Abstract
Normal aging is associated with a significant reduction in cognitive function across primate species. However, the structural and molecular basis for this age-related decline in neural function has yet to be defined clearly. Extensive cell loss does not occur as a consequence of normal aging in human and nonhuman primate species. More recent studies have demonstrated significant reductions in functional neuronal markers in subcortical brain regions in primates as a consequence of aging, including dopaminergic and cholinergic systems, although corresponding losses in cortical innervation from these neurons have not been investigated. In the present study, we report that aging is associated with a significant 25% reduction in cortical innervation by cholinergic systems in rhesus monkeys (P < 0.001). Further, these age-related reductions are ameliorated by cellular delivery of human nerve growth factor to cholinergic somata in the basal forebrain, restoring levels of cholinergic innervation in the cortex to those of young monkeys (P = 0.89). Thus, (i) aging is associated with a significant reduction in cortical cholinergic innervation; (ii) this reduction is reversible by growth-factor delivery; and (iii) growth factors can remodel axonal terminal fields at a distance, representing a nontropic action of growth factors in modulating adult neuronal structure and function (i.e., administration of growth factors to cholinergic somata significantly increases axon density in terminal fields). These findings are relevant to potential clinical uses of growth factors to treat neurological disorders.
Keywords: aging, Alzheimer's disease, gene therapy, plasticity, cholinergic systems
Aging in primates is associated with clear declines in cognitive function (1–4). The structural and cellular bases for these changes remain poorly defined. Extensive neuronal loss as a function of aging does not occur in either humans or nonhuman primates (5–10). However, declines in neuronal function have been described as a consequence of aging in two subcortical neural systems that modulate cortical function, namely basal forebrain cholinergic and mesencephalic dopaminergic neurons. In the nonhuman primate, 43% of basal forebrain cholinergic neurons exhibit age-related declines in somal immunolabeling for cholinergic markers (11) together with declines in cortical levels of choline-acetyltransferase activity (12). Similar changes have been found in rodent species (13–16). Dopaminergic systems also exhibit declines in neurotransmitter expression in the aged primate substantia nigra, and in dopaminergic transmitter markers in both striatal and cortical target regions (17–19). To date, however, reliable structural changes in axonal projections or connectivity in cortical circuitry have not been described in the context of normal aging, despite the strong possibility that such changes could account for observed age-related declines in cognition.
In the present study, we examined whether aging in the primate brain is associated with an alteration of cortical innervation from basal forebrain cholinergic cell populations. Further, we determined whether age-related changes in axonal terminal fields, if present, could be ameliorated by cellular delivery of nerve growth factor (NGF) to cholinergic neuronal somata in the basal forebrain. Previous studies have reported that NGF exerts specific neurite-promoting actions only along chemotropic (concentration) gradients whereby cholinergic axons grow toward the source of NGF (20–22). In the present paradigm, we determined whether NGF delivery to the cholinergic neuronal soma would influence terminal axonal morphology, thereby representing a nonchemotropic action of neurotrophins on intact axons. Findings of the present study indicate that primate aging indeed is associated with a significant reduction of cortical cholinergic innervation, and that this reduction is reversed by NGF gene delivery to the neuronal soma.
Methods
All experimental subjects were born at the California Regional Primate Center (CRPC). Before the present study, animals were maintained under similar social conditions and were not involved in behavioral studies, surgical procedures, or pharmacological experiments. All procedures and animal care adhered strictly to American Association for the Accreditation of Laboratory Animal Care, Society for Neuroscience, and institutional guidelines for experimental animal health, safety, and comfort.
Four groups of rhesus monkeys (Macaca mulatta) were studied: 7 young-adult nonoperated subjects (mean age 9.5 ± 0.9 years); 2 aged nonoperated subjects (mean age 27.5 ± 1.2 years); 4 aged control subjects that received intraparenchymal grafts of autologous fibroblasts genetically modified to produce the reporter gene β-galactosidase (mean age 22.1 ± 0.6 years); and 5 aged subjects that received intraparenchymal grafts of autologous fibroblasts genetically modified to produce and secrete the full-length β component of human NGF (mean age 22.5 ± 0.5 years).
For NGF delivery to aged subjects, autologous fibroblasts genetically modified to produce and secrete human NGF were prepared as described (23, 24). Briefly, primary fibroblasts obtained from skin biopsies were modified genetically in vitro to produce the active, β subunit of human NGF. Transduction procedures were carried out by using replication-incompetent retroviral vectors derived from Moloney murine leukemia virus. Transduced cells were selected by growth in the neomycin analog G418. Production of full-length NGF mRNA was determined by Northern blot, and NGF secretion from transfected cells was monitored by using a two-site ELISA as described (25). In vitro, before grafting, fibroblasts from NGF-grafted monkeys secreted a mean of 12 ± 2.0 ng human NGF per 106 cells per day, and the biological activity of secreted NGF was verified by using a PC12 bioassay (26). Optimal NGF-producing bulk clones were amplified to numbers sufficient for in vivo grafting by serial passaging. Cells for grafting to control monkeys were prepared similarly, except the reporter gene β-galactosidase was substituted for the NGF gene as described (23, 24). Cells were harvested, suspended at a final concentration of 105 cells per μl, and grafted into five sites spanning the rostral-to-caudal extent of the intermediate division of the nucleus basalis of Meynert (Ch4i) bilaterally (10 grafts total). Ch4i was targeted by grafts to potentially influence specifically those cholinergic neurons that project to cortical regions influencing learning and memory (27). After being killed 3 months later, all aged subjects exhibited surviving grafts within the target region (see Fig. 1). Previously, grafts of NGF-secreting fibroblasts have been shown to sustain in vivo gene expression for at least 8 months in the monkey brain (11).
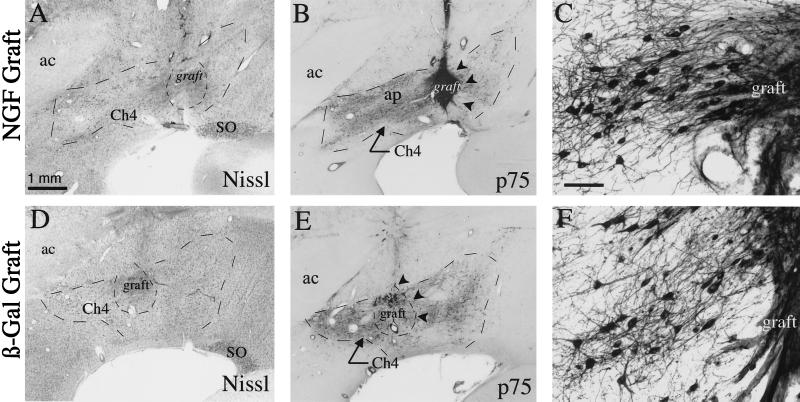
Figure 1.
NGF-secreting and control grafts within Ch4i. (A) Thionin-stained coronal section showing NGF-secreting cell graft within the Ch4i. The graft is well integrated within host tissue. (B) p75 immunolabeled section adjacent to A reveals dense graft penetration by cholinergic axons. (C) Higher magnification of p75 labeled cholinergic neurons adjacent to an NGF-secreting graft demonstrates fiber growth directed toward the graft. (D–F) Comparable thionin and p75 immunolabeled sections from a control aged monkey that received β-galactosidase-expressing fibroblasts. Graft survival is comparable to that of NGF grafts, but fewer axons penetrate the grafts. ac, anterior commissure; ap, Ansa peduncularis; SO, supraoptic nucleus. (Scale bar in A = 1 mm and applies to A, B, D, E; bar in C = 100 μm and applies to C and F.)
Surgery.
Genetically modified cell grafts were targeted to cholinergic somata located in the Ch4i, which primarily innervates insular and inferior temporal cortices (27). Monkeys underwent preoperative MRI scans to generate stereotaxic coordinates for basal forebrain grafting sites. For surgery, monkeys were preanesthetized with 25 mg/kg ketamine i.m. and then were anesthetized with isofluorane. After performing a parasagittal craniotomy and retracting the dura, cells were injected bilaterally into each of five sites equally spaced over the rostral-caudal extent of Ch4i (10 grafts total per animal). Grafts were targeted to a position slightly dorsal to, but within 500 μm of, the Ch4i-cell population. Cell suspensions, each consisting of 10-μl volume (1.0 × 105 cells per μl), were injected into the 10 grafting sites with a 25-gauge Hamilton syringe at a rate of 1 μl/min. Postoperatively, all experimental subjects were observed closely for signs of discomfort or toxicity and received the analgesic buprenorphine. Grafted monkeys survived for 3 months and then were killed by transcardial perfusion as described below.
Perfusion and Histology.
Animals were sedated with 25 mg/kg ketamine i.m. and were anesthetized deeply with Nembutal (30 mg/kg i.p.) before being killed. All reflex responses to cutaneous stimulation were absent verifiably before perfusion procedures were begun. Subjects were perfused transcardially for 1 hour with a solution containing 4% (vol/vol) paraformaldehyde in 0.1 M phosphate buffer at 4°C and 5% sucrose solution in the same buffer for 20 min.
Serial coronal sections through the brain were cut on a freezing microtome set to a 40-μm thickness. Every sixth section was Nissl-stained for identifying graft placement within the basal forebrain. In each case, graft survival and placement relative to the Ch4-cell group were confirmed. An adjacent series of sections (240-μm spacing) was processed for acetylcholinesterase (AChE) histochemistry according to described protocols (28). AChE staining was done routinely on sections either the same day the brain was sectioned or on the next day. Care was taken to be consistent with all incubation times in the staining procedure, including the time in the Karnovsky–Roots solution (65 mM sodium acetate/0.2 mM ethopropazine/1.7 mM acetylthiocholine iodide/5 mM sodium citrate/3 mM cupric sulfate/0.2 mM potassium ferricynide; 30 min), ammonium sulfide (1 min), and silver nitrate (1 min). AChE has been demonstrated to be a reliable marker of cholinergic terminals, and recent studies using selective immunotoxic lesions in rats (29, 30) and primates (31) have demonstrated that almost all AChE fiber labeling in the cortex arises from the basal forebrain cholinergic system.
Quantification of Cholinergic Innervation Density.
An intersect analysis was used to obtain a quantitative estimate of cholinergic axon density in several different cortical regions described below, using standardized methods developed by Geula and Mesulam (32). This method was chosen because it is relatively insensitive to intersubject variability in AChE-staining intensity and is thought to reflect axon density reliably (32). Briefly, very high-resolution digital images of AChE-stained, 40-μm-thick sections were captured from an Olympus AX-70 microscope at ×360 magnification (×600 for hippocampus) with high numerical aperture optics using a digital Spot camera (Diagnostics Instruments, Sterling Heights, MI) with a computer interface. Images were displayed on a high-resolution Sony monitor and a 6 × 6 grid (175 μm × 175 μm for ×360) was superimposed on each quantification frame by using Adobe photoshop 5.0 (see Fig. 2). The number of AChE-stained axons intersecting all gridlines in the field were quantified and summed in each image. This method of analysis was chosen because it is capable of quantifying cholinergic fiber numbers independent of the intensity of the AChE-staining intensity (32). Two fields per animal were quantified in this manner from each of the following cortical regions (nomenclature of Paxinos et al., ref. 33): inferior temporal cortex layers II and V; insular cortex layers II and V; cingulate cortex layer II; frontal cortex layer II; and hippocampal formation (stratum radiatum of CA1). Comparable sections were chosen from each experimental subject by using fiduciary landmarks by an investigator blinded to the group identity, and images were captured by placing the counting grid over the appropriate region manually. Cortical layers II and V were chosen for analysis because they were defined easily on the basis of morphological criteria. Layer II was always sampled parallel to the cortical surface, placing the grid ≈25-μm below the cell-sparse molecular layer I. Layer V was sampled by placing the grid parallel to the cortical surface, directly over the large pyramidal neurons that occupy this region. Hippocampal sampling was accomplished by placing the grid within the center of stratum radiatum of the CA1 hippocampal subfield, parallel to the pyramidal cell layer. All samples were captured by a single experimenter from coded slides, and the analysis of captured images was always carried out by a second experimenter; both experimenters were blinded to the details of the experimental groups until after the study was completed.
Figure 2.
Quantification of cholinergic innervation densities. Cholinergic axon density was determined in multiple cortical regions. Quantified regions included: inferior temporal cortex layers II (IT-II) and V (IT-V); insular cortex layers II (INS-II) and V (INS-V); cingulate cortex layer II (CING); frontal cortex layer II (FR); and hippocampal formation, stratum radiatum of CA1 (HF). Axon densities were determined by superimposing a 6 × 6 grid over a highly magnified image captured from one of the defined regions (see Inset). All AChE-stained fibers crossing the gridlines (arrows in Inset) were counted to yield an index of innervation density, as described by Guela and Mesulam (32). (Scale bar = 5 mm.) (Scale bar in Inset = 35 μm.)
To determine further whether differences in AChE fiber-intensity staining may have influenced the outcome of this analysis, two investigators blinded to the identity of the experimental subjects, independently rated the subjective darkness/intensity of AChE-stained sections in those regions used in the preceding quantitative analysis. By using a rank scale of 0–5 (0 = lightly stained, 5 = very darkly stained), the mean overall intensity/darkness of the AChE staining was measured.
Statistics.
Multiple group comparisons were made by analysis of variance (ANOVA) with post hoc analysis by using Fisher's least-square difference (lsd). Biological significance was established at the 95% confidence level. Data are presented as mean ± SEM.
Results
Aging Is Associated with a Significant Reduction in Cholinergic Fiber Density in the Neocortex.
Aged β-galactosidase-grafted animals did not differ significantly from aged nonoperated animals in cholinergic innervation of the various cortical fields examined in this study and therefore were combined into a single group “aged controls” aged/intact = −17.6 ± 2.8%; aged/Veh = −28.4 ± 3.4% relative to young animals; P = 0.07). When averaged across all cortical regions, overall group differences in cholinergic axon innervation density were present (P < 0.0001, ANOVA; Fig. 3). Aged control monkeys exhibited a significant 24.8 ± 2.6% decline in AChE axon terminal density averaged across all cortical regions compared with nonaged subjects (P < 0.0001, post hoc Fisher's lsd; Fig. 3). These differences were consistent and significant across several individual cortical regions when analyzed independently (Fig. 4), including insular cortex, cingulate cortex, and frontal cortex. Strong trends toward similar age-related differences in cholinergic innervation were present in the inferior temporal cortex and in the hippocampal formation (Fig. 4).
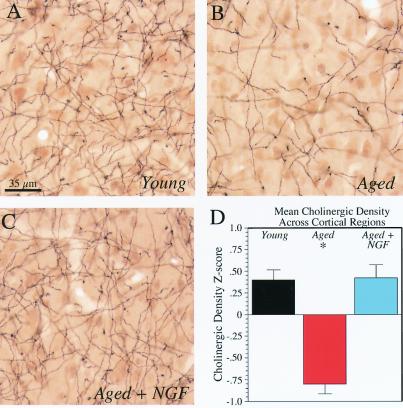
Figure 3.
Age-related decline in mean cortical cholinergic innervation is reversed by NGF gene delivery to cholinergic somata in the basal forebrain. AChE staining in the insular cortex of young, aged-control, and aged-NGF-grafted rhesus monkeys. (A) The normal density of cholinergic axons is illustrated in young subjects. (B) Axon density is reduced in aged-control grafted subjects. (C) AChE-stained fiber density is increased significantly in aged monkeys that received grafts of autologous NGF-secreting fibroblasts into Ch4i. (A–C, scale bar = 35 μm.) (D) Quantification of cholinergic axon density. To compare cholinergic innervation densities across multiple cortical regions, normalized z scores of density measurements from each cortical region were calculated and then averaged. A significant overall group effect was present by one-way ANOVA (P < 0.0001). Aging was associated with a significant reduction in overall cholinergic fiber density (*, P < 0.0001, post hoc Fisher's lsd), which was restored in recipients of NGF-secreting cells. Black bars, young monkeys; red bars, aged controls; blue bars, aged NGF-grafted subjects. Error bars represent SEM.
Figure 4.
Changes in cholinergic axon density across cortical regions. Aged control monkeys (red bars) exhibit a significant decline in cortical cholinergic innervation compared with young intact animals (black bars) in most cortical regions. Aged recipients of NGF-secreting grafts (blue bars) exhibit a significant reversal of age-related loss in cholinergic innervation; however, this effect is significant only in cortical regions (insula and inferior temporal cortex) innervated primarily by cholinergic neurons of Ch4i, which was targeted for grafting. Numbers in parentheses below each cortical region indicate P value for ANOVA. *, significantly reduced compared with young animals (P < 0.05, post hoc Fisher's lsd); #, significantly increased compared with aged control animals (P < 0.05, post hoc Fisher's lsd). INS, insular cortex; IT, inferior temporal cortex; CING, cingulate cortex; FR, frontal cortex; HF, hippocampal formation.
Cellular NGF Delivery Reverses Age-Related Reductions in Cholinergic Innervation.
Aged monkeys that received grafts of NGF-secreting cells exhibited a substantial and significant reversal of age-related declines in cortical cholinergic innervation (Figs. 3 and 4). When averaged across all cortical regions examined in this study, NGF-grafted animals had levels of cholinergic innervation that were significantly greater than values of aged control monkeys (P < 0.0001, post hoc Fisher's lsd; Fig. 3D) and were equal to intact young monkeys (P = 0.89, post hoc Fisher's lsd). Further, in NGF-grafted aged subjects, cortical regions (insular and inferior temporal cortices) receiving innervation primarily from Ch4i, the cholinergic subdivision targeted for grafting, demonstrated levels of cholinergic innervation significantly exceeding those of normal young monkeys (overall 13.4 ± 4.5% increase relative to young monkeys; P = 0.01, post hoc Fisher's lsd; Fig. 4). Levels of cholinergic innervation in these regions also significantly exceeded control aged monkeys (overall 43.6 ± 3.0% increase; P < 0.0001, post hoc Fisher's lsd). Cholinergic fiber densities in cortical regions (cingulate and frontal cortex, hippocampus) not heavily innervated by the targeted Ch4i-cell population also exhibited slight reversal of age-related losses after NGF-cell grafting, although the magnitude of the reversal relative to aged control monkeys (20.6 ± 4.1% increase; P = 0.01, post hoc Fisher's lsd) was more modest than that observed in the temporal and insular cortex. These effects of cellularly delivered NGF on cortical cholinergic innervation were exerted at a distance, because the growth factor was presented to the cholinergic soma yet influenced terminal axon density in the distant cortex.
To determine whether NGF delivery simply increased AChE expression in neurons, thereby enhancing the intensity of the AChE stain rather than actually increasing axon density, AChE-staining intensity (darkness) was compared in experimental groups by using the rating scale of 1–5 defined in Methods (5 = darkest). The mean intensity of AChE staining was equal in aged vehicle-infused (4.1 ± 0.29) and aged NGF-infused (4.1 ± 0.18) subjects. Further, AChE-staining intensity was slightly darker in aged animals compared with young subjects (3.2 ± 0.39), indicating that reductions in axon density with age were not an artifact of lighter AChE staining. It was noted also that AChE-stained fibers were labeled equally throughout the z plane of individual sections.
Discussion
The present findings indicate that normal aging in the nonhuman primate brain is associated with a significant reduction in cholinergic innervation of the cortex. Cholinergic systems contribute only a small fraction to the total number of axons and synapses in the cortex (34), yet exert an important role in modulating neuronal excitability throughout the neocortex and hippocampus (35, 36). Thus, alterations in this system can exert wide-ranging effects on various aspects of cognition, including attention and memory (37–40). Although prior studies have reported age-related declines in some transmitter systems in the cortex (12–19), structural alterations in neocortical systems have not been described previously. The present report indicates that system-specific structural markers reveal extensive reductions in cortical cholinergic axonal terminals in the primate brain with aging.
Notably, these age-related changes in cortical cholinergic innervation were reversed substantially by cellular gene delivery of NGF. Whereas previous studies had reported that NGF reverses age-related atrophy of the cholinergic neuronal soma in the rodent (41–44) and primate brain (11), effects of NGF on distant cholinergic terminals have not been reported. Findings of the present study indicate that NGF reverses the effects of aging on cholinergic innervation in diverse cortical regions. The effects of NGF on cholinergic axon densities were most prominent within cortical regions innervated preferentially by the Ch4i-cell population targeted by the grafts. Ch4i-targeted grafts only marginally affected cholinergic axon densities within cortical regions innervated primarily by either the Ch4 anterior-cell group (cingulate and frontal cortex) or the Ch1 and Ch2 cholinergic-cell group (hippocampus). Taken together, these results strongly suggest that graft-derived NGF acts primarily within the region immediately adjacent the grafted cells.
The possibility that NGF delivery merely up-regulated AChE expression rather than truly augmenting cholinergic density has been excluded largely by the demonstration that the intensity of the AChE stain was not affected by NGF delivery. The lack of a direct effect of NGF on AChE expression is consistent with prior studies. NGF can activate the cholinergic gene locus strongly, thereby increasing expression of choline acetyltransferase, stimulating vesicular acetylcholine transporter and enhancing acetylcholine release but does not directly alter AChE expression (45–47). Thus, aging is associated with a true reduction in the number of cholinergic axons, and this reduction is ameliorated substantially by NGF-secreting cell grafts placed adjacent to projecting cholinergic somata.
These findings also indicate for the first time that neurotrophins can influence axonal morphology at a distance. To the present, neurotrophic factors have been reported to influence axonal growth and structure in a concentration-dependent fashion. Experiments both in vitro (48, 49) and in vivo (20–22) have demonstrated that local application of neurotrophic factors to axons and their growth cones elicit concentration-dependent changes in axonal growth. Indeed, a local chemotropic sprouting of cholinergic axons in NGF-secreting grafts was present in this study (Fig. 1). Yet in addition, aged monkeys with NGF-secreting cell grafts placed adjacent to the cholinergic soma exhibited a significant increase in the number or complexity of cholinergic terminals in the cortex, representing a nonchemotropic, distant action of NGF. Further, this reversal of age-related axonal attenuation occurred after just 3 months of NGF delivery to the primate brain. Thus, the aged brain remains sensitive to growth-factor delivery at both somal (21, 41–44) and axonal levels. The mechanism through which growth factors influence axonal morphology at a distance may involve activation of diverse intracellular downstream signaling pathways, including PI3-kinase and ras/extracellular signal-regulated kinase (50, 51), leading to increases in cellular transport of growth-related proteins. Thus, to the growing list of growth factor effects in the adult brain can be added nonchemotropic modulation of axonal structure.
The mechanism of age-related loss of cortical cholinergic innervation and atrophy of cholinergic somata in the primate brain (11) remains to be established. Age-related reductions in NGF production in the cortex have not been reported either in the context of normal or pathological aging (52–55). On the other hand, reductions in expression of neurotrophin receptors in basal forebrain cholinergic neurons have been reported in Alzheimer's disease (56, 57) but not in normal aging. Alterations in expression of other metabolic components of neural-cell function including immediate early gene activation, neuronal cytoskeletal integrity, and ability to buffer calcium and manage oxidative damage, are currently being examined in the aged brain (reviewed in ref. 1). Regardless of the mechanism of age-related atrophy and loss in neuronal systems, growth-factor delivery reverses these changes in the primate basal forebrain cholinergic system, supporting a potential rationale for growth-factor delivery in the context of such neurodegenerative disorders as Alzheimer's disease (58, 59). Further insights into basic mechanisms of age-related dysfunction in the brain will contribute to the design of the most rational approaches for treating age-related pathological neuronal degeneration.
Abbreviations
- NGF
nerve growth factor
- Ch4i
intermediate division of Ch4
- AChE
acetylcholinesterase
- lsd
least-square difference
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Morrison J H, Hof P R. Science. 1997;278:412–419. doi: 10.1126/science.278.5337.412. [DOI] [PubMed] [Google Scholar]
- 2.Peters A, Morrison J H, Rosene D L, Hyman B T. Cereb Cortex. 1998;8:295–300. doi: 10.1093/cercor/8.4.295. [DOI] [PubMed] [Google Scholar]
- 3.Burke D M, Mackay D G. Philos Trans R Soc London B. 1997;352:1845–1856. doi: 10.1098/rstb.1997.0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davis R T. In: Behavior and Pathology of Aging in Rhesus Monkeys, Monographs in Primatology. Davis R T, Leathers C W, editors. Vol. 8. New York: Liss; 1985. pp. 57–82. [Google Scholar]
- 5.West M J, Coleman P D, Flood D G, Troncoso J C. Lancet. 1994;344:769–772. doi: 10.1016/s0140-6736(94)92338-8. [DOI] [PubMed] [Google Scholar]
- 6.Gómez-Isla T, Price J L, McKeel D W, Jr, Morris J C, Growdon J H, Hyman B T. J Neurosci. 1996;16:4491–4500. doi: 10.1523/JNEUROSCI.16-14-04491.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pakkenberg B, Gundersen H J. J Comp Neurol. 1997;384:312–320. [PubMed] [Google Scholar]
- 8.Šimić G, Kostović I, Winblad B, Bogdanović N. J Comp Neurol. 1997;379:482–494. doi: 10.1002/(sici)1096-9861(19970324)379:4<482::aid-cne2>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 9.West M J, Gundersen H J. J Comp Neurol. 1990;296:1–22. doi: 10.1002/cne.902960102. [DOI] [PubMed] [Google Scholar]
- 10.West M J. Neurobiol Aging. 1993;14:287–293. doi: 10.1016/0197-4580(93)90113-p. [DOI] [PubMed] [Google Scholar]
- 11.Smith D E, Roberts J, Gage F H, Tuszynski M H. Proc Natl Acad Sci USA. 1999;96:10893–10898. doi: 10.1073/pnas.96.19.10893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wenk G L, Pierce D J, Struble R G, Price D L, Cork L C. Neurobiol Aging. 1989;10:11–19. doi: 10.1016/s0197-4580(89)80005-3. [DOI] [PubMed] [Google Scholar]
- 13.Gallagher M, Burwell R D, Kodsi M H, McKinney M, Southerland S, Vella-Rountree L, Lewis M H. Neurobiol Aging. 1990;11:507–514. doi: 10.1016/0197-4580(90)90111-c. [DOI] [PubMed] [Google Scholar]
- 14.Fischer W, Gage F H, Bjorklund A. Eur J Neurosci. 1989;1:34–45. doi: 10.1111/j.1460-9568.1989.tb00772.x. [DOI] [PubMed] [Google Scholar]
- 15.Fischer W, Chen K S, Gage F H, Bjorklund A. Neurobiol Aging. 1992;13:9–23. doi: 10.1016/0197-4580(92)90003-g. [DOI] [PubMed] [Google Scholar]
- 16.Luine V, Hearns M. Brain Res. 1990;523:321–324. doi: 10.1016/0006-8993(90)91507-d. [DOI] [PubMed] [Google Scholar]
- 17.Siddiqi Z, Kemper T L, Killiany R. J Neuropathol Exp Neurol. 1999;58:959–971. doi: 10.1097/00005072-199909000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Siddiqi Z A, Peters A. J Neuropathol Exp Neurol. 1999;58:903–920. doi: 10.1097/00005072-199909000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Emborg M E, Ma S Y, Mufson E J, Levey A I, Taylor M D, Brown W D, Holden J E, Kordower J H. J Comp Neurol. 1998;401:253–265. [PubMed] [Google Scholar]
- 20.Hagg T, Varon S. Exp Neurol. 1993;119:37–45. doi: 10.1006/exnr.1993.1005. [DOI] [PubMed] [Google Scholar]
- 21.Kordower J H, Winn S R, Liu Y-T, Mufson E J, Sladek J R, Hammang J P, Baetge E E, Emerich D F. Proc Natl Acad Sci USA. 1994;91:10898–10902. doi: 10.1073/pnas.91.23.10898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tuszynski M H, U, H-S, Gage F H. Ann Neurol. 1991;30:625–636. doi: 10.1002/ana.410300502. [DOI] [PubMed] [Google Scholar]
- 23.Tuszynski M H, Senut M-C, Ray J, U, H-S, Gage F H. Neurobiol Dis. 1994;1:67–78. doi: 10.1006/nbdi.1994.0009. [DOI] [PubMed] [Google Scholar]
- 24.Tuszynski M H, Roberts J, Senut M-C, Gage F H. Gene Ther. 1996;3:305–314. [PubMed] [Google Scholar]
- 25.Conner J M, Varon S. J Neurosci Methods. 1996;65:93–99. doi: 10.1016/0165-0270(95)00151-4. [DOI] [PubMed] [Google Scholar]
- 26.Greene L A, Aletta J M, Rukenstein A, Green S H. Methods Enzymol. 1987;147:207–216. doi: 10.1016/0076-6879(87)47111-5. [DOI] [PubMed] [Google Scholar]
- 27.Mesulam M-M, Mufson E J, Levey A I, Wainer B H. J Comp Neurol. 1983;214:170–197. doi: 10.1002/cne.902140206. [DOI] [PubMed] [Google Scholar]
- 28.Hedreen J C, Bacon S J, Price D L. J Histochem Cytochem. 1985;33:134–140. doi: 10.1177/33.2.2578498. [DOI] [PubMed] [Google Scholar]
- 29.Heckers S, Ohtake T, Wiley R G, Lappi D A, Geula C, Mesulam M-M. J Neurosci. 1994;14:1271–1289. doi: 10.1523/JNEUROSCI.14-03-01271.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berger-Sweeney J, Heckers S, Mesulam M-M, Wiley R G, Lappi D A, Sharma M. J Neurosci. 1994;14:4507–4519. doi: 10.1523/JNEUROSCI.14-07-04507.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ridley R M, Pugh P, Maclean C J, Baker H F. Brain Res. 1999;836:120–138. doi: 10.1016/s0006-8993(99)01641-8. [DOI] [PubMed] [Google Scholar]
- 32.Geula C, Mesulam M M. Cereb Cortex. 1996;6:165–177. doi: 10.1093/cercor/6.2.165. [DOI] [PubMed] [Google Scholar]
- 33.Paxinos G, Huang X-F, Toga A W. The Rhesus Monkey Brain in Stereotaxic Coordinates. San Diego: Academic; 2000. [Google Scholar]
- 34.Chen K S, Masliah E, Mallory M, Gage F H. Neurosci. 1995;68:19–27. doi: 10.1016/0306-4522(95)00099-5. [DOI] [PubMed] [Google Scholar]
- 35.Sillito A M, Murphy P C. Cereb Cortex. 1987;6:161–185. [Google Scholar]
- 36.Sarter M, Bruno J P. Neurosci. 2000;95:933–952. doi: 10.1016/s0306-4522(99)00487-x. [DOI] [PubMed] [Google Scholar]
- 37.Sarter M, Bruno J P. Brain Res Brain Res Rev. 1997;23:28–46. doi: 10.1016/s0165-0173(96)00009-4. [DOI] [PubMed] [Google Scholar]
- 38.Everitt B J, Robbins T W. Annu Rev Psycol. 1997;45:649–684. doi: 10.1146/annurev.psych.48.1.649. [DOI] [PubMed] [Google Scholar]
- 39.Jerusalinsky D, Edgar K, Izquierdo I. Neurochem Res. 1997;22:507–515. doi: 10.1023/a:1027376230898. [DOI] [PubMed] [Google Scholar]
- 40.Whitehouse P J. J Clin Psychiatry. 1998;13, Suppl. 59:19–22. [PubMed] [Google Scholar]
- 41.Fischer W, Wictorin K, Bjorklund A, Williams L R, Varon S, Gage F H. Nature (London) 1987;329:65–68. doi: 10.1038/329065a0. [DOI] [PubMed] [Google Scholar]
- 42.Chen K S, Gage F H. J Neurosci. 1995;15:2819–2825. doi: 10.1523/JNEUROSCI.15-04-02819.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martinez-Serrano A, Fischer W, Bjorklund A. Neuron. 1995;15:473–484. doi: 10.1016/0896-6273(95)90051-9. [DOI] [PubMed] [Google Scholar]
- 44.Markowska A L, Koliatsos V E, Breckler S J, Price D L, Olton D S. J Neurosci. 1994;14:4815–4823. doi: 10.1523/JNEUROSCI.14-08-04815.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Williams L R, Rylett R J. J Neurochem. 1990;55:1042–1049. doi: 10.1111/j.1471-4159.1990.tb04594.x. [DOI] [PubMed] [Google Scholar]
- 46.Rylett R J, Goddard S, Schmidt B M, Williams L R. J Neurosci. 1993;13:3956–3963. doi: 10.1523/JNEUROSCI.13-09-03956.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Berse B, Lopez-Coviella I, Blusztajn J K. Biochem J. 1999;342:301–308. [PMC free article] [PubMed] [Google Scholar]
- 48.Campenot R B. Proc Natl Acad Sci USA. 1977;74:4516–4519. doi: 10.1073/pnas.74.10.4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Campenot R B. J Neurobiol. 1994;25:599–611. doi: 10.1002/neu.480250603. [DOI] [PubMed] [Google Scholar]
- 50.Segal R A, Greenberg M E. Annu Rev Neurosci. 1996;19:463–489. doi: 10.1146/annurev.ne.19.030196.002335. [DOI] [PubMed] [Google Scholar]
- 51.Klesse L J, Parada L F. Microsc Res Tech. 1999;45:210–216. doi: 10.1002/(SICI)1097-0029(19990515/01)45:4/5<210::AID-JEMT4>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 52.Jette N, Cole M S, Fahnstock M. Mol Brain Res. 1994;25:242–250. doi: 10.1016/0169-328x(94)90159-7. [DOI] [PubMed] [Google Scholar]
- 53.Crutcher K A, Scott S A, Liang S, Everson W V, Weingartner J. J Neurosci. 1993;13:2540–2550. doi: 10.1523/JNEUROSCI.13-06-02540.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mufson E J, Conner J M, Kordower J H. NeuroReport. 1995;6:1063–1066. doi: 10.1097/00001756-199505090-00028. [DOI] [PubMed] [Google Scholar]
- 55.Scott S A, Crutcher K A. Rev Neurosci. 1994;5:179–211. doi: 10.1515/revneuro.1994.5.3.179. [DOI] [PubMed] [Google Scholar]
- 56.Mufson E J, Li J-M, Sobreviela T, Kordower J H. NeuroReport. 1996;8:25–29. doi: 10.1097/00001756-199612200-00006. [DOI] [PubMed] [Google Scholar]
- 57.Mufson E J, Lavine N, Jaffar S, Kordower J H, Quirion R, Saragovi H U. Exp Neurol. 1997;146:91–103. doi: 10.1006/exnr.1997.6504. [DOI] [PubMed] [Google Scholar]
- 58.Hefti F, Weiner W J. Ann Neurol. 1986;20:275–281. doi: 10.1002/ana.410200302. [DOI] [PubMed] [Google Scholar]
- 59.Yuen E C, Mobley W C. Ann Neurol. 1996;40:346–354. doi: 10.1002/ana.410400304. [DOI] [PubMed] [Google Scholar]