Abstract
Context: HIV-infected patients on antiretroviral therapy are at increased risk for excess visceral adiposity and insulin resistance. Treatment with GH decreases visceral adiposity but worsens glucose metabolism. IGF-I, which mediates many of the effects of GH, improves insulin sensitivity in HIV-negative individuals.
Objective: Our objective was to determine whether IGF-I, complexed to its major binding protein, IGF-binding protein-3 (IGFBP-3), improves glucose metabolism and alters body fat distribution in HIV-infected patients with abdominal obesity and insulin resistance.
Methods: We conducted a pilot, open-label study in 13 HIV-infected men with excess abdominal adiposity and insulin resistance to assess the effect of 3 months of treatment with IGF-I/IGFBP-3 on glucose metabolism and fat distribution. Glucose metabolism was assessed by oral glucose tolerance test and hyperinsulinemic-euglycemic clamp. Endogenous glucose production (EGP), gluconeogenesis, whole-body lipolysis, and de novo lipogenesis (DNL) were measured with stable isotope infusions. Body composition was assessed by dual-energy x-ray absorptiometry and abdominal computed tomography scan.
Results: Glucose tolerance improved and insulin-mediated glucose uptake increased significantly during treatment. EGP increased under fasting conditions, and suppression of EGP by insulin was blunted. Fasting triglycerides decreased significantly in association with a decrease in hepatic DNL. Lean body mass increased and total body fat decreased, whereas visceral adipose tissue did not change.
Conclusions: Treatment with IGF-I/IGFBP-3 improved whole-body glucose uptake and glucose tolerance, while increasing hepatic glucose production. Fasting triglycerides improved, reflecting decreased DNL, and visceral adiposity was unchanged.
IGF-I/IGFBP-3 treatment improved glucose tolerance and peripheral insulin sensitivity while increasing hepatic glucose production in HIV-infected men with insulin resistance.
Patients with HIV infection on antiretroviral (ARV) treatment are at increased risk for metabolic and morphological disturbances including visceral fat accumulation and abnormal glucose metabolism (1). Disturbances in body fat distribution may adversely impact quality of life and increase risk of cardiovascular disease (2).
Experimental therapies that have been used to ameliorate these disturbances include GH and GH-releasing factor. GH reduced visceral adipose tissue but worsened glucose metabolism (3,4). Because many patients with HIV infection on ARVs are insulin resistant, GH may lead to development of frank diabetes. GH-releasing factor reportedly did not worsen glucose metabolism, but neither did it improve insulin resistance (5) .
IGF-I mediates many of the anabolic effects of GH (6) but, unlike GH, improved glucose metabolism in patients with type 1 and type 2 diabetes or GH deficiency (7). IGF-I treatment also decreased fat and increased lean mass in patients with GH insensitivity syndrome while increasing lipolysis (8). Due to its short half-life, IGF-I normally requires continuous infusion or multiple daily injections. In this study, we used a novel preparation in which IGF-I is complexed to its major binding protein, IGF-binding protein-3 (IGFBP-3), to prolong its half-life (9). We performed a pilot, open-label study to determine whether 3 months of treatment with IGF-I/IGFBP-3 could improve glucose metabolism and reduce visceral fat in patients with HIV.
Subjects and Methods
Subjects and study design
Thirteen men with excess central fat (waist circumference >100 cm and waist-to-hip ratio >0.95) and insulin resistance [homeostasis model assessment of insulin resistance (HOMA-IR) (10) score >2.77] were enrolled. Subjects were required to be on a stable ARV regimen for at least 6 months and not on antidiabetic agents or other treatments that could affect fat distribution or glucose metabolism. The Committee on Human Research of the University of California, San Francisco, approved the study. Informed consent was obtained from each subject.
Subjects were admitted to the Clinical Research Center (CRC) at San Francisco General Hospital for 5 d, consumed a constant metabolic diet, and underwent metabolic assessments including oral glucose tolerance testing, body composition measurements, and hyperinsulinemic-euglycemic clamp with stable isotope tracer infusions. After baseline testing, subjects started treatment with IGF-I/IGFBP-3 (mecasermin rinfate, iPLEX; Insmed, Inc., Richmond, VA). Ten subjects received 0.5 mg/kg · d sc and three subjects 1.0 mg/kg · d. The dose of IGF-I/IGFBP-3 was increased in the last three subjects after we found no important safety issues and little change in visceral adiposity with the lower dose. After 3 months of treatment, the inpatient assessments were repeated.
Glucose metabolism
For the oral glucose tolerance testing, subjects drank 75 g of glucose after fasting overnight (3); the area under the curve (AUC) for glucose, insulin, and HOMA-IR (10) was calculated. A 3-h hyperinsulinemic-euglycemic clamp was performed as reported previously (3).
Stable isotope tracer methods
Endogenous glucose production (EGP), gluconeogenesis (GNG), and whole-body lipolysis [rate of appearance of glycerol (Ra glycerol)] were measured under fasting and hyperinsulinemic conditions and hepatic de novo lipogenesis (DNL) under fasting and fed conditions using primed infusions of sodium [1-13C]acetate (0.34–0.40 g/h), [U-13C]glucose (1.2 mg/kg · h), and [2-13C]glycerol (15 mg/kg lean body mass/h) as described previously (11).
Body composition
Total and regional fat and lean mass were measured by dual-energy x-ray absorptiometry (DEXA) (Lunar DPX, Madison, WI) (3). Visceral and sc adipose tissue (VAT and SAT) was measured with a helical computed tomography (CT) scanner (General Electrics Medical Systems, Milwaukee, WI) using a single-slice at L4–L5 (12). Intramyocellular lipid content was measured in the soleus and tibialis anterior muscles as described previously (13).
Other laboratory measurements
Fasting total cholesterol and high-density lipoprotein (HDL)-cholesterol were measured using reagents from Fisher Scientific (Pittsburgh, PA), triglycerides (TG) from Sigma Chemical Co. (St. Louis, MO), and free fatty acids using Wako NEFA kit (Wako Chemicals, Richmond, VA). Low-density lipoprotein cholesterol was calculated (14). The San Francisco General Hospital CRC Core Laboratory measured serum insulin by RIA (Linco Research Inc., St. Charles, MO) and glucose by the glucose oxidase method (YSI STAT2300, Yellow Springs, OH). Fasting morning plasma cortisol was measured by competitive immunoassay (Siemens Centaur, Deerfield, IL). Free and total IGF-I and IGFBP-3 concentrations were measured by Insmed, Inc., using ELISA (Diagnostic Systems Laboratories, Webster, TX). Serum IGFBP-1 and IGFBP-2 were measured by RIA and GH by immunochemiluminometric assay at Esoterix Laboratories.
Statistical methods
Analyses were performed using the STATA version 9.0 software. Data are mean ± sem. Student’s paired t test was used to compare changes from baseline to month 3 using two-tailed P values.
Results
Patients and clinical course
Mean age was 53 ± 2.6 yr, duration of HIV infection 16 ± 1.7 yr, and CD4 count 460 ± 54 cells/μl (Supplemental Table 1, published on The Endocrine Society’s Journals Online web site at http://jcem.endojournals.org). All subjects had viral loads under 2000 copies/ml. ARV regimens consisted of at least two nucleoside reverse transcriptase inhibitors with at least one protease inhibitor or nonnucleoside reverse transcriptase inhibitor; one subject was also on a fusion inhibitor. Five subjects were taking medications for hyperlipidemia, and four were on replacement testosterone.
Of the 13 men who received at least one dose of IGF-I/IGFBP-3, one had an allergic reaction after the first dose and was discontinued from the study. Another withdrew at d 23 for an injection site reaction. A third, who had missed at least 20% of study doses, was considered unevaluable. Of the 10 evaluable subjects, seven were treated with 0.5 mg/kg · d of IGF-I/IGFBP-3 and three with 1.0 mg/kg · d.
Hormones and binding proteins
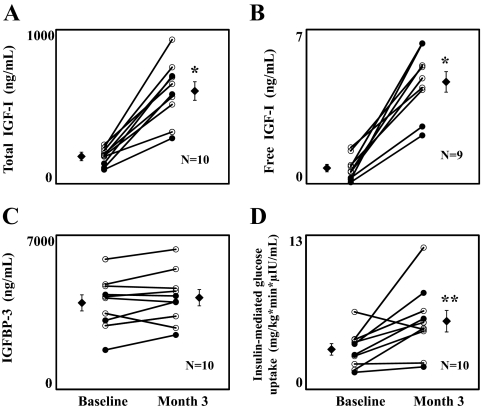
Total IGF-I levels increased in all evaluable subjects (P < 0.001; Fig. 1A), as did free IGF-I (P < 0.001; Fig. 1B). IGFBP-3 levels did not change significantly (Fig. 1C and Table 1). IGFBP-1 levels also did not change significantly, whereas IGFBP-2 increased by 78% (P = 0.007; Table 1). Cortisol levels increased by 38%, but this change did not reach significance (P = 0.09; Table 1). GH was undetectable in five subjects at baseline and eight subjects at month 3; in all subjects with detectable values at baseline, GH levels decreased at month 3 (Table 1).
Figure 1.
Effects of IGF-1/IGFBP-3 treatment on total IGF-I (164 ±17 vs. 594 ± 61 ng/ml) (A), free IGF-I (0.67 ± 0.17 vs. 4.35 ± 0.46 ng/ml) (B), IGFBP-3 (3865 ± 336 vs. 4082 ± 341 ng/ml) (C), and insulin-mediated glucose uptake (3.60 ± 0.46 vs. 5.86 ± 0.86 mg/kg · min · μIU/ml) (D). Individual results and group mean ± sem are shown. *, P < 0.001; **, P < 0.05. ○, 0.5 mg/kg · d dose; •, 1.0 mg/kg · d dose.
Table 1.
Effects of IGF-1/IGFBP-3 on glucose metabolism, lipid metabolism, body composition, and hormones
| Baseline | Month 3 | Change | P value | |
|---|---|---|---|---|
| Oral glucose tolerance test (n = 10) | ||||
| Glucose (mg/dl) t = 0 min | 105 ± 3 | 98 ± 2 | −8 ± 2 | 0.005 |
| Glucose (mg/dl) t = 120 min | 166 ± 11 | 134 ± 13 | −32 ± 9 | 0.008 |
| Glucose AUC | 481 ± 24 | 433 ± 23 | −48 ± 20 | 0.04 |
| Insulin (μIU/ml) t = 0 min | 21.0 ± 2.6 | 15.1 ± 1.9 | −5.9 ± 2.8 | 0.06 |
| Insulin (μIU/ml) t = 120 min | 160.5 ± 37.9 | 68.6 ± 14.9 | −91.9 ± 32.7 | 0.02 |
| Insulin AUC | 390.8 ± 73.5 | 233.2 ± 38.8 | −157.6 ± 58.4 | 0.03 |
| HOMA-IR | 5.45 ± 0.69 | 3.69 ± 0.54 | −1.76 ± 0.73 | 0.04 |
| Serum lipids (n = 10) | ||||
| Total cholesterol (mg/dl) | 165 ± 11 | 153 ± 12 | −11.9 ± 6.3 | 0.09 |
| TG (mg/dl) | 228 ± 37 | 175 ± 25 | −52.6 ± 23.1 | 0.049 |
| HDL-cholesterol (mg/dl) | 32 ± 3.4 | 32 ± 3.5 | −0.5 ± 2.4 | 0.85 |
| LDL-cholesterol (mg/dl) | 87 ± 10 | 86 ± 11 | −0.93 ± 5.1 | 0.85 |
| Non-HDL-cholesterol (mg/dl) | 133 ± 11 | 122 ± 11 | −11.5 ± 5.4 | 0.06 |
| Fasting FFA (mmol/liter) | 0.48 ± 0.07 | 0.42 ± 0.05 | −0.05 ± 0.03 | 0.14 |
| DEXA scan (n = 10) | ||||
| Weight (kg) | 85 ± 2.6 | 84 ± 2.7 | −0.9 ± 0.8 | 0.28 |
| Lean body mass (kg) | 57.1 ± 2.7 | 58.1 ± 2.7 | 1.0 ± 0.9 | 0.02 |
| Total body fat (kg) | 24.1 ± 2.1 | 22.4 ± 2.1 | −1.7 ± 0.7 | 0.045 |
| Truncal fat (kg) | 16.3 ± 1.0 | 15.3 ± 1.1 | −1.1 ± 0.4 | 0.04 |
| Appendicular fat (kg) | 6.7 ± 1.1 | 6.2 ± 1.1 | −0.5 ± 0.3 | 0.20 |
| CT scan (n = 10) | ||||
| VAT (cm2) | 327 ± 30 | 323 ± 35 | −4 ± 19 | 0.80 |
| SAT (cm2) | 144 ± 30 | 138 ± 27 | −3 ± 4 | 0.30 |
| EGP (n = 9) | ||||
| Fasting EGP (mg/kg · min) | 2.03 ± 0.09 | 2.21 ± 0.09 | 0.18 ± 0.05 | 0.006 |
| Hyperinsulinemic EGP (mg/kg · min) | 0.35 ± 0.05 | 0.52 ± 0.06 | 0.17 ± 0.05 | 0.01 |
| EGP suppression by insulin (%) | 83.2 ± 2.1 | 76.6 ± 2.8 | −6.6 ± 2.7 | 0.04 |
| GNG (n = 9) | ||||
| Fasting GNG (mg/kg/min) | 0.53 ± 0.04 | 0.65 ± 0.01 | 0.12 ± 0.04 | 0.01 |
| Hyperinsulinemic GNG (mg/kg/min) | 0.03 ± 0.01 | 0.04 ± 0.01 | 0.01 ± 0.01 | 0.29 |
| GNG suppression by insulin (%) | 94.0 ± 1.1 | 93.6 ± 0.9 | −0.4 ± 0.9 | 0.69 |
| Glycogenolysisa (n = 9) | ||||
| Fasting (mg/kg · min) | 1.50 ± 0.06 | 1.56 ± 0.09 | 0.06 ± 0.06 | 0.33 |
| Hyperinsulinemic (mg/kg · min) | 0.32 ± 0.05 | 0.48 ± 0.05 | 0.16 ± 0.05 | 0.01 |
| Suppression by insulin (%) | 79.4 ± 2.6 | 68.6 ± 4.3 | −10.7 ± 4.3 | 0.04 |
| De novo lipogenesis (n = 10) | ||||
| Fasting DNL (%) | 8.64 ± 1.34 | 6.07 ± 1.05 | −2.57 ± 1.2 | 0.06 |
| Postprandial DNL (%) | 12.68 ± 2.12 | 9.83 ± 1.98 | −2.85 ± 1.7 | 0.14 |
| IGFBPs (n = 10) | ||||
| IGFBP-1 (ng/mL) | 8.7 ± 1.8 | 9.0 ± 2.1 | 0.3 ± 1.9 | 0.88 |
| IGFBP-2 (ng/mL) | 409 ± 61 | 727 ± 129 | 318 ± 91 | 0.007 |
| IGFBP-3 (ng/mL) | 3865 ± 336 | 4082 ± 341 | 217 ± 139 | 0.15 |
| Other hormones | ||||
| Cortisol (μg/ml) (n = 10) | 9.7 ± 1.9 | 13.4 ± 2.6 | 3.7 ± 2.0 | 0.09 |
| GH (ng/ml)b | 0.23 ± 0.96 | 0.075 ± 0.018 | −0.16 ± 0.09 | N/A |
Data are mean ± sem, and P values are by Student’s t test. Values in bold are statistically significant (P < 0.05). The mean and sem values were calculated by setting the undetectable values at 0.05 ng/ml. Statistical analysis was not performed due to the few subjects with detectable values (n = 2) at month 3. LDL, Low-density lipoprotein.
Glycogenolysis is a calculated value (EGP − GNG).
GH levels were undetectable (i.e. <0.05 ng/ml) in 50% of subjects at baseline and 80% of subjects at month 3.
Glucose metabolism
Fasting glucose, HOMA-IR, and 2-h glucose and insulin decreased significantly, as did glucose and insulin AUC (Table 1). Insulin-stimulated glucose uptake (M/I) increased by 63% (P = 0.02; Fig. 1D). Fasting EGP increased by 10% (P = 0.006), and suppression of EGP by insulin was blunted during treatment (P = 0.04; Table 1). A 23% increase in fasting GNG (P = 0.01) explained most of the increase in fasting EGP.
Lipid metabolism
Fasting TG levels decreased by 23% (P = 0.049), whereas HDL-cholesterol was unchanged (Table 1). Both total and non-HDL-cholesterol levels tended to decrease. Fasting free fatty acids and whole-body lipolysis, measured both fasting and during hyperinsulinemia, did not change significantly. Fasting and postprandial rates of fractional DNL decreased by 30% and 20%, respectively (Table 1), but neither achieved statistical significance.
Body composition
Lean body mass increased and total body and truncal fat decreased, whereas changes in limb fat were minor and not significant (Table 1). VAT and SAT did not change significantly; there was no apparent difference between the two dosing levels (Table 1). There was no significant change in the intramyocellular lipid content/water ratio in either the soleus (5.1 ± 1.3 vs. 4.5 ± 1.1%, P = 0.55) or tibialis anterior (0.5 ± 0.2 vs. 0.7 ± 0.1%, P = 0.37).
Other laboratory data
Standard chemistries and liver function tests were not altered during treatment. There was a mild, nonsignificant decrease in hemoglobin (15.2 ± 0.3 vs. 14.7 ± 0.4 g/dl, P = 0.15) and white blood cell count (6.5 ± 0.6 vs. 5.5 ± 0.4 × 103 cells/μl, P = 0.15), and a statistically significant decrease in platelet count (266 ± 18 vs. 237 ± 19 × 103 cells/μl, P = 0.03). Total lymphocyte count decreased (2.0 ± 0.1 vs. 1.7 ± 0.1 × 103 cells/μl, P = 0.03), as did absolute CD4 count (565 ± 61 vs. 516 ± 67 cells/μl, P = 0.21).
Safety parameters
Apart from the aforementioned two subjects who were unable to complete the study due to allergic reactions, side effects were minimal. One subject developed mild arthralgias and lower-extremity edema that improved spontaneously. A subject with a preexisting papule on his philtrum underwent a biopsy of the lesion while on study and was diagnosed with local squamous cell carcinoma. There were no instances of hypoglycemia.
Discussion
In this pilot study, 3 months of IGF-I/IGFBP-3 treatment was associated with significant improvements in glucose tolerance and peripheral insulin sensitivity. These improvements were observed in subjects who received both the lower (0.5 mg/kg · d) and higher (1.0 mg/kg · d) doses of IGF-I/IGFBP-3 and are consistent with results of other studies using IGF-I/IGFBP-3 (15). However, the improvement in extrahepatic insulin sensitivity was accompanied by worsening hepatic glucose control as evidenced by increases in EGP and GNG. In diabetics, 7 d of treatment with IGF-I alone decreased EGP (16), as did 2 d of treatment with IGF-I/IGFBP-3 (15). However, in our study, which had a longer period of treatment, administration of IGF-I/IGFBP-3 was associated with less suppression of EGP and GNG during the clamp. Increased GNG may be a compensation for the decrease in glucose levels; it may also be a consequence of increased cortisol (17). We observed a 38% increase in plasma cortisol levels; however, there was no correlation with the increase in GNG or EGP. GH decreased in all five subjects with detectable levels, which may help explain the observed increase in cortisol levels. IGFBP-1, which may reflect portal insulin levels (18), was not significantly changed. Finally, IGF-I/IGFBP-3 may alter hepatic substrate fluxes, resulting in increased substrate channeling into the gluconeogenic pathway, as discussed further below.
As with GH, IGF-I/IGFBP-3 tended to improve lipid metabolism, including a significant decrease in fasting TG levels and fractional hepatic DNL. IGF-I/IGFBP-3 also decreased truncal and total body fat while increasing lean body mass. However, unlike GH, IGF-I/IGFBP-3 did not decrease VAT. DEXA and CT measure different fat compartments, so the decrease in truncal fat could occur because sc fat was lost in a region of the trunk not adequately captured in a single-slice CT scan, such as upper trunk fat, which is independently associated with insulin resistance (19). A decrease in upper trunk fat could contribute to the changes in metabolism seen here.
IGF-I/IGFBP-3 decreased fasting DNL by 30% (P = 0.06). We previously observed a comparable decrease in DNL and concomitant increase of GNG and EGP after GH treatment (11). These simultaneous changes in hepatic fluxes are consistent with the hypothesis that during treatment with IGF-I/IGFBP-3, fewer gluconeogenic substrates are channeled into hepatic DNL, leading to a modest surge into the gluconeogenic pathway and consequent increase in glucose production (11) as has been observed in other hyperinsulinemic states (20,21). In our study, the reduction of hepatic DNL is consistent with the reduction of fasting insulin.
Our detailed metabolic studies in HIV-infected patients with central fat accumulation have shown a number of similarities and differences between the effects of GH and IGF-I/IGFBP-3 (3,11). Within the liver, both IGF-I/IGFBP-3 and GH treatment increased glucose production and GNG while simultaneously decreasing DNL. Unlike GH, IGF-I/IGFBP-3 significantly improved insulin-mediated glucose uptake in the periphery. GH increased lipolysis and reduced visceral fat, whereas IGF-I/IGFBP-3 treatment did not appreciably alter VAT or lipolysis.
In conclusion, 3 months of treatment with IGF-I/ IGFBP-3 in HIV-infected patients with excess visceral adiposity and insulin resistance improved glucose tolerance and peripheral insulin sensitivity. Interestingly, EGP increased, whereas hepatic DNL and serum TG decreased. Further studies are needed to clarify the effects of IGF-I/IGFBP-3 on hepatic glucose and lipid metabolism.
Supplementary Material
Acknowledgments
We thank Jonathan Cheng for his help in analyzing MRS scans, the nurses of the CRC for their assistance in performing the inpatient assessments, and Geoff Allan and Insmed Corp. for the donation of the study medication and performing the IGF assays.
Footnotes
This work was supported by grant and fellowship numbers DK69185, DK54615, DK66999, RR00083, and RR24131 and a grant from Insmed, Inc. and with resources and the use of facilities of the Veterans Affairs Medical Center, San Francisco, CA.
Disclosure Statement: M.N.R., K.M., V.T., M.J.W., A.D., M.W., X.L., T.L., C.G., and J.-M.S. have nothing to declare. M.S. received the drug used in this National Institutes of Health-funded study and a small research grant from Insmed, Inc.
First Published Online July 7, 2010
Abbreviations: ARV, Antiretroviral; AUC, area under the curve; CRC, Clinical Research Center; CT, computed tomography; DEXA, dual-energy x-ray absorptiometry; DNL, de novo lipogenesis; EGP, endogenous glucose production; GNG, gluconeogenesis; HDL, high-density lipoprotein; HOMA-IR, homeostasis model assessment of insulin resistance; IGFBP-3, IGF-binding protein-3; SAT, sc adipose tissue; TG, triglyceride; VAT, visceral adipose tissue.
References
- Schambelan M, Benson CA, Carr A, Currier JS, Dubé MP, Gerber JG, Grinspoon SK, Grunfeld C, Kotler DP, Mulligan K, Powderly WG, Saag MS 2002 Management of metabolic complications associated with antiretroviral therapy for HIV-1 infection: recommendations of an International AIDS Society-USA panel. J Acquir Immune Defic Syndr 31:257–275 [DOI] [PubMed] [Google Scholar]
- Friis-Møller N, Sabin CA, Weber R, d'Arminio Monforte A, El-Sadr WM, Reiss P, Thiébaut R, Morfeldt L, De Wit S, Pradier C, Calvo G, Law MG, Kirk O, Phillips AN, Lundgren JD 2003 Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med 349:1993–2003 [DOI] [PubMed] [Google Scholar]
- Lo JC, Mulligan K, Noor MA, Schwarz JM, Halvorsen RA, Grunfeld C, Schambelan M 2001 The effects of recombinant human growth hormone on body composition and glucose metabolism in HIV-infected patients with fat accumulation. J Clin Endocrinol Metab 86:3480–3487 [DOI] [PubMed] [Google Scholar]
- Kotler DP, Muurahainen N, Grunfeld C, Wanke C, Thompson M, Saag M, Bock D, Simons G, Gertner JM 2004 Effects of growth hormone on abnormal visceral adipose tissue accumulation and dyslipidemia in HIV-infected patients. J Acquir Immune Defic Syndr 35:239–252 [DOI] [PubMed] [Google Scholar]
- Falutz J, Allas S, Blot K, Potvin D, Kotler D, Somero M, Berger D, Brown S, Richmond G, Fessel J, Turner R, Grinspoon S 2007 Metabolic effects of a growth hormone-releasing factor in patients with HIV. N Engl J Med 357:2359–2370 [DOI] [PubMed] [Google Scholar]
- Clemmons DR, Underwood LE 1992 Role of insulin-like growth factors and growth hormone in reversing catabolic states. Horm Res 38(Suppl 2):37–40 [DOI] [PubMed] [Google Scholar]
- Kim RJ, Grimberg A 2007 Potential non-growth uses of rhIGF-1. Growth Genet Horm 23:1–7 [PMC free article] [PubMed] [Google Scholar]
- Mauras N, Martinez V, Rini A, Guevara-Aguirre J 2000 Recombinant human insulin-like growth factor I has significant anabolic effects in adults with growth hormone receptor deficiency: studies on protein, glucose, and lipid metabolism. J Clin Endocrinol Metab 85:3036–3042 [DOI] [PubMed] [Google Scholar]
- Clemmons DR, Moses AC, McKay MJ, Sommer A, Rosen DM, Ruckle J 2000 The combination of insulin-like growth factor 1 and insulin-like growth factor-binding protein-3 reduces insulin requirements in insulin-dependent type 1 diabetes: evidence for in vivo biological activity. J Clin Endocrinol Metab 85:1518–1524 [DOI] [PubMed] [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC 1985 Homeostasis model assessment: insulin resistance and B-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419 [DOI] [PubMed] [Google Scholar]
- Schwarz JM, Mulligan K, Lee J, Lo JC, Wen M, Noor MA, Grunfeld C, Schambelan M 2002 Effects of recombinant human growth hormone on hepatic lipid and carbohydrate metabolism in HIV-infected patients with fat accumulation. J Clin Endocrinol Metab 87:942–945 [DOI] [PubMed] [Google Scholar]
- Snijder MB, Visser M, Dekker JM, Seidell JC, Fuerst T, Tylavsky F, Cauley J, Lang T, Nevitt M, Harris TB 2002 The prediction of visceral fat by dual-energy X-ray absorptiometry in the elderly: a comparison with computed tomography and anthropometry. Int J Obes Relat Metab Disord 26:984–993 [DOI] [PubMed] [Google Scholar]
- Li X, Youngren JF, Hyun B, Sakkas GK, Mulligan K, Majumdar S, Masharani UB, Schambelan M, Goldfine ID 2008 Technical evaluation of in vivo abdominal fat and IMCL quantification using MRI and MRSI at 3 T. Magn Reson Imaging 26:188–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedewald WT, Levy RI, Fredrickson DS 1972 Estimation of the concentration of low-density lipoprotein cholesterol in plasma without use of the preparative ultracentrifuge. Clin Chem 18:499–502 [PubMed] [Google Scholar]
- Saukkonen T, Shojaee-Moradie F, Williams RM, Amin R, Yuen KC, Watts A, Acerini CL, Umpleby AM, Dunger DB 2006 Effects of recombinant human IGF-I/IGF-binding protein-3 complex on glucose and glycerol metabolism in type 1 diabetes. Diabetes 55:2365–2370 [DOI] [PubMed] [Google Scholar]
- Cusi K, DeFronzo R 2000 Recombinant human insulin-like growth factor I treatment for 1 week improves metabolic control in type 2 diabetes by ameliorating hepatic and muscle insulin resistance. J Clin Endocrinol Metab 85:3077–3084 [DOI] [PubMed] [Google Scholar]
- Tayek JA and Katz J 1997 Glucose production, recycling, Cori cycle, and gluconeogenesis in humans: relationship to serum cortisol. Am J Physiol Endo Metab 272(35):E476–E484 [DOI] [PubMed] [Google Scholar]
- Yki-Järvinen H, Mäkimattila S, Utriainen T, Rutanen EM 1995 Portal insulin concentrations rather than insulin sensitivity regulate serum sex hormone-binding globulin and insulin-like growth factor binding protein 1 in vivo. J Clin Endocrinol Metab 80:3227–3232 [DOI] [PubMed] [Google Scholar]
- Grunfeld C, Rimland D, Gibert CL, Powderly WG, Sidney S, Shlipak MG, Bacchetti P, Scherzer R, Haffner SM, Heymsfield SB 2007 Association of upper trunk and visceral adipose tissue volume with insulin resistance in control and HIV-infected subjects in the FRAM study. J Acquir Immun Defic Syndr 46:283–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimomura I, Matsuda M, Hammer RE, Bashmakov Y, Brown MS, Goldstein JL 2000 Decreased IRS-2 and increased SREBP-1c lead to mixed insulin resistance and sensitivity in livers of lipodystrophic and ob/ob mice. Mol Cell 6:77–86 [PubMed] [Google Scholar]
- Schwarz JM, Linfoot P, Dare D, Aghajanian K 2003 Hepatic de novo lipogenesis in normoinsulinemic and hyperinsulinemic subjects consuming high-fat, low-carbohydrate and low-fat, high-carbohydrate isoenergetic diets. Am J Clin Nutr 77:43–50 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.