Abstract
Improved syntheses of 7-methyl-2-exo-[3′-(2-bromopyridin-3-yl)-5′-pyridinyl]-7-azabicyclo[2.2.1]heptanes (3) and 7-methyl-2-exo-[3′-(6-bromopyridin-2-yl)-5′-pyridinyl]-7-azabicyclo[2.2.1]heptanes (4), precursors for PET radioligands [18F]XTRA (1) and [18F]AZAN (2), involving a key Stille coupling step followed by deprotection of Boc group and N-methylation are described. The new synthetic procedures provided the title compounds in more than 40% overall yields.
Keywords: nAChR, PET radioligand, Stille coupling, N-methylation
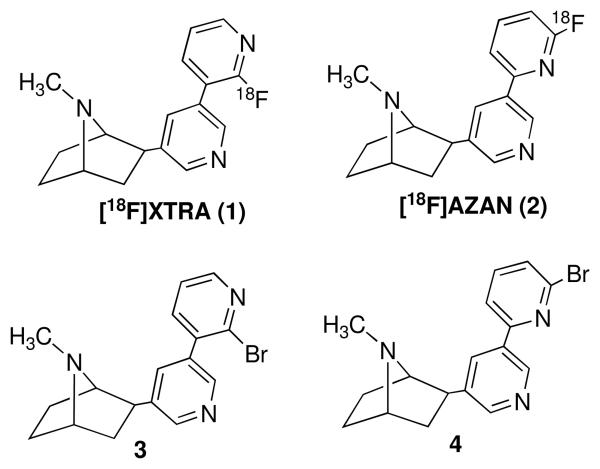
The neuronal nicotinic acetylcholine receptors (nAChRs) are a family of ligand gated ion channels in the central nervous system (CNS), and regulate a variety of neuronal activities. It is well documented that nAChRs play important role in tobacco dependence and various disorders including Alzheimer's disease, Parkinson's disease, schizophrenia, anxiety, depression, Tourette's syndrome, attention-deficit hyperactivity disorder and pain.1-5 nAChRs include many subtypes. Development of specific α4β2-nAChRs antagonist is of current interest, which could lead to useful diagnosis and therapeutics for many disorders.6-9 Recently, we discovered that XTRA (1) and AZAN (2) exhibited exceptionally high affinity and selectivity at α4β2-nAChRs.10,11 Pharmacological studies showed that they are α4β2-nAChRs antagonists with low side effects in mice. Their corresponding radioligands [18F]XTRA ([18F]1) and [18F]AZAN ([18F]2),10,11 were found to be excellent positron emission tomography (PET) radioligands in baboon. Their optimal imaging properties make them attractive candidates for further PET studies in human subjects. [18F]XTRA ([18F]1) and [18F]AZAN ([18F]2) were prepared from their corresponding bromo analogues, 3 and 4 and [18F] fluoride ion (Figure 1).
Figure 1.
Chemical structures of nAChR PET radioligands and their precursors.
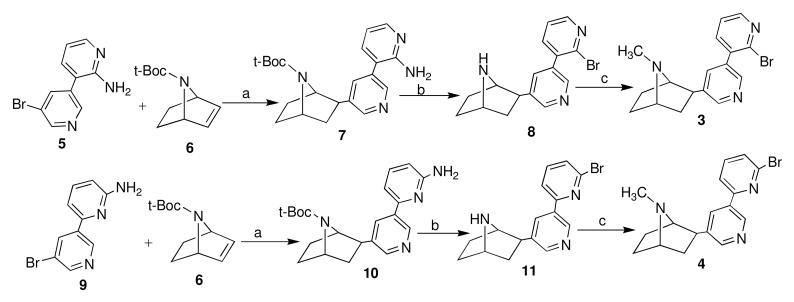
As part of our ongoing research program, large quantities of compounds 3 and 4 were required. Compounds 3 and 4 were previously prepared in 14.5% and 19% overall yields using multi-step procedures10 (Scheme 1). The previous syntheses suffered from some drawbacks. The critical one is the low reaction yields resulted from the Heck coupling of compound 6 with aromatic amine containing bromide 5 and 9, and the following Sandmeyer diazotiation of compound 7 and 10. Furthermore, difficulties in the removal of byproducts and tedious purification procedure during the Heck coupling leaded to product quality issues for the manufacturing processes of the products.
Scheme 1.
Previous syntheses of compound 3 and 4. Reagents and conditions: (a) Pd(PPh3)4, piperidine/HCOOH; (b) HBr, CuBr, NaNO2; (c) NaH2PO3, 37% HCHO.
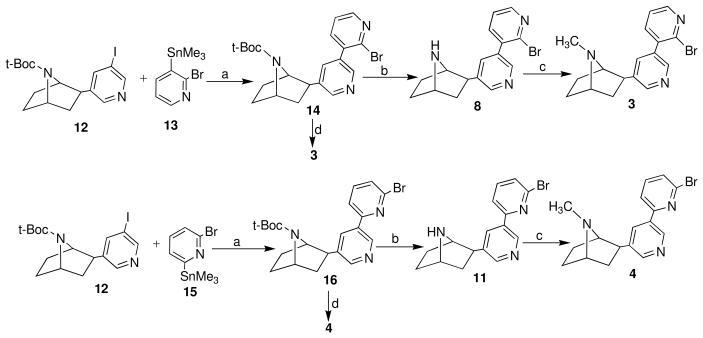
Experienced with the difficulties above, we therefore decided to attempt to develop a more practical and scaleable route to compound 3 and 4. Encouraged by our recent improvement on synthesis of Boc-protected compound 1212 we decided to explore Stille coupling reaction as the key step for our new strategy since the Stille reaction has proved to be particularly efficient for the synthesis of biaryl systems13-15. Herein we wish to report that compound 3 and 4 can be easily and efficiently prepared by Stille cross-coupling of pyridyl stannes with iodopyridine followed by Boc deprotection and N-methylation. (Scheme 2).
Scheme 2.
New syntheses of compound 3 and 4. Reagents and conditions: “(a) For compound 14, Pd(PPh3)4, Ag2O, DMF; for compound 16, Pd(PPh3)4, toluene; (b) CF3COOH (TFA); (c) NaH2PO3, 37% HCHO; (d) HCOOH, 37% HCHO.
Compounds 13 and 15 were obtained in excellent yields from 2-bromopyridine and 2,6- dibromopyridine respectively.l16,17. The Stille cross-coupling of 12 with stannes 1316 in DMF in the presence of Pd(Ph3P)4 and Ag2O leaded to 14. The Stille coupling of 12 with stannes 1517 was effected reproducibly by using Pd(Ph3P)4 as catalyst and toluene as solvent. Compound 14 and 16 were obtained in 73% and 77% yields respectively after column chromatography18. TFA deprotection of 14 and 16 gave 8 and 11 in 86% and 90% yields19. Compound 3 and 4 were synthesized by reductive methylation of amines 8 and 11 with formaldehyde in the presence of sodium phosphite10,20. Alternatively, compound 3 and 4 can be obtained by simultaneous deprotection of Boc group and N-methylation with HCOOH/HCHO under refluxing21. In both procedures, the overall yields are strikingly improved. The final products can be potentially scaled up to obtain multigram quantities since all reactions are very clean and reproducible.
In summary, we have developed an efficient 3-step (and or 2 step) strategy for synthesis of compound 3 and 4 starting from iodo derivaties and stannanes. A main improvement from the original route was the adopting Stille coupling reaction as key step to be performed in good yields and facilitate the isolation of intermediates. Combined with other improvements to optimize conditions for individual reaction step, this approach should enable us to obtain multigram quantities for further studies of our PET radioligands [18F]XTRA and [18F]AZAN.
Acknowledgment
This research was supported by the National Institutes of Health (DA020777) (A.G.H.). Y.G. thanks NARSAD for a Young Investigator Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Karlin A. Nat. Rev. Neurosci. 2002;3(2):102–104. doi: 10.1038/nrn731. (2002) [DOI] [PubMed] [Google Scholar]
- 2.Clementi F, Fornasari D, Gotti C. Trans. Pharmacol. Sci. 2000;2:35–37. doi: 10.1016/s0165-6147(99)01423-6. [DOI] [PubMed] [Google Scholar]
- 3.Loyd GK, Williams M. J. Pharmacol. Exp. Ther. 2000;292:461–467. [PubMed] [Google Scholar]
- 4.Romanelli MN, Gualtieri F. Med. Res. Rev. 2003;23:393–426. doi: 10.1002/med.10037. [DOI] [PubMed] [Google Scholar]
- 5.Graham AJ, Martin-Ruiz CM, Teaktong T, Ray MA, Court JA. Curr. Drug Targets CNS Neurol. Disord. 2002;1:387–397. doi: 10.2174/1568007023339283. [DOI] [PubMed] [Google Scholar]
- 6.Valette H, Dolle F, Saba W, Roger G, Hinnen F, Coulon C, Ottaviani M, Syrota A, Bottlaender M. Synapse. 2007;61:764–770. doi: 10.1002/syn.20426. [DOI] [PubMed] [Google Scholar]
- 7.Abdrakhmanova GR, Damaj MI, Carroll FI, Martin BR. Mol. Pharmacol. 2006;69:1945–1952. doi: 10.1124/mol.105.021782. [DOI] [PubMed] [Google Scholar]
- 8.Carroll FI, Lee JR, Navarro HA, Ma W, Brieaddy LE, Abraham P, Damaj MI, Martin BR. J. Med.Chem. 2002;45:4755–4761. doi: 10.1021/jm0202268. [DOI] [PubMed] [Google Scholar]
- 9.Gao Y, Horti AG, Kuwabara H, Ravert HT, Hilton J, Holt DP, Kumar A, Alexander M, Endres CJ, Wong DF, Dannals RF. J. Med.Chem. 2007;50:3814–3824. doi: 10.1021/jm070224t. [DOI] [PubMed] [Google Scholar]
- 10.Gao Y, Kuwabara H, Spivak CE, Xiao Y, Kellar K, Ravert HT, Kumar A, Alexander M, Hilton J, Wong DF, Dannals RF, Horti AG. J. Med. Chem. 2008;51:4751–4764. doi: 10.1021/jm800323d. [DOI] [PubMed] [Google Scholar]
- 11.Gao Y, Horti AG. Life Sciences. 2009:575–584. doi: 10.1016/j.lfs.2009.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao Y, Horti AG, Kuwabara H, Ravert HT, Holt DP, Kumar A, Alexander M, Wong DF, Dannals RF. Bioorg. Med. Chem. Lett. 2008;18:6168–6170. doi: 10.1016/j.bmcl.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 13.Moreno I, Tellitu I, Herrero MT, SanMartin R, Dominguez E. Curr. Org. Chem. 2002;6:1433–1452. [Google Scholar]
- 14.Hassan Jwanro, Sevignon Marc, Gozzi Christel, Schulz Emmanuelle, Lemaire Marc. Chem. Rev. 2002;102:1359–1469. doi: 10.1021/cr000664r. [DOI] [PubMed] [Google Scholar]
- 15.Stanforth SP. Tetrahedron. 1998;54:263–303. [Google Scholar]
- 16.Muratake H, Tonegawa M, Natsume M. Chem. Pharm. Bull. 1998;46:400–412. doi: 10.1248/cpb.46.559. [DOI] [PubMed] [Google Scholar]
- 17.Benaglia M, Toyota S, Woods CR, Siegel JS. Tetrahedron Lett. 1997;38:4737–4740. [Google Scholar]
- 18.A mixture of 7-tert-Butoxycarbonyl-2-exo-(3′-iodo-5′-pyridinyl)-7-azabicyclo[2.2.1]heptanes (12) (900 mg, 2.5 mmol), tetrakis(triphenylphosphine)palladium(0)(129 mg, 0.11 mmol) and silver (I)oxide (510 mg, 2.25 mmol) in 10 ml of dimethylformamide was stirred at 95 °C. After 5 min, 2-bromo-3-trimethyltinpyridine 13 (freshly prepared, 870 mg, 2.7 mmol, 1.2 equiv) dissolved in 5 mL of dimethylformamide was added, and the mixture was heated in a sealed reaction vessel and stirred at 95 °C for 0.5 h. The reaction mixture was allowed to attain room temperature, the precipitate was filtered off and the filtrate was finally evaporated. The residue was subject to silica gel chromatography to give product 14 as yellow oil (708 mg, 73%). 1H NMR (400 MHz, CDCl3/TMS) d 8.53 (bs, 1H), 8.49 (bs, 1H), 8.40 (dd, J=4.4 Hz, 2.0 Hz, 1H), 7.81 (m, 1H), 7.63 (dd, J=7.2 Hz, 2.0 Hz 1H), 7.37 (m, 1H), 4.39 (bs, 1H), 4.25 (s, 1H), 2.96 (m, 1H), 2.01-2.06 (m, 1H), 1.87-1.93 (m, 3H), 1.53-1.63 (m, 2 H), 1.45 (s, 9H). HRMS calcd for C21H25BrN3O2 [M+H]+: 430.1125, found 430.1114. Compound 16 was prepared in similar manner as yellow oil (77%) by using Pd(Ph3P)4 as catalyst and refluxing in toluene. 1H NMR (400 MHz, CDCl3/TMS) δ 9.00 (s, 1H), 8.58 (s, 1H), 8.22 (s, 1H), 7.71 (d, 7.6 Hz, 1H), 7.63 (t, 1H), 7.47 (d, J=8.0 Hz, 1H), 4.42 (bs, 1H), 4.29 (s, 1H), 2.99 (m, 1H), 1.88-2.07 (m, 4H), 1.54-1.68 (m, 2H), 1.43 (s, 9H). HRMS calcd for C21H25BrN3O2 [M+H]+: 430.1125, found 430.1121.
- 19.To a solution of 14 (675 mg, 1.58 mmol) in CH2Cl2 (12 mL) was added TFA (5 mL). The mixture was stirred at room temperature for 2 h until TLC (Hexane/EtOAc 1:2) showed the starting material disappeared. The reaction mixture was poured into NH4OH:water (50 mL, 1:1). The water layer was extracted with CHCl3 (3 × 40 mL). The organic layers were dried with Na2SO4, filtered and concentrated to give a residue which was purified by silica gel chromatography (CHCl3/MeOH 3:1) to give the product 8 as oil (452 mg, 86%). 1H NMR (400 MHz, CDCl3/TMS) d 8.61 (d, J=2.4 Hz, 1H), 8.55 (d, J=1.6 Hz, 1H), 8.39 (dd, J=2.0 Hz, 4.8 Hz, 1H), 7.95 (m, 1H), 7.82 (dd, J=2.0 Hz, 7.2 Hz, 1H), 7.36 (dd, J=4.8 Hz, 7.6 Hz, 1H), 4.20 (br s, 1H), 4.10 (br s, 1H), 3.14 (m, 1H), 2.08-2.17 (m, 5H), 1.65-1.82 (m, 2H). HRMS calculated for C16H17BrN3, [M+H] m/z=330.0607, found, 330.0596. Compound 11 was prepared similarly in 90% yield as oil; 1H NMR (400 MHz, CDCl3/TMS) δ 8.96 (br s, 1H), 8.62 (br s, 1H), 8.30 (m, 1H), 7.72 (d, J = 8.0 Hz, 1H), 7.63 (t, J = 7.8 Hz, 1H), 7.46 (d, J = 7.6 Hz, 1H), 3.84 (m, 1H), 3.67 (m, 1H), 2.92 (m, 1H), 1.74-1.97 (m, 3H), 1.53-1.79 (m, 4H).
- 20.The secondary amine (8, or 11) (462 mg, 1.4 mmol) was dissolved in 1 M sodium phosphite solution (25 mL). Aqueous formaldehyde (37%) (2.2 mL) was added, and the reaction mixture was heated with stirring at 60 °C in an oil bath until the reaction was complete (about 3 h). The reaction flask was cooled, and 5% K2CO3 (25 mL) was added. The mixture was extracted with CHCl3 (4 × 40 mL). The CHCl3 extracts were dried over sodium sulfate, filtered, and evaporated to give a residue that was purified by silica gel chromatography (CHCl3/MeOH 10:1), giving tertiary amines 3 and 4 in 76% and 85% yields, respectively.
- 21.The compound 14 (200 mg, 0.46 mmol) was dissolved in formic acid (0.5 mL) and aqueous formaldehyde (37%) (1.0 mL), heated at reflux until the completion of the reaction (about 4 h), and cooled to room temperature. The reaction mixture was poured into 5% K2CO3 solution (30 mL). The aqueous mixture was extracted with CHCl3 (4 × 20 mL), the combined extracts were washed with water (20 mL), dried over Na2SO4, and the solvent was removed. The residue was chromatographed on silica gel (CHCl3/MeOH 10:1) to give the product 3 as colorless oil (103 mg, 65%). Compound 4 was prepared similarly as colorless oil (70%). The spectral features of compound 3 and 4 were consistent with previously published values.