 μmol/liter) or ERαβ antagonist ICI182780 (ICI; 10 μmol/liter) or ICI (10 μmol/liter) plus GW (5 μmol/liter) and the stimulatory effects of 2.5% FCS on DNA synthesis assessed after 24 h and as described above.
Expression studies
Western blotting (7) was used to investigate the expression of the MAPK phosphorylation, p21, cyclin A, cyclin D, ER-α, ER-β, CYP1A1, and CYP1B1 (see supplemental material for details).
Metabolism studies
HPLC with UV detection and metabolic conversion of [2-3H]estradiol to 3H-H2O by VSMCs or microsomal preparations (14) were used to assess the inhibitory effects of resveratrol on the metabolism of estradiol to 2-hydroxyestradiol and 2-methoxyestradiol (14,15) (see supplemental material for details).
Due to assay sensitivity limitation, we used [2-3H]estradiol to assess the metabolism of estradiol to 2-hydroxyestradiol in cultured VSMCs. As shown previously (16), for every molecule of estradiol metabolized to 2-hydroxyestradiol, one molecule of 3H2O is released. Hence, quantifying 3H2O in the supernatant serves as a surrogate marker for 2-hydroxyestradiol formation. To study whether VSMCs metabolize estradiol to 2-hydroxyestradiol and whether resveratrol inhibits this conversion, confluent monolayers of VSMCs were treated for 12 h with [2-3H]estradiol in the presence or absence of resveratrol (0–100 nmol/liter), and formation of 3H2O was assessed after extraction by liquid scintillation counting as described previously (16).
Statistics
All experiments were conducted in triplicate or quadruplicate and repeated three to four times using separate cultures. Results are presented as means ± sem. Statistical analyses were performed using ANOVA, paired Student’s t test, or Fishers’ least significant difference test as appropriate. A value of P < 0.05 was considered statistically significant.
Results
Low concentrations of resveratrol abrogate the antimitogenic effects of estradiol
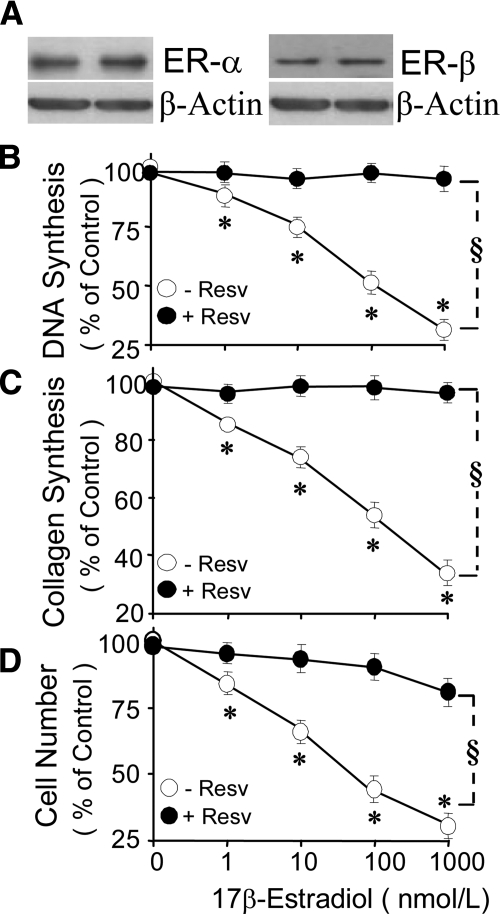
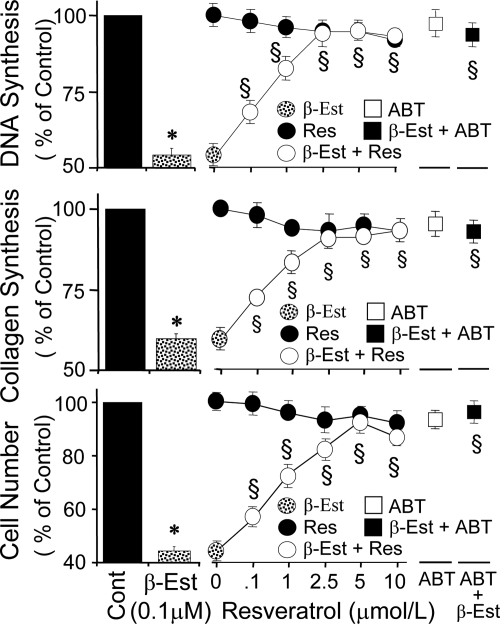
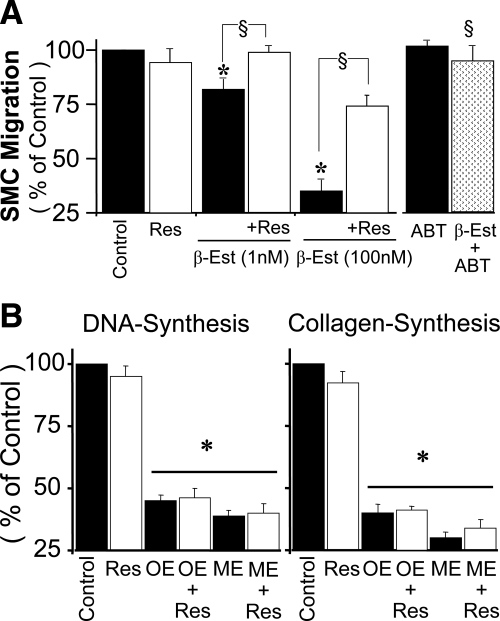
ER-α and ER-β were expressed in VSMCs used for the experiments (Fig. 1A). Treatment with 2.5% FCS stimulated [3H]thymidine incorporation and [3H]proline incorporation by approximately 11- and 14-fold (P < 0.001 vs. 0.25% albumin), respectively. Treatment with 1–1000 nmol/liter of estradiol concentration-dependently inhibited [3H]thymidine incorporation (Fig. 1B), [3H]proline incorporation (Fig. 1C), and cell proliferation (Fig. 1D). The concentration at which estradiol inhibited FCS-induced [3H]thymidine (Fig. 1B), [3H]proline (Fig. 1C), and cell number by 50% (EC50) was approximately 100 nmol/liter. In VSMCs treated for 4 d, a 57% inhibition in cell number by estradiol was observed at 100 nmol/liter (Fig. 1D). Resveratrol (5 μmol/liter) abrogated the concentration-dependent inhibitory effects of estradiol on [3H]thymidine incorporation (Fig. 1B) and [3H]proline incorporation (Fig. 1C) as well as cell proliferation (Fig. 1D). Moreover, treatment with resveratrol 0.1–10 μmol/liter alone did not alter FCS-induced effects on [3H]thymidine and [3H]proline incorporation and cell proliferation (Fig. 2) but reversed the inhibitory effect of 100 nmol/liter estradiol on VSMC growth, with an IC50 being approximately 1 μmol/liter. The lowest concentration of resveratrol that abrogated the inhibitory effects of estradiol was 0.1 μmol/liter. At 5 μmol/liter, resveratrol reversed the inhibitory effects of 100 nmol/liter estradiol on [3H]thymidine incorporation from 48 ± 3.8 to 5.6 ± 1.1% (P < 0.05; Fig. 2), on [3H]proline incorporation from 44 ± 2.8 to 8.6 ± 1.9% (P < 0.05 Fig. 2), and on cell proliferation from 56 ± 3.1 to 7.6 ± 1.7% (P < 0.05; Fig. 2). The inhibitory effects of estradiol were also reversed by ABT (5 μmol/liter; CYP450 inhibitor; Fig. 2). ABT alone did not influence FCS-induced growth. Resveratrol (5 μmol/liter) abrogated the inhibitory effects of 1 nmol/liter (physiological) and 100 nmol/liter (pharmacological) estradiol on PDGF-BB-induced VSMC migration from 18.2 ± 4.7 to 1.46 ± 2.5% and from 69 ± 3.7 to 13 ± 1.2%, respectively (P < 0.05; Fig. 3A). Resveratrol alone did not influence PDGF-BB-induced migration of VSMCs (Fig. 3A). The inhibitory effects of estradiol (100 nmol/liter) on VSMC migration were blocked by ABT (5 μmol/liter; Fig. 3A).
Figure 1.
Expression of ER-α and ER-β in coronary artery smooth muscle cells (A) and attenuation by resveratrol (Resv; 5 μmol/liter) of the concentration-dependent inhibitory effects of estradiol (1–100 nmol/liter) on 2.5% serum (FCS)-induced DNA synthesis (B), [3H]proline incorporation (C), and cell proliferation (D) in human VSMCs. Values represent mean ± sem from at least four independent experiments, each conducted in triplicate. *, P < 0.05 vs. control cells treated with FCS alone; §, significant reversal of the inhibitory effects of estradiol.
Figure 2.
Modulatory effects of resveratrol on the inhibitory effects of estradiol on VSMC proliferation (DNA synthesis and cell number) and collagen synthesis. Top panel depicts the inhibitory effects of 100 nmol/liter of estradiol (β-Est) on 2.5% serum (FCS)-induced DNA synthesis in the presence and absence of low concentrations of resveratrol (Res; 0, 0.1, 1, 2.5, 5, and 10 μmol/liter) and 5 μmol/liter ABT. Middle and bottom panels show the concentration-dependent attenuation by resveratrol (Res) and ABT of the inhibitory effects of estradiol (β-Est; 100 nmol/liter) on collagen synthesis and cell number, respectively. Values represent mean ± sem from at least four independent experiments, each conducted in triplicate. *, P < 0.05 vs. control cells (Cont) treated with FCS or PDGF-BB alone; §, P < 0.05 vs. cells treated with estradiol (significant reversal of the inhibitory effects).
Figure 3.
A, Abrogation by resveratrol (Res; 5 μmol/liter) and ABT (a broad spectrum CYP450 inhibitor; 5 μmol/liter) on the inhibitory actions of estradiol (β-Est; 1 and 100 nmol/liter) on PDGF-BB (25 ng/ml)-induced VSMC migration. Values represent mean ± sem from at least three independent experiments, each conducted in triplicate. *, P < 0.05 vs. control cells treated with FCS or PDGF-BB alone; §, P < 0.05 vs. cells treated with estradiol (significant reversal of the inhibitory effects). B, Modulatory effects of resveratrol (Res; 5 μmol/liter) on the inhibitory effects 2-hydroxyestradiol (OE; 0.1 μmol/liter) and 2-methoxyoestradiol (ME; 0.1 μmol/liter) on 2.5% serum (FCS)-induced DNA synthesis and collagen synthesis. Values represent mean ± sem from at least three independent experiments, each conducted in triplicate. *, P < 0.05 vs. control (VSMCs treated with FCS alone).
In contrast to estradiol, resveratrol did not block the inhibitory effects of 2-hydroxyestradiol on FCS-induced DNA synthesis (Fig. 3B) or collagen synthesis (Fig. 3B). Similar to 2-hydroxyestradiol, FCS-induced VSMC growth and collagen synthesis was inhibited by 2-methoxyestradiol, and the inhibitory effects of 2-methoxyestradiol were not blocked by resveratrol (Fig. 3), suggesting that resveratrol abrogates the effects of estradiol by blocking the conversion of estradiol to 2-hydroxyestradiol, a precursor of 2-methoxyestradiol.
Resveratrol inhibits estradiol metabolism to 2-hydroxyestradiol and 2-methoxyestradiol
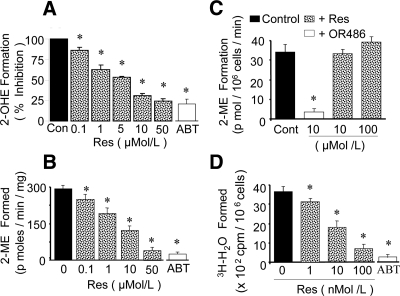
Microsomal preparations incubated with estradiol (25 μmol/liter) metabolized estradiol to 2-hydroxyestradiol. Cotreatment with resveratrol concentration-dependently inhibited the conversion of estradiol to hydroxyestradiol with an IC50 of approximately 5 μmol/liter (Fig. 4A). At concentrations of 1, 5, and 10 μmol/liter, resveratrol inhibited the conversion of estradiol to 2-hydroxyestradiol by 28 ± 3.5, 51 ± 1.4, and 67 ± 2.8%, respectively. In VSMCs treated for 1 h with supernatants of microsomes incubated with estradiol, significant amounts of 2-methoxyestradiol were formed. Moreover, the formation of 2-methoxyestradiol was significantly inhibited by resveratrol (Fig. 4B). The formation of 2-methoxyestradiol from estradiol was inhibited by resveratrol in a concentration-dependent manner with an IC50 of approximately 1 μmol/liter (Fig. 4B). At a concentration of 0.1, 1, and 10 μmol/liter, resveratrol inhibited 2-methoxyestradiol formation by 29 ± 3.1, 47 ± 1.7, and 71 ± 2.6%, respectively (Fig. 4B). VSMCs also metabolized 2-hydroxyestradiol to 2-methoxyestradiol, and resveratrol was unable to inhibit methylation of 2-hydroxyestradiol (Fig. 4C). Moreover, in VSMCs incubated with [2-3H]estradiol, the formation of 3H2O, a marker of 2-hydroxyestradiol formation, was evident, and this conversion was concentration-dependently blocked by resveratrol with an EC50 of approximately 10 nmol/liter (Fig. 4D).
Figure 4.
A, Bar graph showing the concentration-dependent inhibitory effects of resveratrol (Res; 0.1–50 μmol/liter) and CYP450 inhibitor ABT (10 μmol/liter) on the conversion of estradiol (25 μmol/liter) to 2-hydroxyestradiol (2-OHE) in microsomes. B, Concentration-dependent inhibitory effects of resveratrol on 2-methoxyestradiol (2-ME) formation in VSMCs incubated with microsomal extracts (1 mg/ml) that were incubated for 2 h with estradiol (25 μmol/liter). C, Inhibitory effects of 10 μmol/liter of COMT inhibitor OR486 and resveratrol (Res; 10 and 100 μmol/liter) on the conversion of 2-hydroxyestradiol (0.25 μmol/liter) to 2-methoxyestradiol (2-ME) formation in VSMCs. D, Inhibitory effects of resveratrol (1–100 nmol/liter) and ABT (10 μmol/liter) on the conversion of [2-3H]estradiol to 2-hydroxyestradiol and 3H2O in VSMCs incubated for 12 h. Values represent mean ± sem from at least three independent experiments each conducted in triplicate. *, P < 0.05 vs. 2-methoxyestradiol or 2-hydroxyestradiol formation in absence of inhibitors.
Resveratrol abrogates the inhibitory effects of estradiol on intracellular mechanisms
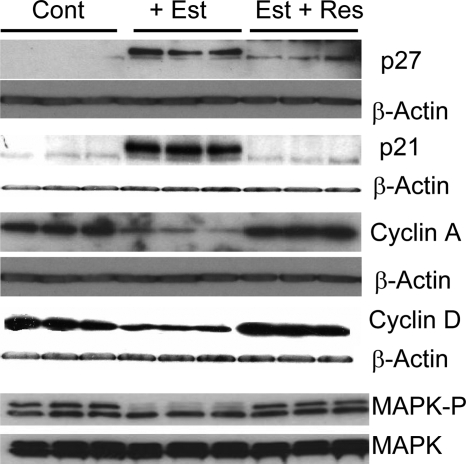
Treatment of VSMCs with 100 nmol/liter of estradiol decreased the expression of cyclin D1 and cyclin A, abrogated MAPK phosphorylation, and induced protein levels of the cdk inhibitors p27 and p21. Resveratrol abrogated the inhibitory effects estradiol on cyclin D1, cyclin A, and MAPK phosphorylation and blocked the stimulatory effects of estradiol on p21 and p27 expression (Fig. 5).
Figure 5.
Western blots depicting the abrogatory effects of resveratrol (Res; 5 μmol/liter) on the modulatory actions of estradiol (Est; 100 nmol/liter) on key signal transduction pathways (MAPK phosphorylation) that trigger G0/G1 cell cycle activation and on positive (cyclin A, cyclin D) and negative (p27, p21) pathways involved in cell cycle progression. Both β-actin or total MAPK, as appropriate, were used as internal standard to assess the changes in expression. Expression studies for each treatment were conducted in triplicate. Cont, Control.
Resveratrol antagonizes ER-α-mediated effects
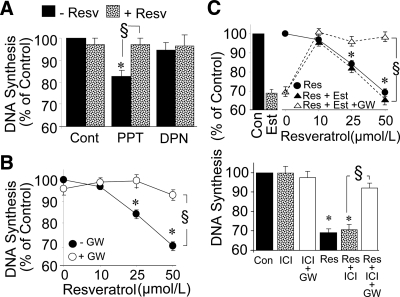
Compared with PPT (ER-α agonist), estradiol was more potent in inhibiting VSMC growth (Supplemental Fig. 1). At a concentration of 100 nmol/liter, PPT inhibited DNA synthesis by 16.8% whereas estradiol inhibited by 48%. Moreover, the effects of PPT, but not estradiol, were blocked by methylpiperidinopyrazole (ER-α antagonist; methylpiperidinopyrazole decreased the inhibitory effects of PPT from 16.8 to 2.3% and estradiol from 48 to 37.5%), suggesting that the antimitogenic effects of estradiol are in part ER-α independent (Supplemental Fig. 1). Indeed, consistent with our previous findings (5,6), ABT at a concentration of 1 μmol/liter reversed estradiol (100 nmol/liter)-mediated inhibition of [3H]thymidine incorporation from 48 ± 1.3 to 4 ± 2.3% but did not influence the inhibitory effects of PPT, suggesting that the antimitogenic effects of estradiol are mediated via its downstream metabolites hydroxyestradiols and methoxyestradiols.
FCS-induced growth of VSMCs was inhibited by PPT (ER-α agonist) but not diarylpropionitrile (ER-β agonist). Moreover, 5 μmol/liter resveratrol abrogated the inhibitory effects of PPT on DNA synthesis from 19 ± 2.8 to 3.2 ± 1.4% and cell proliferation from 26 ± 3.3 to 6 ± 1.3% (Fig. 6A). Resveratrol did not change the expression of ER-α and ER-β in VSMCs (Supplemental Fig. 2, top panel), and both CYP1A1 and CYP1B1 were expressed in VSMCs pretreated with CYP450 inducer 3-methylcholanterene (Supplemental Fig. 2, bottom panel).
Figure 6.
A, Modulatory effects of resveratrol (Resv; 5 μmol/liter) on the growth-modulatory effects of ER-α and ER-β agonists PPT (100 nmol/liter) and diarylpropionitrile (DPN; 10nmol/liter), respectively, on 2.5% FCS-induced DNA synthesis. Values represent mean ± sem from at least three independent experiments, each conducted in triplicate. *, P < 0.05 vs. control (VSMCs treated with FCS alone); §, P < 0.05 vs. PPT (significant reversal of the inhibitory effects). Cont, Control. B, Modulatory effects of PPARγ antagonist (GW; 5 μmol/liter) on the inhibitory effects of high concentrations of resveratrol (10, 25, and 50 μmol/liter) on 2.5% FCS-induced VSMC growth (DNA synthesis). C, Modulatory effects of ER antagonist (ICI; 10 μmol/liter) and PPARγ antagonist (GW; 5 μmol/liter) on the inhibitory effects of high concentrations of resveratrol (Res; 50 μmol/liter) and resveratrol (10, 25, and 50 μmol/liter) plus estradiol (Est; 10 nmol/liter) on 2.5% FCS-induced VSMC growth (DNA synthesis). Values represent mean ± sem from at least four independent experiments, each conducted in triplicate. Con, Control. *, P < 0.05 vs. control (VSMCs treated with FCS alone); §, P < 0.05 vs. resveratrol alone (significant reversal of the inhibitory effects).
Effects of high concentrations (not attained in vivo) of resveratrol on VSMC growth: role of PPARγ
Treatment of VSMCs with 0.1–10 μmol/liter resveratrol did not influence 2.5% FCS-induced VSMC growth. However, at concentrations of 25 and 50 μmol/liter, resveratrol inhibited DNA synthesis (Fig. 6B), collagen synthesis and cell number (data not shown). At a concentration of 50 μmol/liter, resveratrol inhibited [3H]thymidine incorporation, [3H]proline incorporation, and cell number by 26 ± 2.8, 21 ± 1.4, and 28 ± 3.1%, respectively. The inhibitory effects of resveratrol at these concentrations were not enhanced by 10 nmol/liter estradiol (Fig. 6, top panel). Interestingly, the inhibitory effects of resveratrol on 2.5% FCS-induced VSMC growth were abrogated by GW (PPARγ antagonist) but not by ICI (ER-α/β antagonist) (Fig. 6 C, bottom panel). Moreover, the modulatory effects of high concentrations of resveratrol were blocked by the PPARγ antagonist GW in both the presence and absence of estradiol (Fig. 6, B and C).
Discussion
Here we provide the first evidence that: 1) dietarily relevant concentrations (0.1–10 μmol/liter) of resveratrol block the inhibitory effects of physiological and pharmacological concentrations of estradiol on FCS-stimulated VSMC growth and signaling pathways responsible for VSMC cell cycle progression; 2) the abrogatory effects of resveratrol are largely mediated via its inhibitory effects on the sequential metabolism of estradiol to hydroxyestradiols and methoxyestradiols and partly via blockade of ER-α; and 3) high concentrations (>10 μmol/liter) of resveratrol, unlikely to be attained by normal dietary consumption, inhibit VSMC growth and that these effects are PPARγ mediated.
Several observations suggest that low concentrations of resveratrol interfere with the growth inhibitor effects of estradiol by attenuating the metabolism of estradiol by CYP450: 1) low concentrations of resveratrol block the inhibitory effects of estradiol, but not 2-hydroxyestradiol or 2-methoxyestradiol, on VSMC growth; 2) the abrogatory effects of resveratrol are mimicked by the CYP450 inhibitor 1-aminobenzotriazole; 3) VSMCs metabolize estradiol to 2-hydroxyestradiol and 2-methoxyestradiol and express CYP450 enzymes responsible for converting estradiol to 2-hydroxyestradiol; 4) in VSMCs, resveratrol blocked the conversion of estradiol to 2-hydroxyestradiol and 2-methoxyestradiol, a finding consistent with the fact that resveratrol is an inhibitor of CYP450 isozymes that metabolize estradiol to hydroxyestradiols in VSMCs (2,5).
The inhibitory effects of estradiol on VSMC growth are also in part mediated by ER-α (4). Indeed, we observe that VSMC growth is inhibited by the ER-α agonist PPT but not by the ER-β agonist diarylpropionitrile. Importantly, the inhibitory effects of PPT are blocked by both the ER-α antagonist methylpiperidinopyrazole and by resveratrol. Together these findings suggest that resveratrol can also block the antimitogenic actions of estradiol mediated via ER-α.
Although resveratrol blocks the antimitogenic effects of estradiol on VSMC growth via antagonizing ER-α and by inhibiting estradiol metabolism, we hypothesize that the latter mechanism is dominant because: 1) ER-α antagonism only partially abrogates the inhibitory effects of estradiol; 2) estradiol is more efficacious than PPT in inhibiting VSMC growth; 3) the metabolic inhibitor 1-aminobenzotriazole is more efficacious than methylpiperidinopyrazole in abrogating the effects of estradiol (Supplemental Fig. 1); 4) estradiol metabolites are more efficacious than PTT in inhibiting VSMC growth (Supplemental Fig. 3); 5) compared with estradiol, resveratrol is a low-affinity ER antagonist (IC50 >10 μmol/liter) and a low-affinity ER agonist (EC50 >3 μmol/liter) (10); 6) treatment with resveratrol does not down-regulate the expression of ERs in VSMCs (Supplemental Fig. 2); 7) low concentrations (100 nmol/liter) of resveratrol block the inhibitory effects of estradiol (100 nmol/liter); 8) resveratrol blocks the inhibitory effects of estradiol, but not 2-hydroxyestradiol or 2-methoxyestradiol, on VSMC growth; 9) the abrogatory effects of resveratrol are mimicked by a broad-spectrum CYP450 inhibitor; 10) VSMCs efficiently metabolize estradiol to 2-hydroxyestradiol and 2-methoxyestradiol and expressed CYP450 enzymes (Supplemental Fig. 2) responsible for converting estradiol to 2-hydroxyestradiol; and 11) in VSMCs, resveratrol blocks the conversion of estradiol to 2-hydroxyestradiol and 2-methoxyestradiol. Taken together, our findings provide evidence that dietarily relevant levels of resveratrol block the antimitogenic effects of estradiol in VSMCs largely by inhibiting the sequential metabolism of estradiol to 2-hydroxyestradiol and 2-methoxyestradiol, although the participation of its antagonistic effects on ER-α cannot be ruled out.
Interestingly, high concentrations of resveratrol (≥25 μmol/liter), not attainable by dietary consumption, inhibit VSMC growth, and this effect is blocked by the PPARγ antagonist GW, but not by the ER antagonist ICI. This suggests that the antimitogenic effects of resveratrol at high concentrations are PPARγ mediated. Indeed, resveratrol is known to activate PPARγ (17), a nuclear receptor that acts as a transcription factor controlling the expression of multiple genes involved in cell growth and vascular remodeling associated with cardiovascular disease (16). Because the effects of resveratrol via PPARγ are observed only at high micromolar concentrations, it is unlikely that this action of resveratrol plays a significant role in the pharmacological effects of dietary resveratrol.
Our finding that resveratrol blocks the antimitogenic effects of estradiol on VSMC growth implies that resveratrol may abrogate the cardiovascular protective effects of estradiol. In support of this concept, resveratrol enhances atherosclerosis in hypercholesterolemic rabbits (18). Similarly, red wine polyphenol extracts containing resveratrol fail to inhibit atherogenesis or change cholesterol and low-density lipoprotein levels in C57BL/6 apolipoprotein E-deficient mice (19). In both models, resveratrol does not influence cholesterol, high-density lipoprotein or low-density lipoprotein levels. Interestingly, resveratrol abrogates the cholesterol-lowering effects of estradiol in rats (10). Moreover, resveratrol abrogates the beneficial/lowering effects of estrogen on serum cholesterol (10), worsens dysregulation of genes involved in hepatic lipid homeostasis (20), increases plasma homocysteine levels in hyperhomocysteinemic mice (20), activates inducible nitric oxide synthase (proinflammatory) in VSMCs, and reduces levels of prostaglandin E2 (vasodilator) and inhibits cyclooxygenase-2 expression (2,21). Importantly, most evidence supporting cardiovascular protective effects of resveratrol is based on findings using high concentrations (>5 μmol/liter) of resveratrol, never attained in vivo (21,22,23,24,25). Indeed, resveratrol has been shown to induce both deleterious and protective effects on atherosclerosis (17,18,26,27).
Estradiol mediates its antimitogenic actions on VSMCs by inhibiting mitogen-induced activation of key signal transduction pathways and proteins responsible for cell cycle-governed cell replication (3). In the present study, we demonstrate that estradiol: 1) inhibits the MAPK pathway; 2) inhibits the expression of cyclin D1 responsible for the progression of cells into the DNA replication phase; 3) up-regulates the expression of the cdk inhibitors p27 and p21, which are negative regulators of cell growth; and 4) inhibits expression of cyclin A responsible for the G1/S transition and in the S and G2/M phases of the cell cycle (7). Our finding that low concentrations of resveratrol abrogate the inhibitory effects of estradiol on mitogen-induced MAPK phosphorylation, cyclin D1, and cyclin A expression as well as the stimulatory effects of estradiol on p21 and p27 expression suggests that resveratrol can block the antimitogenic actions of estradiol on key intracellular mechanisms, which promote VSMC growth.
The hypothesis that resveratrol is cardiovascular protective is based largely on in vitro studies using high micromolar (>10 μmol/liter) concentrations. The observational evidence (the French paradox) that resveratrol in red wine may have protective actions has fueled the assumption that resveratrol could protect against cardiovascular disease (2). However, based on pharmacokinetic studies conducted in humans, the average and peak plasma concentrations of resveratrol across four doses (0.5, 1, 2.5, and 5 g) ranged from 0.04 to 0.23 μmol/liter and from 0.3 to 2.4 μmol/liter, respectively (21). Because the resveratrol content in red wine ranges between 1 and 10 mg/liter, it would require hundreds of bottles of red wine to achieve peak levels of 0.3–2.4 μmol/liter, markedly below resveratrol concentrations required to elicit pharmacological effects associated with cardiovascular benefits. This information questions the perceived notion that resveratrol mediates the cardiovascular protective effects of wine.
Ingestion of resveratrol as a health drug may not mimic the effects of red wine and may induce deleterious effects. Our results provide evidence that low concentrations of resveratrol per se are ineffective in modulating VSMC growth and suggest that the cardiovascular protective effects of red wine may be mediated via other molecules in wine. Red wine has other polyphenols, tannins, and alcohol, and they may collectively mediate the cardiovascular protective actions of red wine. However, the effects of resveratrol on vascular endothelium may, in part, contribute to the presumed cardiovascular protective effects of wine. In this context, resveratrol induces endothelium-dependent relaxation via increased generation of nitric oxide and reduced generation of free reactive oxygen species (2,28). Moreover, resveratrol induces endothelial cell growth, stimulates angiogenesis (at low concentrations), protects endothelial cells against oxidative damage, inhibits thrombus formation and platelet aggregation, and protects against ischemia-reperfusion injury (28). Finally, similar to our observation, some in vitro studies provide evidence that high concentrations (≥10 μmol/liter) of resveratrol, not achieved in vivo, inhibit VSMC proliferation (2,24,28), and this may contribute to its vascular protective and antivasoocclusive actions.
Resveratrol is a pleiotropic compound with a complex and concentration-dependent pharmacology and exerts different effects, depending on the concentration of resveratrol. For example, at high concentrations, resveratrol might activate sirtuin 1 (29), although this is presently controversial (30). Also, as shown in the present study, high concentrations of resveratrol directly inhibit vascular smooth muscle cell growth. Therefore, high concentrations of resveratrol may be cardioprotective; however, such concentrations are unlikely to be achieved from dietary intake but could be achieved from pharmaceutical formulations of resveratrol. On the other hand, low concentrations of resveratrol that are achievable from dietary consumption inhibit the metabolism of estradiol to cardioprotective metabolites and antagonize ER-α. This would imply that when estradiol levels are sufficiently high to provide cardiovascular benefits via either direct ER-α activation or conversion of estradiol to cardioprotective metabolites (i.e. in premenopausal women during midcycle when estradiol levels are highest), low levels of resveratrol might be harmful. Therefore, whether resveratrol is beneficial, harmful, or neither is likely dose and context dependent.
Finally, apart form altering the effects of estradiol, the inhibitory effects of resveratrol on CYP450 (a ubiquitous enzyme with multiple isoforms that are expressed in many tissues) may also influence organs other than blood vessels. Because CYP450 is involved in the synthesis of hormones such as estrogens and androgens, resveratrol could alter their levels and physiological actions in target tissues such as reproductive organs, liver, and the cardiovascular system in both males and females. Because CYP450 activation results in the generation of free radicals and carcinogenic metabolites/DNA adducts, the inhibitory actions of resveratrol would be protective against free radical-induced injury and generation of carcinogenic metabolites within the target tissues (liver, breast, ovary, heart, and blood vessels).
Perspectives
Resveratrol, a red wine constituent, is hypothesized to be cardioprotective. However, this notion is based on experimental evidence using high concentrations of resveratrol that are never attainable in vivo. Indeed, circulating levels of resveratrol after ingestion of 25 mg resveratrol in wine ranges between 7.5 and 40 nmol/liter (2,21). In the present study, concentrations of resveratrol as low as 10–100 nmol/liter significantly attenuated the growth-inhibitory effects of 100 nmol/liter estradiol. Because the interaction between resveratrol and estradiol at CYP450 is competitive, even lower levels of resveratrol would be expected to attenuate the antimitogenic effects of lower levels of estradiol. These considerations imply that moderate consumption of wine or resveratrol-containing health supplements (20–500 mg) by premenopausal women or postmenopausal women on estradiol could attenuate the inhibitory effects of estradiol on VSMC growth and abrogate the vasoprotective actions of estradiol.
Footnotes
This work was supported by Swiss National Science Foundation Grants 32-64040.00, 320000-117998; Oncosuisse OCS-01551-08-2004; EMDO Forschung; and National Institutes of Health Grants HL069846, DK079307, and DK068575.
Disclosure Summary: The authors have nothing to disclose.
First Published Online June 9, 2010
Abbreviations: ABT, 1-Aminobenzotriazole; COMT, catechol-O-methyltransferase; ER, estrogen receptor; FCS, fetal calf serum; GW, GW9662; ICI, ICI182780;PDGF, platelet-derived growth factor; PPAR, peroxisomal proliferator-activated receptor; PPT, propylpyrazoletriol; VSMC, vascular smooth muscle cell.
References
- Renaud S, de Lorgeril M 1992 Wine, alcohol platelets, and the French paradox for coronary heart disease. Lancet 339:1523–1526 [DOI] [PubMed] [Google Scholar]
- Saiko P, Szakmary A, Jaeger W, Szekeres T 2008 Resveratrol and its analogs: defense against cancer, coronary disease and neurodegenerative maladies or just a fad? Mutat Res 658:68–94 [DOI] [PubMed] [Google Scholar]
- Dubey RK, Jackson EK 2001 Estrogen-induced cardiorenal protection: potential cellular, biochemical, and molecular mechanisms. Am J Physiol Renal Physiol 280:F365–F388 [DOI] [PubMed] [Google Scholar]
- Pare G, Krust A, Karas RH, Dupont S, Aronovitz M, Chambon P, Mendelsohn ME 2002 Estrogen receptor-α mediates the protective effects of estrogen against vascular injury. Circ Res 90:1087–1092 [DOI] [PubMed] [Google Scholar]
- Dubey RK, Gillespie DG, Zacharia LC, Barchiesi F, Imthurn B, Jackson EK 2003 CYP450- and COMT-derived estradiol metabolites inhibit activity of human coronary artery SMCs. Hypertension 41:807–813 [DOI] [PubMed] [Google Scholar]
- Zacharia LC, Gogos JA, Karayiorgou M, Jackson EK, Gillespie DG, Barchiesi F, Dubey RK 2003 Methoxyestradiols mediate the antimitogenic effects of 17β-estradiol. Direct evidence from catechol-O-methyltransferase knockout mice. Circulation 108:2974–2978 [DOI] [PubMed] [Google Scholar]
- Barchiesi F, Jackson EK, Fingerle J, Gillespie DG, Odermatt B, Dubey RK 2006 2-Methoxyestradiol, an estradiol metabolite, inhibits neointima formation and smooth muscle cell growth via double blockade of the cell cycle. Circ Res 99:266–274 [DOI] [PubMed] [Google Scholar]
- Dubey RK, Jackson EK 2001 Cardiovascular protective effects of 17β-estradiol metabolites. J Appl Physiol 91:1868–1883 [DOI] [PubMed] [Google Scholar]
- Bowers JL, Tyulmenkov VV, Jernigan SC, Klinge CM 2000 Resveratrol acts as a mixed agonist/antagonist for estrogen receptor α and β. Endocrinology 141:3657–3667 [DOI] [PubMed] [Google Scholar]
- Turner RT, Evans GL, Zhang M, Maran A, Sibonga JD 1999 Is resveratrol an estrogen agonist in growing rats ? Endocrinology 140:50–54 [DOI] [PubMed] [Google Scholar]
- Lu R, Serrero G 1999 Resveratrol, a natural product derived from grape, exhibits antiestrogenic activity and inhibits the growth of human breast cancer cells. J Cell Physiol 179:297–304 [DOI] [PubMed] [Google Scholar]
- Basly JP, Marre-Fournier F, Le Bail JC, Habrioux G, Chulia AJ 2000 Estrogenic/antiestrogenic and scavenging properties of (E)- and (Z)-resveratrol. Life Sci 66:769–777 [DOI] [PubMed] [Google Scholar]
- Dubey RK, Jackson EK, Gillespie DG, Zacharia LC, Imthurn B, Keller PJ 2000 Clinically used estrogens differentially inhibit human aortic smooth muscle cell growth and MAP kinase activity. Arterioscler Thromb Vasc Biol 20:964–972 [DOI] [PubMed] [Google Scholar]
- Yusufi AN, Cheng J, Thompson MA, Burnett JC, Grande JP 2002 Differential mechanisms of Ca2+ release from vascular smooth muscle cell microsomes. Exp Biol Med 227:36–44 [DOI] [PubMed] [Google Scholar]
- Zacharia LC, Jackson EK, Gillespie DG, Dubey RK 2001 Increased 2-methoxyestradiol production in human coronary versus aortic vascular cells. Hypertension 37:658–662 [DOI] [PubMed] [Google Scholar]
- Brueggemeier RW, Tseng K, Katlic NE, Beleh MA, Lin YC 1990 Estrogen metabolism in primary kidney cell cultures from Syrian hamsters. J Steroid Biochem 36:325–331 [DOI] [PubMed] [Google Scholar]
- Ulrich S, Loitsch SM, Rau O, von Knethen A, Brüne B, Schubert-Zsilavecz M, Stein JM 2006 Peroxisome proliferator-activated receptor-γ as a molecular target of resveratrol-induced modulation of polyamine metabolism. Cancer Res 66:7348–7354 [DOI] [PubMed] [Google Scholar]
- Wilson T, Knight TJ, Beitz DC, Lewis DS, Engen RL 1996 Resveratrol promotes atherosclerosis in hypercholesteromic rabbits. Life Sci 59:PL15–PL21 [DOI] [PubMed] [Google Scholar]
- Soulat T, Philippe C, Bal dit Sollier C, Brézillon C, Berge N, Teissedre PL, Callebert J, Rabot S, Drouet L 2006 Wine constituents inhibit thrombosis but not atherogenesis in C57BL/6 apolipoprotein E-deficient mice. Br J Nutr 96:290–298 [DOI] [PubMed] [Google Scholar]
- Noll C, Hamelet J, Ducros V, Belin N, Paul JL, Delabar JM, Janel N 2009 Resveratrol supplementation worsen the dysregulation of genes involved in hepatic lipid homeostasis observed in hyperhomocysteinemic mice. Food Chem Toxicol 47:230–236 [DOI] [PubMed] [Google Scholar]
- Cignarella A, Minici C, Bolego C, Pinna C, Sanvito P, Gaion RM, Puglisi L 2006 Potential pro-inflammatory action of resveratrol in vascular smooth muscle cells from normal and diabetic rats. Nutr Metab Cardiovasc Dis 16:322–329 [DOI] [PubMed] [Google Scholar]
- Boocock DJ, Faust GE, Patel KR, Schinas AM, Brown VA, Ducharme MP, Booth TD, Crowell JA, Perloff M, Gescher AJ, Steward WP, Brenner DE 2007 Phase 1 dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemopreventive agent. Cancer Epidemiol Biomarkers Prev 16:1246–1252 [DOI] [PubMed] [Google Scholar]
- Park DW, Baek K, Kim JR, Lee JJ, Ryu SH, Chin BR, Baek SH 2009 Resveratrol inhibits foam cell formation via NADPH oxidase 1-mediated reactive oxygen species and monocyte chemotactic protein-1. Exp Mol Med 41:171–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poussier B, Cordova AC, Bacquemin JP, Sumpio BE 2005 Resveratrol inhibits vascular smooth muscle cell proliferation and induces apoptosis. J Vasc Surg 42:1190–1197 [DOI] [PubMed] [Google Scholar]
- Olson ER, Naugle JE, Zhang X, Bosmer JA, Meszaros JG 2005 Inhibition of cardiac fibroblast proliferation and myofibroblast differentiation by resveratrol. Am J Physiol Heart Circ Physiol 288:H1131–H1138 [DOI] [PubMed] [Google Scholar]
- Li Y, Cao Z, Zhu H 2006 Upregulation of endogenous antioxidants and phase 2 enzymes by the red wine polyphenol, resveratrol in cultured aortic smooth muscle cells leads to cytoprotection against oxidative and electrophilic stress. Pharmacol Res 53:6–15 [DOI] [PubMed] [Google Scholar]
- Do GM, Kwon EY, Kim HJ, Jeon SM, Ha TY, Park T, Choi MS 2008 Long-term effects of resveratrol supplementation on suppression of atherogenic lesion formation and cholesterol synthesis in apo E-deficient mice. Biochem Biophys Res Commun 374:55–59 [DOI] [PubMed] [Google Scholar]
- Baur JA, Sinclair DA 2006 Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev 5:493–502 [DOI] [PubMed] [Google Scholar]
- Alcaín FJ, Villaba JM 2009 Sirtuin activators. Expert Opin Ther Pat 19:403–414 [DOI] [PubMed] [Google Scholar]
- Beher D, Wu J, Cumine S, Kim KW, Lu SC, Atangan L, Wang M 2009 Resveratrol is not a direct activator of SIRT1 enzyme activity. Chem Biol Drug Des 74:619–624 [DOI] [PubMed] [Google Scholar]