Abstract
Unsuspected adrenal masses, or incidentalomas, are increasingly found with the widespread use of thoracic and abdominal imaging. These masses may be hormonally active or nonfunctional and malignant or benign. Clinicians must determine the nature of the mass to decide what treatment, if any, is needed. Measurement of precontrast Hounsfield units (HU) and contrast washout on computed tomography scan provide useful diagnostic information. All patients should undergo biochemical testing for pheochromocytoma, either with plasma or urinary catecholamine measurements. This is particularly important before surgical resection, which is routinely recommended for masses larger than 4 cm in diameter without a clear-cut diagnosis and for others with hormonal secretion or ominous imaging characteristics. Hypertensive patients should undergo biochemical testing for hyperaldosteronism. Patients with features consistent with Cushing’s syndrome, such as glucose intolerance, weight gain, and unexplained osteopenia, should be evaluated for cortisol excess. Here, the dexamethasone suppression test and late-night salivary cortisol may be preferred over measurement of urine cortisol. The ability of surgical resection to reverse features of mild hypercortisolism is not well established. For masses that appear to be benign (<10 HU; washout, >50%), small (<3 cm), and completely nonfunctioning, imaging and biochemical reevaluation (pheochromocytoma and hypercortisolism only) at 1–2 yr (or more) is appropriate. For more indeterminate lesions, repeat evaluation for growth after 3–12 months is useful, with subsequent testing intervals based on the rate of growth.
After pheochromocytoma evaluation, incidentally found adrenal masses >4 cm should be resected and smaller masses should have endocrine evaluation.
Accreditation and Credit Designation Statements
The Endocrine Society is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians. The Endocrine Society has achieved Accreditation with Commendation.
The Endocrine Society designates this educational activity for a maximum of 1 AMA PRA Category 1 Credit(s)™. Physicians should only claim credit commensurate with the extent of their participation in the activity.
Learning Objectives
Upon completion of this educational activity, participants should be able to
Assess the probability of malignancy based on radiographic features.
Obtain pre-operative testing before resection of an incidentaloma.
Predict improvements in patients with subclinical Cushing’s syndrome after resection of an adenoma.
Target Audience
This continuing medical education activity should be of substantial interest to endocrinologists.
Disclosure Policy
Authors, editors, and Endocrine Society staff involved in planning this CME activity are required to disclose to learners any relevant financial relationship(s) that have occurred within the last 12 months with any commercial interest(s) whose products or services are discussed in the CME content. The Endocrine Society has reviewed all disclosures and resolved or managed all identified conflicts of interest, as applicable.
The following individuals reported NO relevant financial relationships:
Lynette Nieman, M.D., and Leonard Wartofsky, M.D., reported no relevant financial relationships.
Endocrine Society staff and the medical writer associated with the development of content for this activity reported no relevant financial relationships.
Acknowledgement of Commercial Support
This activity is not supported by grants, other funds, or in-kind contributions from commercial supporters.
Privacy and Confidentiality Statement
The Endocrine Society will record learner’s personal information as provided on CME evaluations to allow for issuance and tracking of CME certificates. No individual performance data or any other personal information collected from evaluations will be shared with third parties.
Method of Participation
This Journal CME activity is available in print and online as full text HTML and as a PDF that can be viewed and/or printed using Adobe Acrobat Reader. To receive CME credit, participants should review the learning objectives and disclosure information; read the article and reflect on its content; then go to http://jcem.endojournals.org and find the article, click on CME for Readers, and follow the instructions to access and complete the post-activity test questions and evaluation. The estimated time to complete this activity, including review of material, is 1 hour. If you have questions about this CME activity, please direct them to education@endo-society.org.
Activity release date: September 2010
Activity expiration date: September 2011
Introduction
A 45-yr-old woman was referred for evaluation of a 3-cm homogeneous adrenal mass discovered when a computed tomography (CT) scan was obtained to evaluate intermittent epigastric pain. She offered no complaints except for a desire to lose weight. She had long-standing obesity, bipolar disorder, and asthma, and a 3-yr history of diabetes and hypertension treated medically. There was no history of fracture, problem with memory, or menstrual irregularity. On examination, blood pressure was 118/56 mm Hg; heart rate, 78 beats per minute; weight, 160 kg; height, 170 cm; and body mass index, 55.4 kg/m2. She was generally obese and plethoric. There was a small increase only in dorsocervical fat. There were no other skin changes, hirsutism, or muscle weakness. The plethora was probably long-standing (“Just the way I look”). Her appearance had not changed over 3 yr.
On CT, the mass had a regular border and punctate calcifications. No contrast washout was obtained, but precontrast Hounsfield units (HU) were 11.
Background
With widespread use of CT and magnetic resonance imaging (MRI), unexpected adrenal masses with a diameter of more than 1 cm are increasingly found. In 1979, Korobkin et al. (1) reported 15 patients studied on a prototype CT scanner for other indications who had an adrenal mass. In a prescient remark, they commented, “It is clear that the capacity of CT to image both adrenal glands will lead to occasional discovery of asymptomatic adrenal disease” (1). A large study at the Mayo Clinic from 1985 through 1989 found a 3.4% prevalence (2,066 of 61,054) of incidentally found adrenal masses (2). Masses were seen in 4.2% of 520 patients studied later with higher resolution machines (3). The frequency of incidentaloma increases from about 0.2 to 7% from youth to old age (4).
In adults, these masses represent many disorders (Table 1). They may be hormonally active or nonfunctional and malignant or benign. Clinicians must determine the nature of the mass to decide what treatment, if any, is needed.
Table 1.
Causes of adrenal masses
| Hormone excess—up to 15% (5) |
| Adenoma (aldosterone or cortisol) |
| Carcinoma (any adrenal hormone) |
| Pheochromocytoma |
| Congenital adrenal hyperplasiaa |
| Massive macronodular adrenal diseasea |
| Nodular variant of Cushing’s disease |
| No hormonal excess |
| Adenoma |
| Myelolipoma |
| Neuroblastoma |
| Ganglioneuroma |
| Hemangioma |
| Carcinoma |
| Metastasisa |
| Cyst |
| Hemorrhagea |
| Granulomaa |
| Amyloidosisa |
| Infiltrative diseasea |
Bilateral etiologies.
Diagnostic Strategies
Malignancy is an uncommon cause of incidentaloma in patients not known to have cancer. In two large studies, 4.7 and 5% of patients had primary adrenal carcinoma and 0.7 and 2.5% had nonadrenal metastasis (5,6). Malignancy is suggested (but not excluded) on CT by a large diameter (>6 cm), irregular border, inhomogeneity, a “washout” of contrast after 15 min of less than 40%, and calcifications (7,8). However, except for the washout characteristics, many malignant lesions look benign. A large diameter is not diagnostic; only about 25% of masses larger than 4 cm were adrenal cancer in one large study (5). Despite this, given the high mortality rate associated with adrenal carcinoma, most centers recommend excision of lesions sized 4 cm or more. However, if CT characteristics point to a myelolipoma or cyst, and the patient is asymptomatic, nonsurgical management is appropriate, regardless of mass size (9,10). Similarly, other clear-cut diagnoses do not dictate surgery (e.g. an acute hematoma, tuberculosis, myelolipoma, and possibly a nonfunctioning mass in an older individual).
Additional imaging techniques may suggest a diagnosis (11). If the initial CT studies are not diagnostic, measurement of HU and washout of contrast should be done. A low attenuation on CT before contrast administration reflects high lipid content and is found in myelolipomas (less than −30 HU) and lipid-rich adenomas (less than 10 HU) (11). In a study of 151 patients who had pathologically proven diagnoses, all nonadenomas had a precontrast HU of more than 10. However, lipid-poor adenomas can have higher attenuation, accounting for the poor sensitivity (about 58% for a 20-HU criterion) for their identification (12).
Other more expensive tests generally are not needed. Chemical shift imaging on MRI also reflects the lipid content of tissues, with lipid-rich adenomas losing signal intensity on out-of-phase images (7). 18-Fluoro-2-deoxy-d-glucose positron emission tomography has high sensitivity for malignancy but is not specific. A more recently developed ligand, 11C-metomidate, detects nonnecrotic adrenocortical tumors (benign and malignant) and is negative in other masses (13). However, it is not widely available.
Fine-Needle Aspiration (FNA)
What is the diagnostic role of FNA? If hormonal evaluation is done first (as it should be, at least to exclude pheochromocytoma), the question is whether FNA provides a diagnosis that would change management. Because cytology cannot discriminate adrenal cancer from adenoma, FNA is usually recommended when a nonadrenal malignancy is suspected in the setting of a known tumor elsewhere, or when infection is a possibility. If either diagnosis would change treatment, it is reasonable to consider biopsy, bearing in mind that there is a small but important risk of an adverse event, most commonly adrenal hemorrhage, pneumothorax, and pain (∼3%) (14,15). Rarely, cancer recurrence along the needle track or death has been reported (15). In a recent retrospective analysis of a 10-yr experience, FNA revealed pheochromocytoma in two of 15 patients with incidentaloma; 12 had nondiagnostic results. Biopsies were helpful in two of four patients with suspected metastasis and in one of three patients with a large symptomatic mass. Adverse events included liver hematoma, hemothorax, and duodenal hematoma. However, FNA results did not alter the treatment plan in any patient (16). Taken together, these data indicate that FNA is rarely indicated.
Evaluation for Hormonal Excess
There is no consensus on the optimal diagnostic approach to the incidentally discovered adrenal mass. A report from an National Institutes of Health (NIH) “state of the science” conference and a review from an experienced clinician both recommend hormonal evaluation (except when the imaging findings are unequivocal, as in a myelolipoma) (17,18).
Hypercortisolism
Cushing’s syndrome represents a spectrum of excess cortisol production in which signs and symptoms increase as hypercortisolism worsens. Patients with the full-blown syndrome often date its appearance to small changes that increased over time. By analogy, some adrenal masses probably secrete so little excess cortisol that minimal signs of Cushing’s syndrome are not recognized. This lack of recognition may reflect the common nature of the presenting features (usually weight gain, hypertension, and/or glucose intolerance in a middle-aged or older patient) and the absence of more “obvious” features. It may be more accurate to refer to this as mild or subtle rather than “subclinical” Cushing’s syndrome. The notion of mild hypersecretion with clinical consequences was substantiated by a study comparing women with “non-secretory” adrenal incidentaloma to healthy women and those with overt Cushing’s syndrome. The women with an incidentaloma were intermediate in terms of fat mass, prevalence of hypertension, lipid abnormalities, and response to an oral glucose tolerance test (19).
Because the diagnostic criteria for subtle Cushing’s syndrome vary between studies, its prevalence is not established (20). A meta-analysis of more than 2000 cases found subclinical Cushing’s syndrome in 5.3% (6), whereas a subsequent large study of 1004 patients found a 9.2% prevalence (5).
The Endocrine Society guidelines for the diagnosis of Cushing’s syndrome advocate screening these patients and suggest that the diagnosis is likely if two screening tests are abnormal, using urine free cortisol (UFC), late-night salivary cortisol, and/or dexamethasone suppression (1 mg overnight or 2 mg 2-day test) (21). By contrast, studies of adrenal incidentaloma often use measures of ACTH suppression, such as the basal morning value, the response to CRH, or the dehydroepiandrosterone sulfate (DHEAS) level (5,22,23,24,25). Furthermore, the criterion for an abnormal response to dexamethasone ranges from 2 to 5 μg/dl, not the 1.8 μg/dl cutoff point in the guidelines or the 5 μg/dl criterion recommended by the NIH state of the science report. Also, the number of abnormal results required for diagnosis is variable.
The dexamethasone suppression test (DST) is often abnormal in patients with incidentaloma, but all reports required additional abnormalities before making the diagnosis of Cushing’s syndrome. In one study, 36 of 39 patients had an abnormal DST response, but only 15 met criteria for Cushing’s syndrome (26). Similarly, in another report, 33 of 98 patients had an abnormal response to dexamethasone, but only eight had clinical or preclinical Cushing’s syndrome. These data suggest that there is a continuum of mild oversecretion, development of autonomy, and the presence of clinical features.
The utility of a late-night salivary cortisol measurement for the detection of hypercortisolism has been tested in few patients. In the largest study, 22 of 104 cases with incidentaloma had subclinical hypercortisolism; of these, the DST and ACTH levels were abnormal in about 86%, whereas UFC was abnormal in about 31%; the sensitivity and specificity of salivary cortisol were 23 and 88%, respectively (27). By contrast, smaller series found it useful as a screening test or during prolonged outpatient sampling (28,29).
Postoperative hypocortisolism, which implies previous hypercortisolism, was evaluated in 60 patients undergoing surgery because of adenoma size or subclinical hypercortisolism. When tested 2 months after surgery, 39 had subnormal cortisol responses to insulin or ACTH. Preoperative lack of suppression to the 2 mg DST, low plasma ACTH, elevated UFC, and/or 2400 h serum cortisol predicted an abnormal response. Postoperative hypocortisolism occurred in all patients with preoperative increased UFC and 2400 h serum cortisol, but also in some with completely normal preoperative results (22).
A few uncontrolled studies described improvement after adenoma resection, most often in blood pressure. In one study, systolic blood pressure decreased but did not normalize, diastolic blood pressure normalized in four of six hypertensive patients, and glucose status normalized in two patients (30). In another study, nearly all nine patients with subclinical Cushing’s syndrome improved in fatigue, weakness, and body mass index, and half had improvement or normalization of hypertension (31). Another report of 11 patients found that hypertension, but not body mass index, presence of diabetes, or hyperlipidemia improved 1 yr after surgery (32).
Few studies have addressed the important question of whether adenoma resection improves metabolic or other features of Cushing’s syndrome compared with medical treatment. One nonrandomized study compared 47 patients who underwent adrenalectomy to 78 treated conservatively and showed that atherosclerotic risk factors did not differ 5–16 yr after diagnosis (33). Another showed deterioration in cardiovascular markers in six of 12 patients with subclinical hypercortisolism who received medical treatment only, compared with improvement in eight of 10 after adrenalectomy (34). Unfortunately, these retrospective studies do not address whether there were differences in treatment groups at baseline.
A single prospective randomized trial suggests that surgery may be more beneficial than medical treatment. In this study, patients with subclinical Cushing’s syndrome were randomly assigned to laparoscopic adrenalectomy (n = 23) or medical management (n = 22). In the surgical group, diabetes normalized or improved in five of eight patients, hypertension in 12 of 18, hyperlipidemia in three of eight, and obesity in three of six. Bone parameters did not improve. The medically managed group had further impairment in glucose tolerance, blood pressure, and lipids (35).
Pheochromocytoma
In most series, 4–7% of incidentalomas are pheochromocytomas (36,37,38), although one Korean study found a 20% prevalence (39). From another perspective, in one series of 201 patients with proven pheochromocytoma, only 10% of cases had the typical triad of symptoms (sweating, headaches, and palpitations), and 12.5% of incidentally found cases were normotensive (40).
Because patients may be asymptomatic and normotensive, each should be screened for pheochromocytoma (except for those with other obvious diagnoses). There is no consensus on the best screening test(s) and no prospective study comparing test regimens. Measurement of urine fractionated metanephrines and catecholamines is less sensitive than that of fractionated plasma free metanephrines, but the latter is less specific. An international symposium report suggests using either as a screening test, while recognizing the increased probability of true pathology with more abnormal results (41). Others suggest measurement of urine catecholamines in patients without strong radiographic evidence for pheochromocytoma and use of plasma free metanephrines in those with a greater pretest probability based on the imaging results (14). If possible, all patients with pheochromocytoma should undergo preoperative medical management to block any effects of catecholamines released during surgery (30).
Hyperaldosteronism
Recent guidelines from The Endocrine Society advocate screening for hyperaldosteronism in patients with an adrenal incidentaloma and hypertension, citing a prevalence of 1.1 to 10% (42). The plasma aldosterone-to-renin ratio is recommended for case detection, using a sensitive renin assay and a diagnostic threshold of 20–40 (42). This should be obtained in the morning from a seated patient. Oral sodium loading, saline infusion, fludrocortisone suppression, or captopril challenge should be used to confirm or exclude the diagnosis. For patients with a mass of less than 2–3 cm who desire surgery, adrenal venous sampling is recommended to exclude adrenal hyperplasia, for which surgery is not recommended (42).
Congenital adrenal hyperplasia
In one study, adrenal hyperplasia was found in all 42 patients and nodule formation in 18 of 22 homozygous patients and nine of 20 heterozygotes (43). However, this is an uncommon cause of incidentaloma (<1% in one series) (44). Young (14) recommends case detection for patients with clinically suggestive features or bilateral masses, whereas Jaresch et al. (43) suggest screening when a large mass(es) would otherwise be excised.
Diagnostic Tests in Our Patient
Based on the CT findings, immediate surgery was not deemed necessary. Endocrine evaluation was delayed because she did not attend appointments. A morning renin:aldosterone ratio and urinary catecholamines were normal. Twenty-four-hour UFC were 81, 182, and 207 μg (normal to 77), with appropriate creatinine and volume. The overnight 1-mg and 8-mg DSTs were normal (cortisol, 3.1 and 3.3 μg/dl) according to the guidelines available at the time (<5 μg/dl) (17). DHEAS was undetectable. Table 2 shows other results.
Table 2.
Preoperative laboratory and test data in the case
| Day 1 | Day 2 | Day 3 | Normal range | ACTH (pg/ml) | Cortisol (μg/dl) | |
|---|---|---|---|---|---|---|
| Serum cortisol (μg/dl) | ||||||
| 0800 h | 19.7 | 10.2 | 13.9 | 5–25 | ||
| 2400 h | 9.5 | 2.6 | 3.1a | < 7.5 | ||
| Ovine CRH test | ||||||
| Baseline | <4 | 15.3 | ||||
| Peak | 15.4 | 47.3 |
Salivary cortisol undetectable at this time.
Our patient had discordant results. One slightly elevated UFC might be explained by her psychiatric disorder or obesity, two of the three 2400 h serum cortisol values were normal, and a 2400 h salivary cortisol was undetectable. She had a normal response to ovine CRH. Her clinical presentation was not striking, and she did not exhibit any clear-cut feature of Cushing’s syndrome (except perhaps for the plethora). On the other hand, her presentation and recent history of diabetes and hypertension were consistent with mild hypercortisolism, the basal ACTH and DHEAS were suppressed, and the UFC and 2400 h cortisol were variably increased. Based on this subtle cortisol excess, we offered surgical resection, with the caveat that clinical improvement was uncertain.
The resected mass appeared to be a benign adenoma. During the first postoperative week, UFC (41 and 63 μg/24 h) and plasma ACTH (26 pg/ml) were normal, morning cortisol levels were 1.6 to 13.6 μg/dl, and she had no symptoms of adrenal insufficiency. An ACTH stimulation test 3 wk later showed a peak cortisol of 24.3 μg/dl. She did not receive hydrocortisone.
Four months later, antihypertensive medications and insulin had been discontinued. Her blood pressure was 100/50 mm Hg, and she had lost 7 kg. She felt “better” but had been placed on an antipsychotic.
This patient illustrates the inconsistency in diagnostic tests, lack of postoperative hypocortisolism, and improvement in cortisol-dependent signs and symptoms after adrenalectomy for subtle cortisol excess. However, psychiatric symptoms did not improve. Was adrenalectomy the correct choice? For her, given variable compliance with appointments, and (possibly) medications, this result was welcome. For more compliant patients, an intensive medical regimen might work as well.
Long-Term Follow-Up
If patients do not undergo surgical resection, most authors recommend continued annual surveillance for hyperfunction, and one to three interval CT scans to evaluate increasing size, based on estimated risks (5,14,17,45). Cawood et al. (46) pose a contrarian view based on a meta-analysis of data from patients with probable adrenal adenoma followed for a median of 2.1 yr. They argue that the actual rates of conversion to hyperfunction are about 1% (95% confidence interval, −0.5 to 2.2), and increased size about 15% (95% confidence interval, 8.0–21.3%), and they show that malignancy or metastasis developed in only three of about 1400 patients. None of the patients developed hyperaldosteronism. Because of the cost of testing and potentially increased risk of cancer due to radiation exposure, they propose no additional follow-up for lesions less than 4 cm that appear to be benign and nonfunctioning, and they recommend a single additional CT scan for 4–6 cm lesions.
Two subsequent series (n = 200) with follow-up of 2 and 5 yr report an increase in size of 0.5 cm or more in 17.4 and 20% of patients, development of overt hyperfunction in 8 and 0%, but development of subclinical hypercortisolism in 6 and 24% (47,48). One patient developed adrenal carcinoma. Another retrospective study (49) suggests that malignancy can be detected by an absolute increase in size of 0.8 cm, 0.64 cm growth per year, or a 25% increase in size per year. These cutoff points tended to have a better specificity (75–85%) than sensitivity (50–50%) in patients with a second imaging at 3–12 months after initial evaluation. However, the study included patients with known malignancy. There were three patients with initially unrecognized adrenal cancer whose masses had a HU more than 10 and grew 1.0, 3.0, and 3.3 cm over 2, 12, and 15 months, respectively (49).
Controversies and Areas of Uncertainty
The long-term natural history of adrenal incidentalomas is not known, and continued follow-up of current cohorts is critical to answer the following questions:
What percentage of incidentalomas ultimately hyperfunction?
What percentage eventually increase in size to 4 cm? If these are resected, what is their pathology—in other words does a gradual increase over time demand resection?
When does hyperfunction or increase in mass last occur? Is there a safe time to discontinue follow-up?
Do any imaging findings predict who should undergo additional testing?
The best diagnostic strategy for determination of hypercortisolism is not known. Ideally, studies would include a consistent set of laboratory tests, perhaps a 1 mg DST, plasma ACTH and DHEAS, late-night salivary cortisol, and UFC. Follow-up evaluations also would need to be consistent. If these data and the results of surgical or medical management were shared, better conclusions could be made about the diagnosis of subclinical hypercortisolism and its management.
When patients have bilateral nodules, one may be nonfunctioning and the other hyperfunctioning. With pheochromocytoma or subclinical hypercortisolism, we do not know how to determine which one should be resected.
Finally, the diagnostic utility of other imaging techniques should be explored.
Summary and Suggested Approach
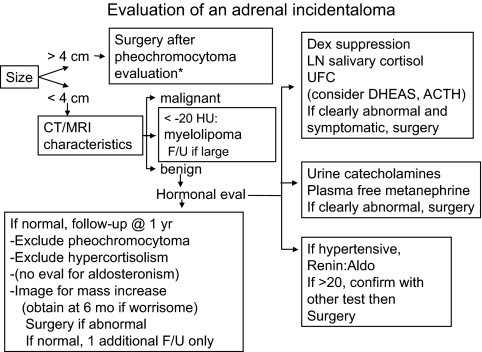
An algorithm for the evaluation of an incidentally discovered adrenal mass is shown in Fig. 1. Evaluation of imaging characteristics is the first step. Most advocate resection of a mass larger than 4 cm if the patient is a surgical candidate, unless there is clearly a transient, infectious, or benign cause. All patients (except for those with another clear diagnosis) should undergo testing for pheochromocytoma.
Figure 1.
Suggested evaluation of an incidentally found adrenal mass. *, Surgery for large masses without a cause that requires resection, e.g. tuberculosis. LN, Late-night; Aldo, aldosterone; Dex, dexamethasone; F/U, follow-up; eval, evaluation; mo, months.
Other CT or MRI characteristics may suggest surgery, regardless of size. Inhomogeneous masses with an irregular border are less likely to be benign, especially if the HU reading is more than 20. The washout characteristics may confirm benignity. Patients with known or likely infection need medical, not surgical treatment, if any, and should be assessed for adrenal insufficiency.
The medical history and physical examination may suggest possible causes. For example, hypertension may suggest an hormonally active mass, whereas congenital adrenal hyperplasia may explain bilateral masses. A known malignancy may account for other masses.
Evaluation of hormonal excess should be done when imaging characteristics do not suggest a need for surgery. As mentioned above, most patients require evaluation to exclude pheochromocytoma. Hyperaldosteronism should be excluded in hypertensive patients, and all should be evaluated for hypercortisolism. There is no consensus on the best evaluation for hypercortisolism. This author follows The Endocrine Society guidelines, placing more weight on the 1-mg DST or late-night cortisol test, rather than UFC, and using a suppressed morning plasma ACTH and DHEAS level as supportive of the diagnosis (21).
Surgical treatment is an option for patients with mild hypercortisolism initially, if medical treatment fails or if other symptoms intervene. When clinical and laboratory results are not striking, or when features seem to be very long-standing without progression, the potential risks of surgery may be greater than the prospect of benefit. A meta-analysis of 2625 laparoscopic adrenalectomy procedures showed a mortality of about 0.2% 1 month after surgery. Causes of death included massive hemorrhage, necrotizing pancreatitis, pulmonary embolism, sepsis, and cardiopulmonary failure. The overall morbidity rate averaged about 9%, with a higher rate in pheochromocytoma and Cushing’s syndrome. Obesity contributes to a more difficult procedure in the latter patients (50). The clinician, patient, and surgeon must make the decision to operate jointly, trying to achieve the best calculus of what is known, valued, and predicted.
There is no consensus on appropriate follow-up evaluation for those who do not have surgery, and one must be guided by clinical judgment and the presumed cause of the mass. For masses that appear to be benign (<10 HU; washout, >50%), small (<3 cm), and completely nonfunctioning, imaging and biochemical reevaluation at 1–2 yr (or more) is appropriate, with subsequent follow-up for clinical changes; the risk of malignancy or subsequent hyperfunction is nearly nil. For more indeterminate lesions, repeat evaluation for growth after 3–12 months is useful. Subsequent testing would occur earlier for lesions showing increasing size, and later for those with no change.
No patients have been reported with a subsequent diagnosis of hyperaldosteronism, so repeat screening is not necessary. The risk for development of pheochromocytoma is very low; unfortunately, its CT characteristics can overlap those of lipid-poor adenomas. Because most of these tumors grow over time (14), biochemical testing could possibly be restricted to those individuals. Patients with laboratory feature(s) of cortisol excess should be rescreened clinically at annual intervals, with biochemical evaluation as warranted. However, although the cost-effectiveness of this strategy is unknown, most authors recommend annual biochemical screening for catecholamine and cortisol excess for 4 yr (14).
Footnotes
Disclosure Summary: L.K.N. has nothing to declare.
Abbreviations: CT, Computed tomography; DHEAS, dehydroepiandrosterone sulfate; DST, dexamethasone suppression test; FNA, fine-needle aspiration; HU, Hounsfield units; MRI, magnetic resonance imaging; UFC, urine free cortisol.
References
- Korobkin M, White EA, Kressel HY, Moss AA, Montagne JP 1979 Computed tomography in the diagnosis of adrenal disease. AJR Am J Roentgenol 132:231–238 [DOI] [PubMed] [Google Scholar]
- Herrera MF, Grant CS, van Heerden JA, Sheedy PF, Ilstrup DM 1991 Incidentally discovered adrenal tumors: an institutional perspective. Surgery 110:1014–1021 [PubMed] [Google Scholar]
- Bovio S, Cataldi A, Reimondo G, Sperone P, Novello S, Berruti A, Borasio P, Fava C, Dogliotti L, Scagliotti GV, Angeli A, Terzolo M 2006 Prevalence of adrenal incidentaloma in a contemporary computerized tomography series. J Endocrinol Invest 29:298–302 [DOI] [PubMed] [Google Scholar]
- Herra MF, Pantoja JP, Espagna N 2005 Adrenal incidentalomas. In: Linos D, van Heerden JA, eds. Adrenal glands: diagnostic aspects and surgical therapy. Berlin: Springer-Verlag; 231–244 [Google Scholar]
- Mantero F, Terzolo M, Arnaldi G, Osella G, Masini AM, Alì A, Giovagnetti M, Opocher G, Angeli A 2000 A survey on adrenal incidentaloma in Italy. Study Group on Adrenal Tumors of the Italian Society of Endocrinology. J Clin Endocrinol Metab 85:637–644 [DOI] [PubMed] [Google Scholar]
- Young Jr WF 2000 Management approaches to adrenal incidentalomas. A view from Rochester, Minnesota. Endocrinol Metab Clin North Am 29:159–185, x [DOI] [PubMed] [Google Scholar]
- Boland GW, Blake MA, Hahn PF, Mayo-Smith WW 2008 Incidental adrenal lesions: principles, techniques, and algorithms for imaging characterization. Radiology 249:756–775 [DOI] [PubMed] [Google Scholar]
- Peña CS, Boland GW, Hahn PF, Lee MJ, Mueller PR 2000 Characterization of indeterminate (lipid-poor) adrenal masses: use of washout characteristics at contrast-enhanced CT. Radiology 217:798–802 [DOI] [PubMed] [Google Scholar]
- Castillo OA, Vitagliano G, Cortes O, Sánchez-Salas R, Arellano L 2007 Laparoscopic adrenalectomy for adrenal myelolipoma. Arch Esp Urol 60:217–221 [DOI] [PubMed] [Google Scholar]
- El-Hefnawy AS, El Garba M, Osman Y, Eraky I, El Mekresh M, Ibrahim el-H 2009 Surgical management of adrenal cysts: single-institution experience. BJU Int 104:847–850 [DOI] [PubMed] [Google Scholar]
- Ilias I, Sahdev A, Reznek RH, Grossman AB, Pacak K 2007 The optimal imaging of adrenal tumours: a comparison of different methods. Endocr Relat Cancer 14:587–599 [DOI] [PubMed] [Google Scholar]
- Hamrahian AH, Ioachimescu AG, Remer EM, Motta-Ramirez G, Bogabathina H, Levin HS, Reddy S, Gill IS, Siperstein A, Bravo EL 2005 Clinical utility of noncontrast computed tomography attenuation value (Hounsfield units) to differentiate adrenal adenomas/hyperplasias from nonadenomas: Cleveland Clinic experience. J Clin Endocrinol Metab 90:871–877 [DOI] [PubMed] [Google Scholar]
- Hennings J, Lindhe O, Bergström M, Långström B, Sundin A, Hellman P 2006 [11C]Metomidate positron emission tomography of adrenocortical tumors in correlation with histopathological findings. J Clin Endocrinol Metab 91:1410–1414 [DOI] [PubMed] [Google Scholar]
- Young Jr WF 2007 Clinical practice. The incidentally discovered adrenal mass. N Engl J Med 356:601–610 [DOI] [PubMed] [Google Scholar]
- Kloos RT, Gross MD, Francis IR, Korobkin M, Shapiro B 1995 Incidentally discovered adrenal masses. Endocr Rev 16:460–484 [DOI] [PubMed] [Google Scholar]
- Quayle FJ, Spitler JA, Pierce RA, Lairmore TC, Moley JF, Brunt LM 2007 Needle biopsy of incidentally discovered adrenal masses is rarely informative and potentially hazardous. Surgery 142:497–502; discussion 502–494 [DOI] [PubMed] [Google Scholar]
- Grumbach MM, Biller BM, Braunstein GD, Campbell KK, Carney JA, Godley PA, Harris EL, Lee JK, Oertel YC, Posner MC, Schlechte JA, Wieand HS 2003 Management of the clinically inapparent adrenal mass (“incidentaloma”). Ann Intern Med 138:424–429 [DOI] [PubMed] [Google Scholar]
- Young Jr WF 2007 Adrenal causes of hypertension: pheochromocytoma and primary aldosteronism. Rev Endocr Metab Disord 8:309–320 [DOI] [PubMed] [Google Scholar]
- Garrapa GG, Pantanetti P, Arnaldi G, Mantero F, Faloia E 2001 Body composition and metabolic features in women with adrenal incidentaloma or Cushing’s syndrome. J Clin Endocrinol Metab 86:5301–5306 [DOI] [PubMed] [Google Scholar]
- Kasperlik-Zeluska AA, Rosłonowska E, Słowinska-Srzednicka J, Migdalska B, Jeske W, Makowska A, Snochowska H 1997 Incidentally discovered adrenal mass (incidentaloma): investigation and management of 208 patients. Clin Endocrinol (Oxf) 46:29–37 [DOI] [PubMed] [Google Scholar]
- Nieman LK, Biller BM, Findling JW, Newell-Price J, Savage MO, Stewart PM, Montori VM 2008 The diagnosis of Cushing’s syndrome: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 93:1526–1540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eller-Vainicher C, Morelli V, Salcuni AS, Torlontano M, Coletti F, Iorio L, Cuttitta A, Ambrosio A, Vicentini L, Carnevale V, Beck-Peccoz P, Arosio M, Ambrosi B, Scillitani A, Chiodini I 2010 Post-surgical hypocortisolism after removal of an adrenal incidentaloma: is it predictable by an accurate endocrinological work-up before surgery? Eur J Endocrinol 162:91–99 [DOI] [PubMed] [Google Scholar]
- Emral R, Uysal AR, Asik M, Gullu S, Corapcioglu D, Tonyukuk V, Erdogan G 2003 Prevalence of subclinical Cushing’s syndrome in 70 patients with adrenal incidentaloma: clinical, biochemical and surgical outcomes. Endocr J 50:399–408 [DOI] [PubMed] [Google Scholar]
- Terzolo M, Bovio S, Reimondo G, Pia A, Osella G, Borretta G, Angeli A 2005 Subclinical Cushing’s syndrome in adrenal incidentalomas. Endocrinol Metab Clin North Am 34:423–439, x [DOI] [PubMed] [Google Scholar]
- Rossi R, Tauchmanova L, Luciano A, Di Martino M, Battista C, Del Viscovo L, Nuzzo V, Lombardi G 2000 Subclinical Cushing’s syndrome in patients with adrenal incidentaloma: clinical and biochemical features. J Clin Endocrinol Metab 85:1440–1448 [DOI] [PubMed] [Google Scholar]
- Katabami T, Obi R, Shirai N, Naito S, Saito N 2005 Discrepancies in results of low-and high-dose dexamethasone suppression tests for diagnosing preclinical Cushing’s syndrome. Endocr J 52:463–469 [DOI] [PubMed] [Google Scholar]
- Masserini B, Morelli V, Bergamaschi S, Ermetici F, Eller-Vainicher C, Barbieri AM, Maffini MA, Scillitani A, Ambrosi B, Beck-Peccoz P, Chiodini I 2009 The limited role of midnight salivary cortisol levels in the diagnosis of subclinical hypercortisolism in patients with adrenal incidentaloma. Eur J Endocrinol 160:87–92 [DOI] [PubMed] [Google Scholar]
- Doi M, Sekizawa N, Tani Y, Tsuchiya K, Kouyama R, Tateno T, Izumiyama H, Yoshimoto T, Hirata Y 2008 Late-night salivary cortisol as a screening test for the diagnosis of Cushing’s syndrome in Japan. Endocr J 55:121–126 [DOI] [PubMed] [Google Scholar]
- Nunes ML, Vattaut S, Corcuff JB, Rault A, Loiseau H, Gatta B, Valli N, Letenneur L, Tabarin A 2009 Late-night salivary cortisol for diagnosis of overt and subclinical Cushing’s syndrome in hospitalized and ambulatory patients. J Clin Endocrinol Metab 94:456–462 [DOI] [PubMed] [Google Scholar]
- Midorikawa S, Sanada H, Hashimoto S, Suzuki T, Watanabe T 2001 The improvement of insulin resistance in patients with adrenal incidentaloma by surgical resection. Clin Endocrinol (Oxf) 54:797–804 [DOI] [PubMed] [Google Scholar]
- Mitchell IC, Auchus RJ, Juneja K, Chang AY, Holt SA, Snyder 3rd WH, Nwariaku FE 2007 “Subclinical Cushing’s syndrome” is not subclinical: improvement after adrenalectomy in 9 patients. Surgery 142:900–905; discussion 905.e1 [DOI] [PubMed] [Google Scholar]
- Erbil Y, Ademođlu E, Ozbey N, Barbaros U, Yanik BT, Salmasliođlu A, Bozbora A, Ozarmađan S 2006 Evaluation of the cardiovascular risk in patients with subclinical Cushing syndrome before and after surgery. World J Surg 30:1665–1671 [DOI] [PubMed] [Google Scholar]
- Sereg M, Szappanos A, Toke J, Karlinger K, Feldman K, Kaszper E, Varga I, Gláz E, Rácz K, Tóth M 2009 Atherosclerotic risk factors and complications in patients with non-functioning adrenal adenomas treated with or without adrenalectomy: a long-term follow-up study. Eur J Endocrinol 160:647–655 [DOI] [PubMed] [Google Scholar]
- Tsuiki M, Tanabe A, Takagi S, Naruse M, Takano K 2008 Cardiovascular risks and their long-term clinical outcome in patients with subclinical Cushing’s syndrome. Endocr J 55:737–745 [DOI] [PubMed] [Google Scholar]
- Toniato A, Merante-Boschin I, Opocher G, Pelizzo MR, Schiavi F, Ballotta E 2009 Surgical versus conservative management for subclinical Cushing syndrome in adrenal incidentalomas: a prospective randomized study. Ann Surg 249:388–391 [DOI] [PubMed] [Google Scholar]
- Bernini GP, Vivaldi MS, Argenio GF, Moretti A, Sgrò M, Salvetti A 1997 Frequency of pheochromocytoma in adrenal incidentalomas and utility of the glucagon test for the diagnosis. J Endocrinol Invest 20:65–71 [DOI] [PubMed] [Google Scholar]
- Bülow B, Ahrén B 2002 Adrenal incidentaloma—experience of a standardized diagnostic programme in the Swedish prospective study. J Intern Med 252:239–246 [DOI] [PubMed] [Google Scholar]
- Mantero F, Masini AM, Opocher G, Giovagnetti M, Arnaldi G 1997 Adrenal incidentaloma: an overview of hormonal data from the National Italian Study Group. Horm Res 47:284–289 [DOI] [PubMed] [Google Scholar]
- Kim HY, Kim SG, Lee KW, Seo JA, Kim NH, Choi KM, Baik SH, Choi DS 2005 Clinical study of adrenal incidentaloma in Korea. Korean J Intern Med 20:303–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopetschke R, Slisko M, Kilisli A, Tuschy U, Wallaschofski H, Fassnacht M, Ventz M, Beuschlein F, Reincke M, Reisch N, Quinkler M 2009 Frequent incidental discovery of phaeochromocytoma: data from a German cohort of 201 phaeochromocytoma. Eur J Endocrinol 161:355–361 [DOI] [PubMed] [Google Scholar]
- Pacak K, Eisenhofer G, Ahlman H, Bornstein SR, Gimenez-Roqueplo AP, Grossman AB, Kimura N, Mannelli M, McNicol AM, Tischler AS 2007 Pheochromocytoma: recommendations for clinical practice from the First International Symposium, October 2005. Nat Clin Pract Endocrinol Metab 3:92–102 [DOI] [PubMed] [Google Scholar]
- Funder JW, Carey RM, Fardella C, Gomez-Sanchez CE, Mantero F, Stowasser M, Young Jr WF, Montori VM 2008 Case detection, diagnosis, and treatment of patients with primary aldosteronism: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 93:3266–3281 [DOI] [PubMed] [Google Scholar]
- Jaresch S, Kornely E, Kley HK, Schlaghecke R 1992 Adrenal incidentaloma and patients with homozygous or heterozygous congenital adrenal hyperplasia. J Clin Endocrinol Metab 74:685–689 [DOI] [PubMed] [Google Scholar]
- Barzon L, Scaroni C, Sonino N, Fallo F, Gregianin M, Macrì C, Boscaro M 1998 Incidentally discovered adrenal tumors: endocrine and scintigraphic correlates. J Clin Endocrinol Metab 83:55–62 [DOI] [PubMed] [Google Scholar]
- Bülow B, Jansson S, Juhlin C, Steen L, Thorén M, Wahrenberg H, Valdemarsson S, Wängberg B, Ahrén B 2006 Adrenal incidentaloma—follow-up results from a Swedish prospective study. Eur J Endocrinol 154:419–423 [DOI] [PubMed] [Google Scholar]
- Cawood TJ, Hunt PJ, O'Shea D, Cole D, Soule S 2009 Recommended evaluation of adrenal incidentalomas is costly, has high false-positive rates and confers a risk of fatal cancer that is similar to the risk of the adrenal lesion becoming malignant; time for a rethink? Eur J Endocrinol 161:513–527 [DOI] [PubMed] [Google Scholar]
- Yener S, Ertilav S, Secil M, Demir T, Akinci B, Kebapcilar L, Comlekci A, Bayraktar F, Yesil S 2010 Prospective evaluation of tumor size and hormonal status in adrenal incidentalomas. J Endocrinol Invest 33:32–36 [DOI] [PubMed] [Google Scholar]
- Vassilatou E, Vryonidou A, Michalopoulou S, Manolis J, Caratzas J, Phenekos C, Tzavara I 2009 Hormonal activity of adrenal incidentalomas: results from a long-term follow-up study. Clin Endocrinol (Oxf) 70:674–679 [DOI] [PubMed] [Google Scholar]
- Pantalone KM, Gopan T, Remer EM, Faiman C, Ioachimescu AG, Levin HS, Siperstein A, Berber E, Shepardson LB, Bravo EL, Hamrahian AH 2010 Change in adrenal mass size as a predictor of malignancy. Endocr Pract 11:1–31 [DOI] [PubMed] [Google Scholar]
- Gumbs AA, Gagner M 2006 Laparoscopic adrenalectomy. Best Pract Res Clin Endocrinol Metab 20:483–499 [DOI] [PubMed] [Google Scholar]