Abstract
The secretion of IL-9, initially recognized as a Th2 cytokine, was recently attributed to a novel CD4 T cell subset termed Th9 in the murine system. However, IL-9 can also be secreted by mouse Th17 cells and may mediate aspects of the proinflammatory activities of Th17 cells. Here we report that IL-9 is secreted by human naive CD4 T cells in response to differentiation by Th9 (TGF-β and IL-4) or Th17 polarizing conditions. Yet, these differentiated naive cells did not coexpress IL-17 and IL-9, unless they were repeatedly stimulated under Th17 differentiation-inducing conditions. In contrast to the naive cells, memory CD4 T cells were induced to secrete IL-9 by simply providing TGF-β during stimulation, as neither IL-4 nor proinflammatory cytokines were required. Furthermore, the addition of TGF-β to the Th17-inducing cytokines (IL-1β, IL-6, IL-21, IL-23) that induce memory cells to secrete IL-17, resulted in the marked coexpression of IL-9 in IL-17 producing memory cells. The proinflammatory cytokine mediating TGF-β–dependent coexpression of IL-9 and IL-17 was identified to be IL-1β. Moreover, circulating monocytes were potent costimulators of IL-9 production by Th17 cells via their capacity to secrete IL-1β. Finally, to determine whether IL-9/IL-17 coproducing CD4 cells were altered in an inflammatory condition, we examined patients with autoimmune diabetes and demonstrated that these subjects exhibit a higher frequency of memory CD4 cells with the capacity to transition into IL-9+IL-17+ cells. These data demonstrate the presence of IL-17+IL-9+ CD4 cells induced by IL-1β that may play a role in human autoimmune disease.
CD4 helper T cells secrete specific cytokines that can regulate effector immune responses of other cells and thus play a pivotal role in protecting the body from pathogenic infection. Dysregulation of Th-mediated immune activation, however, can lead to the development of tissue inflammation and autoimmune disorders (1). Three major Th subsets have been described: Th1 cells that produce IFN-γ; Th2 cells, characterized by secretion of IL-4, IL-5, and IL-13; and Th17 cells that secrete IL-17A and IL-17F. The differentiation into a specific Th subset is directed by polarizing cytokines and expression of master transcription factors. Th1 cells arise in response to IL-12 and require expression of the transcription factor T bet (2); whereas, Th2 cells are driven by IL-4 and controlled by expression of GATA-3 (3). In humans, Th17 cells arise in response to a combination of TGF-β and proinflammatory cytokines (IL-1β, IL-6, IL-21, IL-23) and are controlled by expression of the transcription factor RORγt (4–6). In contrast to the differentiation of naive cells into specific Th populations, memory cells appear to exhibit a greater plasticity as they simultaneously secrete combinations of cytokines that are typically not coexpressed in early differentiated naive populations.
IL-9 is a T cell-derived cytokine that was initially designated a Th2 cytokine. It recently became apparent that a number of CD4 T cell subsets shared the capacity to secrete IL-9 in mice. A subset of IL-9–producing effector T cells distinct from Th1, Th2, or Th17 cells was identified (7, 8). These cells, termed Th9 cells, are characterized by production of IL-9 and IL-10 and develop from naive CD4 precursors in response to TGF-β and IL-4 (7, 8). It was also shown that IL-9 secretion by murine Th2 cells was strongly dependent on exogenous TGF-β, and that TGF-β could redirect committed Th2 cells toward a Th9 phenotype (8). Moreover, regulatory T cells (Tregs) expressing IL-9 were observed in allografts that had undergone tolerance (9). Although subsequent studies failed to detect IL-9 expression in murine natural Tregs (8), it is unclear whether (10) or not (8) TGF-β–induced Tregs can secrete IL-9. Finally, recent reports indicated that murine Th17 cells also may secrete significant amounts of IL-9 (10–12). Yet the human CD4 T cell subsets capable of secreting IL-9 remain to be determined.
IL-9 was shown to promote the survival and activation of various cellular targets, including mast cells, B cells, T cells, and structural cells (13). It is still unclear whether IL-9 mainly exerts proinflammatory or anti-inflammatory activities. Primarily studied in Th2-type immunity, IL-9 was shown to be involved in asthma (14), allergy (15), and host defense against helminth infections (16). Discovery of Th9 cells and IL-9–producing Th17 cells implicated IL-9 as a mediator of inflammation in experimental disease models, such as colitis, peripheral neuritis, and experimental autoimmune encephalomyelitis (7, 10, 12). However, IL-9 was also shown to participate in peripheral tolerance, either through direct production by Tregs (9) or by increasing the survival and activity of Tregs (11).
In this study, we examined the requirements for inducing and regulating IL-9 production in human naive and memory CD4 T cells. We report that TGF-β and IL-4 drove IL-9 but not IL-10 expression in human naive CD4 T cells. Although IL-9 was induced by Th17 polarization, coexpression of IL-17 and IL-9 was acquired only after several rounds of polarization with Th17 conditions. We identify TGF-β as a critical regulator of IL-9 production by memory CD4 T cells and show that IL-9 production by memory Th17 cells was inducible by the combination of TGF-β and proinflammatory cytokines. Finally, we observed an increase in IL-9+IL-17+ cells in patients with type 1 diabetes (T1D), suggesting that IL-9–producing Th17 cells play a role in human autoimmune disease.
Materials and Methods
mAbs and recombinant cytokines
The following mAbs were used as follows: for FACS-sorting, CD45RO (UCHL1), CD25 (M-A251), CD62L (Dreg56; all from BD Biosciences, San Jose, CA), and CD127 (eBioRDR5, eBioscience, San Diego, CA); for T cell stimulation, CD3 (UCHT1 and Hit3a), CD28 (28.2; from BD Biosciences), and CD3 (OKT3); for intracellular staining, IL-9 (MH9A4), IL-17 (BL168), FoxP3 (206D; from BioLegend, San Diego, CA), IFN-γ (B27), IL-4 (MP4-25D2), and IL-10 (JES3-19F1; from BD Biosciences); and for cytokine neutralization, IFN-γ (25718), IL-4 (3007), IL-6 (1936), TNF-α (28401) (R&D Systems, Minneapolis, MN). Recombinant human IL-2 was obtained through AIDS Research and Reference Reagent Program, Division of AIDS, National Institutes of Allergy and Infectious Diseases, National Institutes of Health (Bethesda, MD); human rIL-2 from Dr. Maurice Gately, Hoffmann-La Roche (Nutley, NJ). Recombinant human TGF-β, IL-4, IL-12, IL-1β, IL-6, IL-23, and IL-1R antagonist were purchased from R&D Systems, recombinant human IL-21 was purchased from Cell Sciences (Canton, MA).
T cell isolation and stimulation
Peripheral venous blood was obtained from healthy control volunteers in compliance with Institutional Review Board protocols. PBMCs were separated by Ficoll-Paque PLUS (GE Healthcare, Piscataway, NJ) gradient centrifugation. Total CD4 T cells were isolated from fresh PBMC by negative selection via the CD4+ T cell isolation kit II (Miltenyi Biotec, Auburn, CA). Naive (CD45RO−CD25−CD127+CD62L+) and memory (CD45RO+ CD25medCD127+CD62L+) CD4 T cells were sorted by high-speed flow cytometry with a FACS Aria (BD Biosciences) to typically >98% purity in postsort analysis. Cells were cultured in 96-well round-bottom plates (Costar, Cambridge, MA) at 104 cells/well in serum-free X-Vivo 15 medium (BioWhittaker, Walkersville, MD), and stimulated with plate-bound anti-CD3 (UCHT1, 5 µg/ml) and soluble anti-CD28 (28.2, 1 µg/ml) Abs. Where indicated, recombinant IL-2 (50 U/ml), TGF-β (5 ng/ml), IL-4 (10 ng/ml), IL-12 (5 ng/ml), IL-1β (12.5 ng/ml), IL-6 (25 ng/ml), IL-21 (25 ng/ml), or IL-23 (25 ng/ml) were added at the start of the cultures. Memory cells were stimulated for 5 d and naive cells for 7 d. In Th polarization assays, naive CD4 T cells were stimulated for 6 d with plate-bound anti-CD3 (UCHT1, 5 µg/ml), soluble anti-CD28 (28.2, 1 µg/ml), and recombinant IL-2 (50 U/ml) in the presence of anti–IFN-γ and anti–IL-4 for Th0, IL-12 (10 ng/ml), and anti–IL-4 for Th1, IL-4 (25 ng/ml), and anti–IFN-γ for Th2, TGF-β (5 ng/ml), IL-1β (12.5 ng/ml), IL-6, IL-21, and IL-23 (all at 25 ng/ml) for Th17, or TGF-β (5 ng/ml) with or without IL-4 (25 ng/ml). Neutralizing Abs were used at 10 µg/ml.
T cell/monocyte coculture assays
Monocytes were isolated from PBMCs by negative selection (Miltenyi Biotec). FACS-sorted memory CD4 T cells (104 cells/well) were cultured in 96-well round-bottom plates in complete HL-1 medium containing 5% human AB serum (Cellgro, Herndon, VA) and stimulated with plate-bound anti-CD3 (OKT3, 1 µg/ml) and allogeneic monocytes (104 cells/well). Where indicated, recombinant IL-2 (50 U/ml), TGF-β (5 ng/ml), IL-1R antagonist (125 ng/ml), anti–IL-6, or anti–TNF-α Abs (10 µg/ml) were added. Cells were stimulated for 6 d.
Th17 capture assays
Naive CD4 T cells were sorted by high-speed flow cytometry and stimulated for 6 d with Th17 polarizing conditions. IL-17+ cells were captured using a Th17 capture kit (Miltenyi Biotec) and sorted by high-speed flow cytometry into 96-well plates containing X-vivo 15 medium, irradiated PBMCs (1 × 104/well), and IL-2 (50 U/ml). After 10 d of expansion, cells were restimulated for 3 d with Th17 polarizing conditions, rested for 7 d with IL-2, and stimulated again with Th17 polarizing conditions for a total of four rounds.
Single-cell clones
Memory CD4 T cells were sorted by high-speed flow cytometry at one cell per well in X-Vivo 15 medium containing 5% human AB serum, soluble anti-CD3 (Hit3a, 1 µg/ml), soluble anti-CD28 (28.2, 1 µg/ml), irradiated PBMCs (1 × 104/well), and IL-2 (50 U/ml). Half of the medium was replaced with fresh medium containing IL-2 starting at day 5 and every 3–4 d thereafter. After 4 wk of expansion, each clone was characterized for expression of IFN-γ, IL-4, or IL-17 by intracellular staining, and restimulated for 3 d with plate-bound anti-CD3 (UCHT1, 1 µg/ml), soluble anti-CD28 (28.2, 1 µg/ml), and IL-2 (50 U/ml) with or without TGF-β.
T1D subjects
Peripheral venous blood was obtained from 11 T1D subjects (mean age ± SD, 36 ± 10 y; mean disease duration ± SD, 18 ± 12 y) and 11 healthy controls (mean age ± SD, 32 ± 10 y) in compliance with institutional review board protocols. PBMCs were separated and frozen at a concentration of 1–3 × 107 cells/ml in 10% DMSO (Sigma-Aldrich, St. Louis, MO)/90% FCS (Atlanta Biologicals, Lawrenceville, GA). After thawing, memory CD4 T cells were isolated by negative selection via the memory CD4+ T cell isolation kit (Miltenyi Biotec).
Cytokine measurement
For intracellular staining, cells were stimulated for 4 h with PMA (50 ng/ml) and ionomycin (250 ng/ml; both from Sigma-Aldrich) in the presence of GolgiStop (BD Biosciences), fixed and made permeable (Fix/Perm; eBioscience) according to the manufacturer’s instructions, and incubated at room temperature with mAbs. Data were acquired on a FACSCalibur or LSR II (BD Biosciences) and analyzed with FlowJo software (TreeStar, Ashland, OR). Culture supernatants were measured by ELISA for secretion of IL-9, IL-5, IL-10 (BD Biosciences), IFN-γ (Thermo Scientific, Worcester, MA), and IL-17A (R&D Systems).
Real-time PCR
RNA was isolated using the Stratagene kit (Agilent Technologies, Palo Alto, CA) and converted to cDNA via reverse transcriptase by random hexamers and Multiscribe RT (TaqMan Gold RT-PCR kit, Applied Biosystems, Foster City, CA). The primers used for this study were purchased from Applied Biosystems. The values are represented as the difference in Ct values normalized to β2-microglobulin for each sample as per the following formula: relative RNA expression = (2−dCt) × 103.
Statistics
Bar graphs are represented as mean ± SEM. A standard two-tailed unpaired t test was used for statistical analysis; p values of 0.05 or less were considered significant.
Results
Both Th9 and Th17 polarizations promote IL-9 expression in human naive CD4 T cells
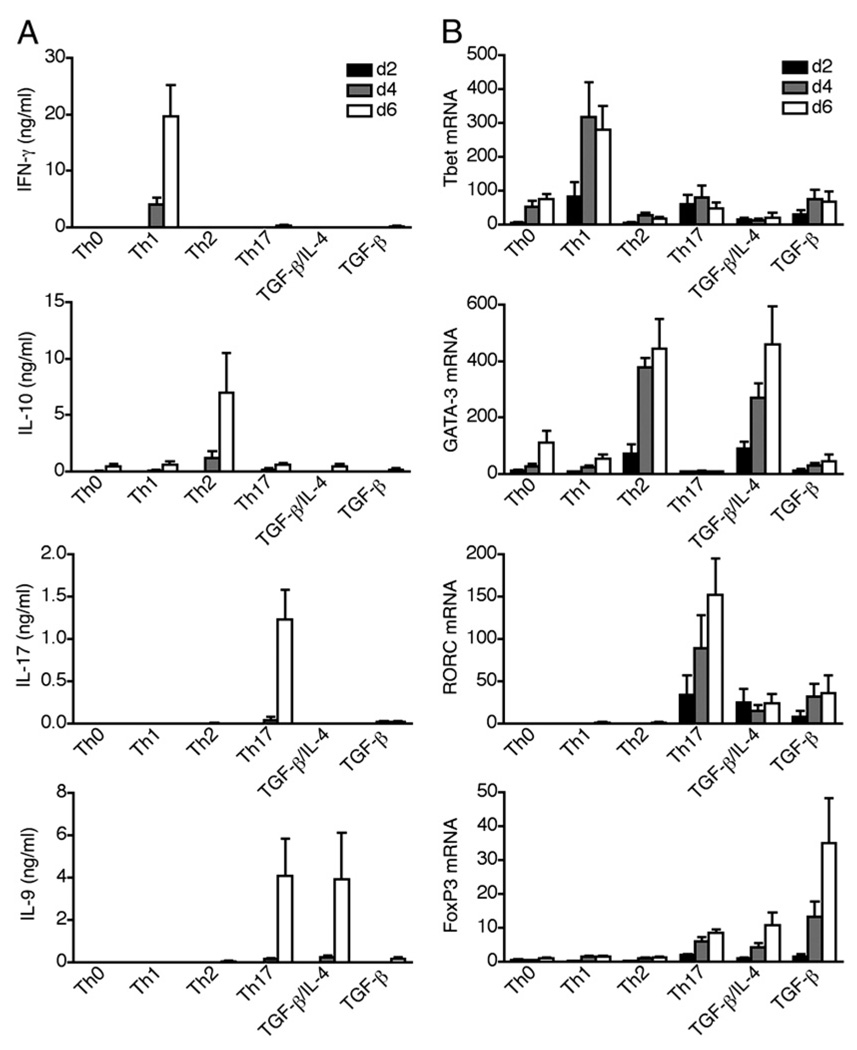
Both Th9 (TGF-β/IL-4) and Th17 (TGF-β/IL-6) polarization conditions can promote IL-9 expression in murine naive CD4 T cells (7, 8, 10–12). To determine whether this also occurs in human cells, we stimulated circulating naive CD4+CD45RA+ T cells in serum-free medium under standard Th polarizing conditions and analyzed cytokine secretion (Fig. 1A) and expression of master transcription factors (Fig. 1B) at various time points. As expected, IFN-γ/T bet, IL-10/GATA-3, and IL-17/RORC were induced in Th1, Th2, and Th17 polarizations, respectively (Fig. 1A, 1B). IL-9 secretion was induced both in TGF-β/IL-4–stimulated cells (Th9 polarization) and in TGF-β/IL-1β/IL-6/IL-21/IL-23–stimulated cells (Th17 polarization); whereas, Th0, Th1, Th2 cells, and TGF-β–stimulated cells failed to produce IL-9 (Fig. 1A).
FIGURE 1.
Both Th9 and Th17 polarizations promote IL-9 expression in human naive CD4 T cells. FACS-sorted naive CD4 T cells were stimulated with anti-CD3, anti-CD28, and IL-2 under Th polarizing conditions: Th0 (anti–IFN-γ/anti–IL-4), Th1 (Il-12/anti–IL-4), Th2 (IL-4/anti–IFN-γ), Th17 (TGF-β/IL-1β/IL-6/IL-21/IL-23), TGF-β/IL-4, or TGF-β. Supernatants and cells were harvested at day 2, 4, and 6 of stimulation. A, Secretion of IFN-γ, IL-10, IL-17, and IL-9 was measured by ELISA and (B) mRNA expression of T Bet, GATA-3, RORC, and FoxP3 was measured by quantitative RT-PCR. Data are represented as mean ± SEM of four different donors.
The transcription factors controlling IL-9 expression in mice and humans CD4 T cells are still unknown. We found that although GATA-3 mRNA was induced in Th9 polarization, it was completely absent during Th17 polarization, which also drove IL-9 secretion (Fig. 1B). IL-9 expression was also independent of RORC, as RORC was induced in Th17 but not in Th9 polarization (Fig. 1B). T bet was induced in Th1 polarization but not in Th17 or Th9 conditions (Fig. 1B). As both conditions driving IL-9 expression contained TGF-β, a cytokine that induces expression of the Treg transcription factor FoxP3, we analyzed FoxP3 mRNA expression in Th polarized cells. As compared with stimulation with TGF-β alone, FoxP3 expression was inhibited in Th17 and Th9 conditions (Fig. 1B).
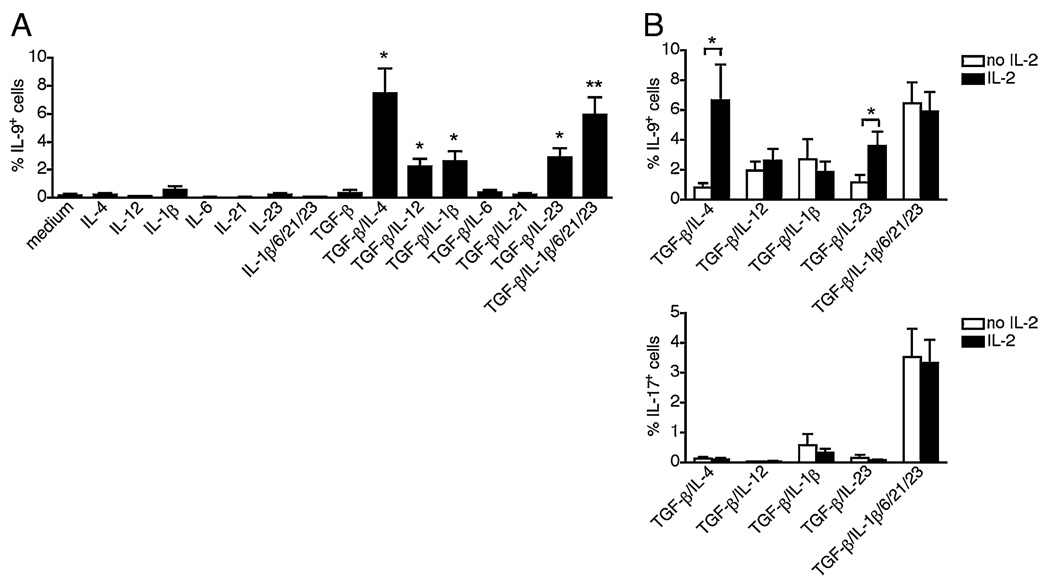
To determine the minimal cytokine interactions required for IL-9 induction, we stimulated human naive CD4 T cells in serum-free medium in the presence of each polarizing cytokine alone or combined with TGF-β (Fig. 1A). Intracellular staining for IL-9 enabled the measurement of the efficiency of differentiation on a per cell basis. Among Th17 polarizing cytokines, TGF-β, IL-1β, and IL-23 exerted major effects on IL-9 induction, as the combination of TGF-β with either IL-1β or IL-23 was sufficient to promote low but significant expression of IL-9 (Fig. 2A). IL-9 expression was more efficiently induced by TGF-β/IL-4 or Th17 conditions (Fig. 2A). The combination of TGF-β and IL-12 also induced low but significant IL-9 expression (Fig. 2A). Thus human naive CD4 T cells can differentiate into IL-9–producing cells in response to various cytokine milieus combining TGF-β and either IL-1β, IL-23, or IL-12, and are optimally induced by Th9 (TGF-β/IL-4) and Th17 (TGF-β/IL-1β/IL-6/IL-21/IL-23) polarizations.
FIGURE 2.
Minimal cytokine requirements for the differentiation of IL-9–producing cells. FACS-sorted naive CD4 T cells were stimulated for 7 d with anti-CD3 and anti-CD28 in the presence of various polarizing cytokines, with (A) or without (B) exogenous IL-2. Intracellular cytokine expression was analyzed by flow cytometry. The frequencies of cells expressing IL-9 or IL-17 are represented as mean ± SEM of five different donors. *p < 0.05; **p < 0.01.
We next examined the role that IL-2 plays in the differentiation of IL-9–producing cells. The above assays (Fig. 2A) were performed in the presence of exogenous IL-2, a cytokine commonly used in human T cell culture to promote cell survival and cell growth. By inducing the differentiation of Th9 cells in the presence or absence of IL-2, we found that the lack of IL-2 greatly reduced IL-9 expression induced by TGF-β/IL-4 stimulation (Fig. 2B). In contrast, exogenous IL-2 was dispensable for the induction of IL-9 with Th17 polarization (Fig. 2B). Similar to previous reports in mouse (17), these data indicate that IL-2 is an important cofactor for the differentiation of human Th9 cells.
Recently differentiated Th9 and Th17 cells are more restrictive in their capacity to coexpress other proinflammatory cytokines
We next examined whether in vitro polarized IL-9–producing cells coexpress other Th cytokines. Although data obtained in mouse models suggested that Th9 cells coexpress IL-9 and IL-10 (7, 8), in our initial assays, we found that human naive CD4 T cells did not produce IL-10 when differentiated with TGF-β/IL-4 (Fig. 1A). Thus, we examined whether TGF-β played a role in modulating IL-10 expression during naive cell differentiation. We found that IL-10 expression was in fact inhibited by TGF-β in a dose-dependent manner, and resulted in reciprocal expression of IL-9 and IL-10 mRNA in human Th9 cells (Fig. 3A). In examining the capacity of Th9 cells to coproduce other cytokines, we found that IL-9–producing cells did not coexpress IFN-γ, IL-4, IL-10, or IL-17 regardless of the stimulus used to induce IL-9 expression (Fig. 3C). Similarly, the ability of Th17 differentiating stimuli to induce the secretion of both IL-9 and IL-17 was found to arise from distinct cells. These data indicate that after initial differentiation of human naive cells, IL-17, and IL-9 secretion is induced in distinct subsets of cells.
FIGURE 3.
Upon differentiation, IL-9–producing cells do not coexpress IFN-γ, IL-4, IL-10, IL-17, or FoxP3. A, FACS-sorted naive CD4 T cells were stimulated for 6 d with anti-CD3, anti-CD28, and IL-2 in the presence of IL-4 (10 ng/ml) and increasing doses of TGF-β. Expression of IL-9 and IL-10 mRNA was measured by quantitative RT-PCR. Data are representative of two different donors. B and C, FACS-sorted naive CD4 T cells were stimulated for 7 d with anti-CD3, anti-CD28, and IL-2 in the presence of various polarizing cytokines, and analyzed for intracellular cytokine expression by flow cytometry. B, The frequencies of FoxP3+ cells are represented as mean ± SEM of five different donors. *p < 0.05; **p < 0.01. C, Expression of IL-9 versus IFN-γ, IL-4, IL-10, IL-17, and FoxP3 is shown. Data are representative of five different donors.
As TGF-β can induce FoxP3 expression and each of the Th9-inducing cytokine combinations contained TGF-β,we examined whether FoxP3 was expressed in the differentiated IL-9–producing cells. In comparison with the level of FoxP3 expressed by naive cells stimulated in the presence of TGF-β alone, FoxP3 expression was inhibited in all conditions that drove IL-9 expression (Fig. 3B) and all IL-9–producing cells were FoxP3neg (Fig. 3C) .
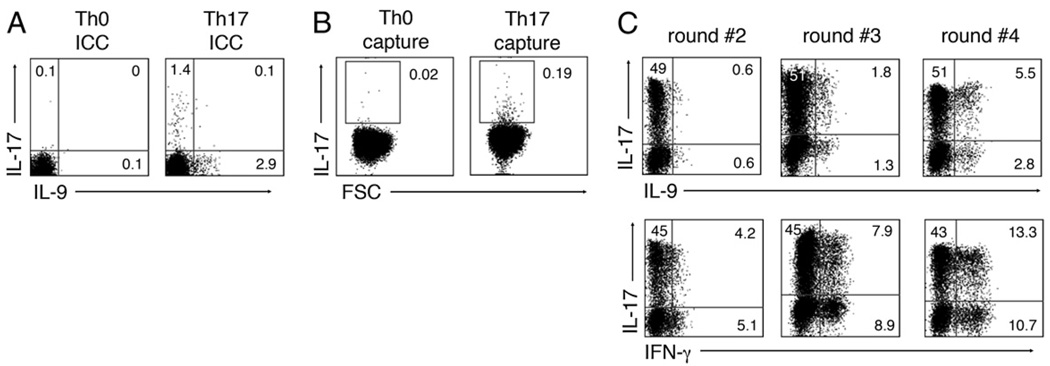
We then examined whether Th17-differentiated cells could acquire the capacity to coexpress IL-9 after multiple rounds of stimulation. After confirming that IL-17 and IL-9 were not coexpressed after Th17 polarization of naive cells (Fig. 4A), we purified IL-17+ cells using an IL-17–capture kit and cell sorting (Fig. 4B). The capacity of these IL-17–secreting cells to secrete IL-9 was then monitored after each of three successive rounds of stimulation in the context of the original Th17-inducing cytokines. These data indicate that although secondary-stimulated Th17 cells did not express IL-9, cells coexpressing IL-17+ and IL-9+ were generated after the third round of polarization (Fig. 4C). As expected, the multiple rounds of stimulation resulted in the appearance of IL-17+IFN-γ+ cells (Fig. 4C). As early differentiated cells did not exhibit the capacity to coexpress IL-17 and IL-9 (Fig. 3), these data suggest that IL-9 expression by human Th17 cells is acquired as a late event, and that IL-9 and IL-17 can, in fact, be coexpressed by the same cell in humans (Fig. 4).
FIGURE 4.
Th17 cells gain IL-9 expression after multiple rounds of polarization in vitro. FACS-sorted naive CD4 T cells were stimulated for 6 d with anti-CD3, anti-CD28, IL-2, and Th17 polarizing conditions (TGF-β/IL-1β/IL-6/IL-21/IL-23) and analyzed for intracellular expression of IL-17 and IL-9 by flow cytometry (A). B, IL-17+ cells were labeled using an IL-17 capture kit (B) and isolated by cell sorting. C, The IL-17+ fraction was repeatedly restimulated using Th17-polarizing conditions and analyzed for intracellular expression of IL-17 and IL-9 after second, third, and fourth rounds of polarization. Data are representative of three different donors.
TGF-β plays a determinant role in inducing memory cell secretion of IL-9
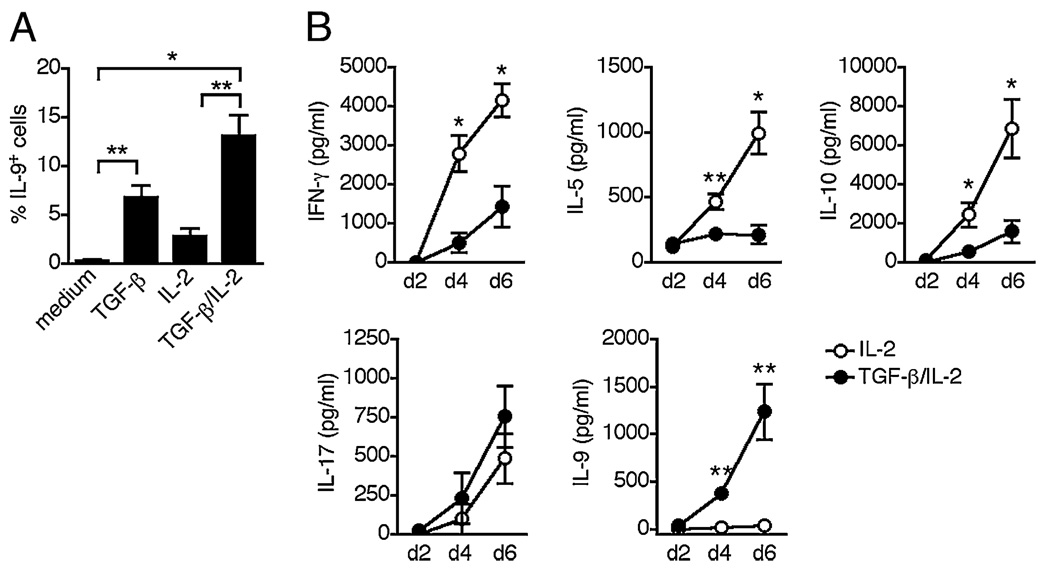
We then examined whether the capacity to coexpress IL-17 and IL-9 after multiple rounds of stimulation could be reflected in the response of memory cells that have undergone expansion and differentiation in vivo. In initial studies to induce IL-9 expression in memory CD4 T cells, we found that anti-CD3/anti-CD28-stimulated CD4+CD45RA− memory T cells did not produce IL-9; whereas, they readily produced IL-17 (Fig. 5A and data not shown). In examining whether IL-9 could be induced via the various cytokine combinations used to differentiate naive cells, we found that IL-9 expression was induced by exogenous TGF-β and could be further amplified by the addition of IL-2 (Fig. 5A). These data indicate that TGF-β is crucial for IL-9 secretion by memory CD4 T cells and further confirms the role of IL-2 as a cofactor for IL-9 production. In contrast to IL-9, other effector cytokines were actively produced by memory CD4 T cells in the absence of exogenous TGF-β, and were either inhibited (IFN-γ, IL-5, IL-10) or unmodified (IL-17) by TGF-β (Fig. 5B). These data indicate that activation in the presence of TGF-β induces a memory CD4 T cell response that is dominated by IL-17 and IL-9, with a loss of Th1 and Th2 cytokines.
FIGURE 5.
IL-9 secretion by memory CD4 T cells is inducible by TGF-β. A, FACS-sorted memory CD4 T cells were stimulated for 5 d with anti-CD3 and anti-CD28 with or without TGF-β or IL-2. The frequencies of cells expressing IL-9 are represented as mean ± SEM of five different donors. B, FACS-sorted memory CD4 T cells were stimulated with anti-CD3, anti-CD28, and IL-2 with or without TGF-β. Supernatants were harvested at day 2, 4, and 6 of stimulation. Secretion of IFN-γ, IL-5, IL-10, IL-17, and IL-9 was measured by ELISA. Data are represented as mean ± SEM of four different donors. *p < 0.05; **p < 0.01.
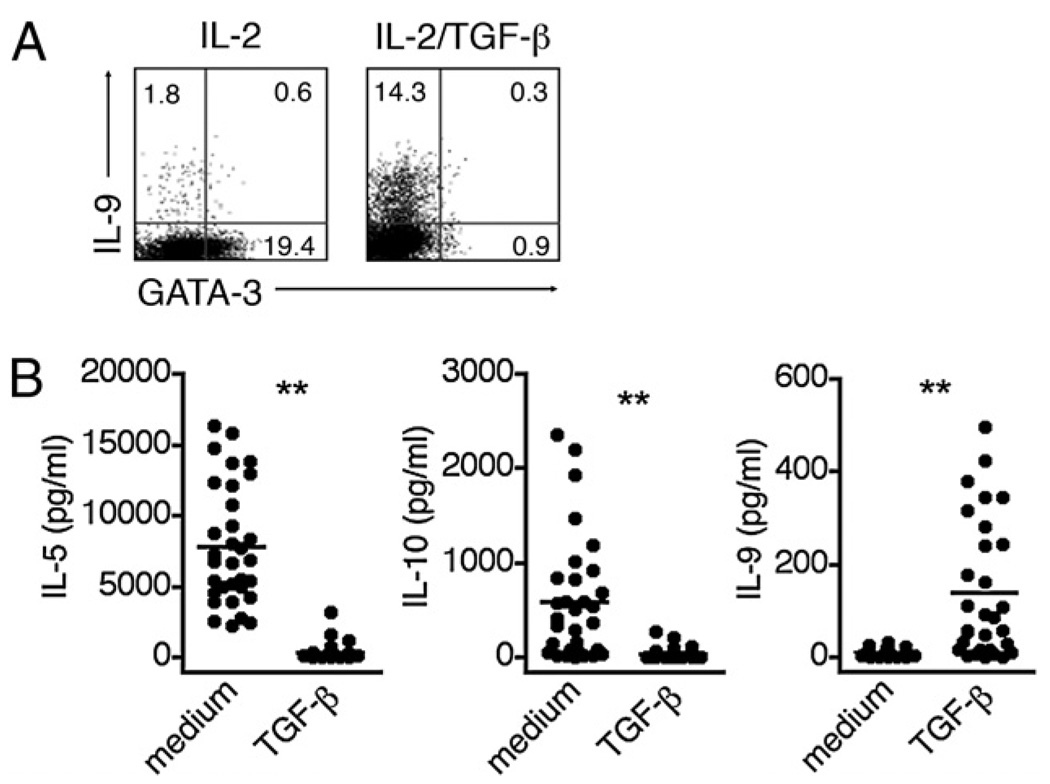
TGF-β can initiate the reprogramming of fully polarized murine Th2 cells into IL-9+IL-10+–producing cells that exhibit reduced expression of the Th2 transcription factor GATA-3 (8). In asking whether TGF-β can exert a similar activity on human Th2 cytokine-secreting CD4 cells, we found that human memory CD4 T cells stimulated in the presence of TGF-β exhibited a concomitant induction of IL-9 secretion and reduction in GATA-3 expression (Fig. 6A). To examine whether established human Th2 cells were able to respond to TGF-β with a similar transition into an IL-9 phenotype, we generated single-cell clones from memory CD4 T cells. Although initially generated under nonpolarizing conditions, we identified those clones that exhibited a classic Th2 phenotype by their ability to secrete IL-4 but not IFN-γ or IL-17 in response to stimulation (Th2 clones, n = 32/90). We then determined the capacity of these clones to secrete IL-9 when stimulated in the absence or presence of TGF-β. As shown in Fig. 6B, 6C, the clones exhibited a marked downregulation of IL-5 and IL-10 secretion, and an increase in IL-9 secretion, as >50% began to secrete IL-9 when stimulated in the presence of TGF-β. Thus TGF-β can promote the conversion of human Th2 cells into IL-9–producing cells. In contrast to mice, however, human Th2 cells redirected toward a Th9 phenotype did not coproduce IL-10.
FIGURE 6.
TGF-β promotes the conversion of Th2 cells into IL-9–producing cells. A, FACS-sorted memory CD4 T cells were stimulated for 5 d with anti-CD3, anti-CD28, and IL-2 with or without TGF-β, and analyzed for intracellular expression of IL-9 and GATA-3. Data are representative of two different donors. B, Single-cell clones were derived from memory CD4 T cells. The clones that expressed IL-4 but not IFN-γ or IL-17 (Th2 clones, n = 32/90) were restimulated for 3 d with anti-CD3, anti-CD28, and IL-2 with or without TGF-β. Secretion of IL-5, IL-10, and IL-9 was measured by ELISA.
The capacity of TGF-β to induce IL-17+IL-9+ secreting cells is primarily due to IL-1β and is enhanced by monocytes
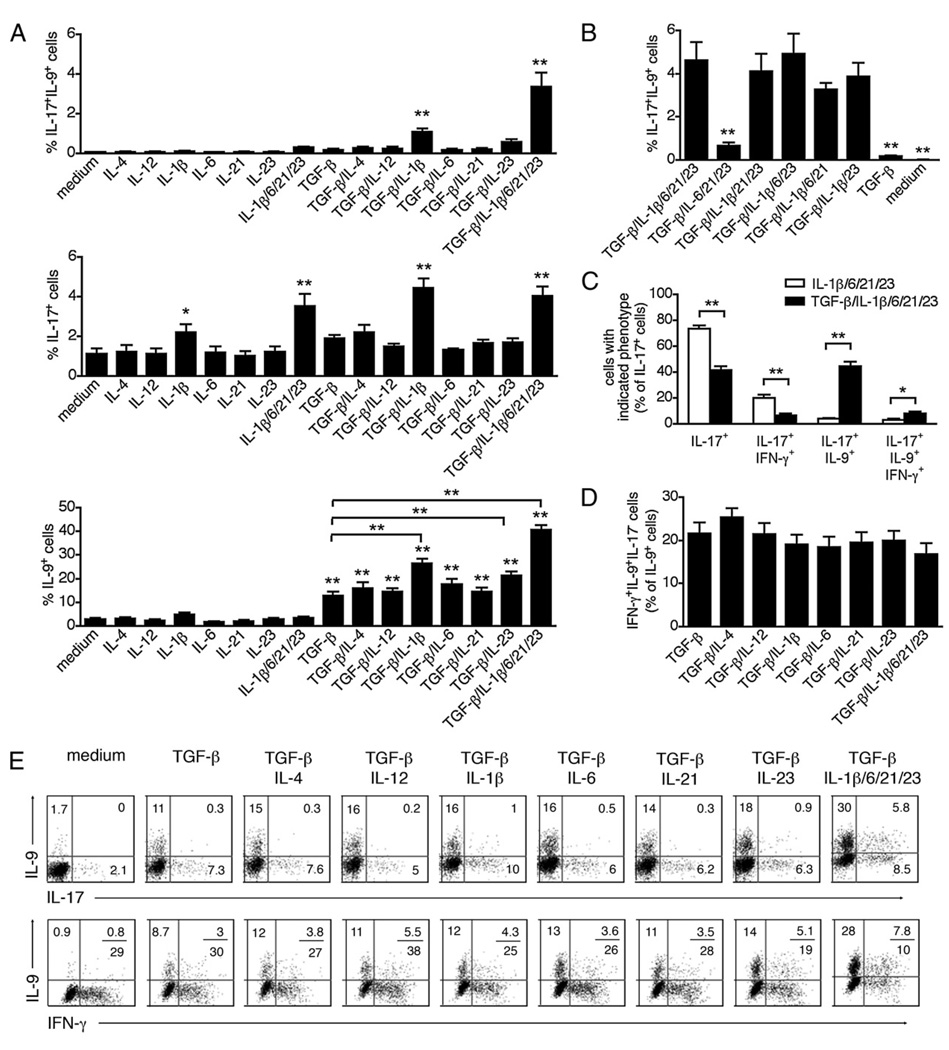
It was evident that the capacity of TGF-β to induce memory CD4 T cells to secrete both IL-9 and IL-17 required its interaction with a proinflammatory milieu. When memory CD4 T cells were stimulated in the presence of TGF-β alone, they expressed IL-9 in the absence of IL-17 (Fig. 7A). Yet, the combination of TGF-β and proinflammatory cytokines (IL-1β/IL-6/IL-21/IL-23) induced a subset of CD4 cells to be IL-17+IL-9+ double- producing cells (Fig. 7A). We examined whether a single proinflammatory cytokine was responsible for directing this outcome and found that IL-1β was the major regulator of IL-9 and IL-17 coexpression. Thus, although stimulation with TGF-β and IL-1β was sufficient to promote IL-17+IL-9+ cells (Fig. 7A), removing IL-1β from the proinflammatory cytokine mixture abolished the induction of IL-17+IL-9+ cells (Fig. 7B). Not only did the addition of TGF-β to the proinflammatory cytokines result in the marked increase in IL-17+IL-9+–secreting cells, it also resulted in a reduction of IL-17+IFN-γ+ cells (Fig. 7C.). Moreover, our data indicated that unlike differentiated naive cells, a subset of memory CD4 T cells can coproduce IL-9 and IFN-γ (Fig. 7D, 7E). It is unknown whether these cells derive from Th9 cells that gained IFN-γ expression or Th1 cells that gained IL-9 expression.
FIGURE 7.
IL-9 secretion by memory Th17 cells requires TGF-β and proinflammatory cytokines. FACS-sorted memory CD4 T cells were stimulated for 5 d with anti-CD3, anti-CD28, IL-2, and various cytokines, and analyzed for intracellular cytokine expression by flow cytometry. Data are represented as mean ± S.E.M. of 5 different donors. A and B, The frequencies of IL-17+IL-9+ cells, total IL-17+, and total IL-9+ cells are shown. C, Frequencies of cells expressing IL-17 only, IL-17/IFN-γ, IL-17/IL-9, or IL-17/IL-9/IFN-γ calculated as a percentage of total IL-17+ cells. D, Frequencies of IL-9+IFN-γ+IL-17− cells were calculated as a percentage of total IL-9+ cells. E, Intracellular expression of IL-9 versus IL-17 or IFN-γ. *p < 0.05; **p < 0.01.
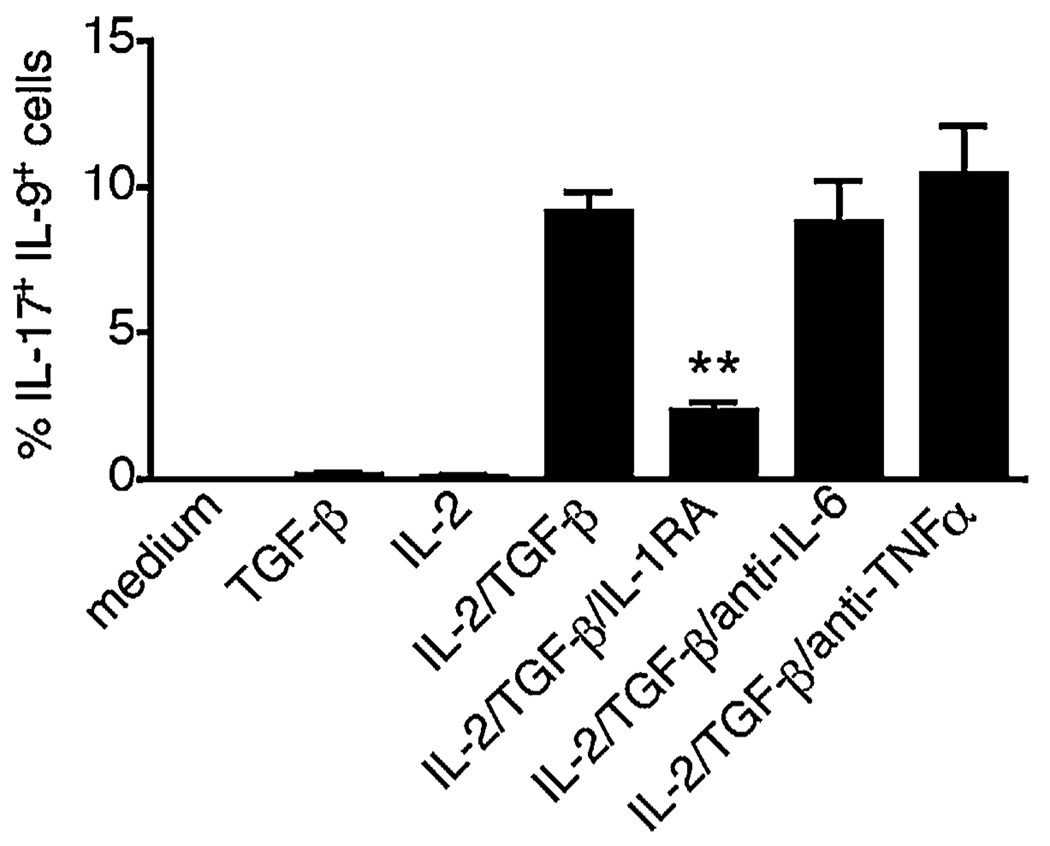
As monocytes are a major cellular source of IL-1β as well as other proinflammatory cytokines, we examined whether they could induce memory cells to transition into an IL-17+IL-9+ phenotype. To determine whether monocytes could provide the proinflammatory environment that promotes IL-9 secretion by Th17 cells, we stimulated memory CD4 T cells with anti-CD3 Abs and allogeneic monocytes. As shown in Fig. 8, IL-17+IL-9+ cells were induced by the presence of monocytes in these cultures, provided that both IL-2 and TGF-β had also been added to the culture. Importantly, no proinflammatory cytokines were added to these cultures. We demonstrated the importance of monocyte-derived IL-1β by establishing replicate cultures that did or did not receive IL-1R antagonist (IL-1RA) to block the binding of IL-1β. The addition of IL-1RA abrogated the costimulatory effect of monocytes. In contrast to IL-1β, the neutralization of IL-6 or TNF-α had no effect. These data confirm the crucial roles of IL-2, TGF-β, and IL-1β in the induction of IL-9 secretion by Th17 cells, and identify circulating monocytes as potent costimulators of IL-17+IL-9+ memory CD4 T cells.
FIGURE 8.
Circulating monocytes are potent costimulators of IL-9 production by Th17 cells. FACS-sorted memory CD4 T cells were stimulated for 6 d with anti-CD3 and allogeneic monocytes, with or without IL-2 or TGF-β. Where indicated, an IL-1RA or neutralizing Abs against IL-6 or TNF-α was added. The frequencies of IL-17+IL-9+ cells are shown. T cells were isolated from two different donors and monocytes from three different donors. Data are represented as mean ± SEM. *p < 0.05; **p < 0.01.
IL-9 secretion by Th17 cells is increased in patients with autoimmune diabetes
Recent reports in mouse experimental models suggested that IL-9 might mediate part of the proinflammatory activities of Th17 cells (10, 12). To determine whether IL-9/IL-17 coproducing CD4 cells were altered in an inflammatory condition, we examined patients with autoimmune diabetes. Specifically, we examined whether Th17 cells from T1D subjects produced more IL-9 than those from healthy individuals. Memory CD4 T cells isolated from 11 patients with T1D and 11 healthy controls were stimulated in the absence of accessory cells, and in the presence of proinflammatory cytokines with or without TGF-β. The cultures were analyzed for frequency of cells expressing IL-9 and IL-17. In cultures of cells isolated from both healthy individuals and diabetic subjects, the combination of TGF-β and proinflammatory cytokines was required for the induction of IL-17+IL-9+ cells (Fig. 9A). There was a significant increase in the frequency of cells exhibiting the IL-17+IL-9+ phenotype in cultures of cells isolated from the diabetic subjects as compared with healthy controls (p = 0.012) (Fig. 9A). The increase in frequency of IL-17+IL-9+ cells was also mirrored by increases in the frequency of total IL-9+ cells (p = 0.027) and total IL-17+ cells, although they did not reach statistical significance (p = 0.059) (Fig. 9A). Although there was no link between the frequencies of IL-17+IL-9+ and total IL-9+ cells, we observed a highly significant correlation between frequencies of IL-17+IL-9+ and total IL-17+ cells (r2 = 0.94, p < 0.001) (Fig. 9B). In healthy and diabetic subjects, IL-17+IL-9+ cells were mostly IFN-γneg (Supplemental Fig. 1A, 1B). The frequencies of IL-17+IFN-γ− and IL-17+IFN-γ+ cells were similar in diabetic subjects and healthy individuals, in the TGF-β/IL-1β/IL-6/IL-21/IL-23 stimulation (Supplemental Fig. 1C, 1D), suggesting a specific expansion of the IL17+IL-9+ cells. Overall, our data indicate that Th17 cells from diabetic subjects exhibit an increased propensity to secrete IL-9 as compared with healthy Th17 cells and provide suggestive evidence for an association of IL-17+IL-9+ cells with inflammatory disorders.
FIGURE 9.
IL-9 secretion by Th17 cells is increased in patients with autoimmune diabetes. Memory CD4+ T cells isolated from the blood of T1D patients (n = 11) and healthy controls (n = 11) were stimulated for 5 d with anti-CD3, anti-CD28, and IL-2 in the presence of various cytokines, and analyzed for intracellular expression of IL-9 and IL-17 by flow cytometry. A, The frequencies of IL-17+IL-9+, total IL-9+, and total IL-17+ cells are showed. B, The frequencies of IL-17+IL-9+ cells in diabetic memory CD4 T cells stimulated with TGF-β/IL-1β/IL-6/IL-21/IL-23 are represented versus the frequencies of total IL-9+ cells or total IL-17+ cells.
Discussion
Although Th9 cells have been described in mice, they are not well characterized in humans or are the conditions required for Th9 differentiation. In this study, we examined the requirements for differentiation to IL-9–secreting cells by human CD4 T cells. As in mice, human Th9 differentiation was driven in naive CD4 cells by the combination of TGF-β and IL-4. In Th17 cells, coexpression of IL-9 and IL-17 was acquired after several rounds of polarization and was inducible by the combination of TGF-β and proinflammatory cytokines. Our study identifies TGF-β as a critical activator of IL-9 secretion in memory CD4 T cells and suggests that additional CD4 T cell subsets, including Th2 and perhaps Th1 cells, are capable of IL-9 secretion in humans.
In contrast to data obtained in the mouse system, suggesting that Th9 cells secrete both IL-9 and IL-10 (7, 8), the combination of TGF-β and IL-4 induced IL-9 but not IL-10 expression in human naive CD4 T cells. This difference between species might be explained by a differential effect of TGF-β on IL-10 production in mice and humans. Although TGF-β has synergistic effects in inducing IL-10–producing Tr1 cells in mice (18, 19), it was shown to inhibit Tr1 cytokine secretion and development in humans (20). Here we demonstrate that TGF-β significantly inhibits IL-10 secretion in human CD4 T cells, resulting in lack of IL-10 expression by in vitro polarized Th9 cells or memory IL-9– producing cells.
Several inflammatory cytokines, including IL-12, synergize with TGF-β to drive IL-9 expression in naive CD4 T cells. As IL-4 and IL-12 are antagonistic cytokines that reciprocally inhibit each other’s activity (21) it is surprising that they have similar effects on IL-9 polarization. We found that the combination of TGF-β/IL-1β/IL-6/IL-21/IL-23, which drives Th17 differentiation, was as effective as TGF-β/IL-4 in inducing IL-9 expression. IL-1β and IL-23 exerted major effects on IL-9 induction, because they synergized with TGF-β to drive IL-9 expression in naive CD4 T cells; whereas, IL-6 and IL-21 did not. Moreover, anti-inflammatory cytokines, such as IL-10 and IL-27, failed to induce IL-9 expression in naive T cells even in the presence of TGF-β (G. Beriou, unpublished data).
We found that TGF-β plays a critical role both in the differentiation of IL-9–producing cells from naive precursors and in the induction of IL-9 secretion by memory T cells. In memory CD4 T cells, TGF-β was essential and sufficient to promote IL-9 secretion. This requirement for TGF-β appears to be unique to IL-9, as other effector cytokines, notably IFN-γ and IL-4 are secreted by memory CD4 T cells in the absence of TGF-β and are even inhibited by TGF-β. As both conditions driving IL-9 expression contained TGF-β, a cytokine that induces expression of the Treg transcription factor FoxP3, we analyzed FoxP3 mRNA expression in T helper polarized cells. As compared with stimulation with TGF-β alone, FoxP3 expression was inhibited in Th17 and Th9 conditions.
The transcription factors controlling IL-9 expression in mice and human CD4 T cells are still unknown. Our data suggest, however, that IL-9 expression by human CD4 T cells is independent of typical Th transcription factors. Indeed, although GATA-3 mRNA was induced in Th9 polarization, it was completely absent after Th17 polarization, which also drove IL-9 secretion. IL-9 expression was also independent of RORC, as RORC was induced in Th17 but not in Th9 polarization; whereas, T bet was induced in Th1 polarization but not in Th17 or Th9 conditions.
Overall, our data indicate that the capacity to produce IL-9 is shared by many CD4 T cell subsets in humans, including Th9, Th17, Th2, and perhaps Th1 cells. However, we did not detect any IL-9 expression in human circulating Tregs. In our assays, memory (CD45RA−;) or naive (CD45RA+) CD25highCD127−FoxP3+ Tregs failed to express IL-9, even with conditions that promoted IL-9 expression in effector T cells (G. Beriou, unpublished data). Although Tregs might not express IL-9, they may promote IL-9 secretion by other T cells through their production of TGF-β, as was shown for IL-17 (22).
Although IL-9 was primarily studied in Th2-type immunity, a novel function of IL-9 in promoting inflammation and mediating autoimmune tissue destruction was recently uncovered (7, 10). It was postulated that in diseased animals, IL-9 could act as a growth factor for pathogenic T cells and/or macrophages. In contrast, our group recently identified an anti-inflammatory activity of IL-9, which acted as a survival factor for murine Tregs and enhanced Treg suppressive activity (11). Preliminary data indicated that the IL-9R was absent on human naive CD4 T cells but was expressed at low levels shortly after activation of memory CD4 T cells and Tregs (G. Beriou, unpublished data). Although previous work suggested that IL-9 was devoid of any activity on freshly isolated human T cells (23), a more careful examination of the effects of IL-9 on human effector and CD4 Tregs will be needed.
We demonstrate that in the presence of TGF-β, IL-2, and anti-CD3 T cell activation, human monocytes are able to induce IL-9 secretion by memory Th17 cells. Moreover, blocking experiments with IL-1RA indicated that monocyte-derived IL-1β was the critical cytokine necessary for monocyte-induced IL-9 secretion by Th17 cells. This is of particular interest as monocytes can secrete significant quantities of proinflammatory cytokines, and we have demonstrated that CD11b+ monocytes from patients with T1D spontaneously secrete high amounts of IL-1β and IL-6 (24).
The Th17 subset has been shown to be an important CD4 T cell subset in human autoimmune diseases, including rheumatoid arthritis (25) and multiple sclerosis (26). We recently demonstrated that monocytes from T1D subjects, through their increased capacity to secrete IL-1β, are more potent at inducing Th17 memory T cells than monocytes from healthy control subjects (24). As we demonstrated that Th17 cells are capable of producing IL-9, we investigated the regulation and levels of IL-17+IL-9+ T cells in diabetic subjects, hypothesizing that the inflammatory monocytes would be driving these IL-17+IL-9+ dual producers. Specifically, we examined whether Th17 cells from T1D subjects produced more IL-9 than those from healthy individuals. We observed a significant increase in the frequency of cells with the IL-17+IL-9+ phenotype in cultures of cells isolated from the diabetic subjects as compared with healthy controls. These data indicate that Th17 cells from diabetic subjects exhibit an increased propensity to secrete IL-9 as compared with healthy Th17 cells and provide suggestive evidence for an association of IL-17+IL-9+ cells with inflammatory disorders. Whether the in vitro models suggesting that monocyte-derived IL-1β is driving the secretion of IL-17+IL-9+ CD4 cells in vivo would require a clinical manipulation that blocked IL-1β in association with changes in this subset of CD4 cells.
In conclusion, we report that IL-9 is secreted by naive CD4 T cells in response to differentiation by Th9 (TGF-β and IL-4) or Th17 polarizing conditions; whereas, differentiated naive cells did not coexpress IL-17 and IL-9 unless they were repeated stimulated under Th17 differentiation-inducing conditions. In contrast, memory CD4 T cells were induced to secrete IL-9 by TGF-β during stimulation. The proinflammatory cytokine mediating TGF-β–dependent coexpression of IL-9 and IL-17 was identified to be IL-1β. Finally, we observed a higher frequency of memory CD4 cells with the capacity to transition into IL-9+IL-17+ cells in patients with autoimmune diabetes. These data demonstrate the presence in humans of IL-17+IL-9+ CD4 cells induced by IL-1β that may play a role in human autoimmune disease.
Supplementary Material
Acknowledgments
We thank D. Kozoriz for cell sorting.
This work was supported by National Multiple Sclerosis Society Grant FG1744A1 (to G.B.) and Grant RG3825A1, a Dana Scholars grant (to C.B.A.), and National Institutes of Health Grants P01 AI045757, U19 AI046130, U19 AI070352, P01 AI039671, (to D.A.H.) and F32 AI651003 (to E.M.B). D.A.H. is also supported by Jacob Javits Merit Award NS2427 from the National Institute of Neurological Disorders and Stroke.
Abbreviations used in this paper
- T1D
type 1 diabetes
- Treg
regulatory T cell
Footnotes
The online version of this article contains supplemental material.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat. Immunol. 2007;8:345–350. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- 2.Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–669. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 3.Zheng W, Flavell RA. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell. 1997;89:587–596. doi: 10.1016/s0092-8674(00)80240-8. [DOI] [PubMed] [Google Scholar]
- 4.Manel N, Unutmaz D, Littman DR. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat. Immunol. 2008;9:641–649. doi: 10.1038/ni.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Volpe E, Servant N, Zollinger R, Bogiatzi SI, Hupé P, Barillot E, Soumelis V. A critical function for transforming growth factor-beta, interleukin 23 and proinflammatory cytokines in driving and modulating human T(H)-17 responses. Nat. Immunol. 2008;9:650–657. doi: 10.1038/ni.1613. [DOI] [PubMed] [Google Scholar]
- 6.Yang L, Anderson DE, Baecher-Allan C, Hastings WD, Bettelli E, Oukka M, Kuchroo VK, Hafler DA. IL-21 and TGF-beta are required for differentiation of human T(H)17 cells. Nature. 2008;454:350–352. doi: 10.1038/nature07021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dardalhon V, Awasthi A, Kwon H, Galileos G, Gao W, Sobel RA, Mitsdoerffer M, Strom TB, Elyaman W, Ho IC, et al. IL-4 inhibits TGF-beta-induced Foxp3+ T cells and, together with TGF-beta, generates IL-9+ IL-10+ Foxp3(−) effector T cells. Nat. Immunol. 2008;9:1347–1355. doi: 10.1038/ni.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Veldhoen M, Uyttenhove C, van Snick J, Helmby H, Westendorf A, Buer J, Martin B, Wilhelm C, Stockinger B. Transforming growth factor-beta ‘reprograms’ the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nat. Immunol. 2008;9:1341–1346. doi: 10.1038/ni.1659. [DOI] [PubMed] [Google Scholar]
- 9.Lu LF, Lind EF, Gondek DC, Bennett KA, Gleeson MW, Pino-Lagos K, Scott ZA, Coyle AJ, Reed JL, Van Snick J, et al. Mast cells are essential intermediaries in regulatory T-cell tolerance. Nature. 2006;442:997–1002. doi: 10.1038/nature05010. [DOI] [PubMed] [Google Scholar]
- 10.Nowak EC, Weaver CT, Turner H, Begum-Haque S, Becher B, Schreiner B, Coyle AJ, Kasper LH, Noelle RJ. IL-9 as a mediator of Th17-driven inflammatory disease. J. Exp Med. 2009;206:1653–1660. doi: 10.1084/jem.20090246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elyaman W, Bradshaw EM, Uyttenhove C, Dardalhon V, Awasthi A, Imitola J, Bettelli E, Oukka M, van Snick J, Renauld JC, et al. IL-9 induces differentiation of TH17 cells and enhances function of FoxP3+ natural regulatory T cells. Proc. Natl. Acad. Sci. USA. 2009;106:12885–12890. doi: 10.1073/pnas.0812530106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jäger A, Dardalhon V, Sobel RA, Bettelli E, Kuchroo VK. Th1, Th17, and Th9 effector cells induce experimental autoimmune encephalomyelitis with different pathological phenotypes. J. Immunol. 2009;183:7169–7177. doi: 10.4049/jimmunol.0901906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hauber HP, Bergeron C, Hamid Q. IL-9 in allergic inflammation. Int. Arch. Allergy Immunol. 2004;134:79–87. doi: 10.1159/000078384. [DOI] [PubMed] [Google Scholar]
- 14.Temann UA, Geba GP, Rankin JA, Flavell RA. Expression of interleukin 9 in the lungs of transgenic mice causes airway inflammation, mast cell hyperplasia, and bronchial hyperresponsiveness. J. Exp. Med. 1998;188:1307–1320. doi: 10.1084/jem.188.7.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soussi-Gounni A, Kontolemos M, Hamid Q. Role of IL-9 in the pathophysiology of allergic diseases. J. Allergy Clin. Immunol. 2001;107:575–582. doi: 10.1067/mai.2001.114238. [DOI] [PubMed] [Google Scholar]
- 16.Faulkner H, Humphreys N, Renauld JC, Van Snick J, Grencis R. Interleukin-9 is involved in host protective immunity to intestinal nematode infection. Eur. J. Immunol. 1997;27:2536–2540. doi: 10.1002/eji.1830271011. [DOI] [PubMed] [Google Scholar]
- 17.Schmitt E, Germann T, Goedert S, Hoehn P, Huels C, Koelsch S, Kühn R, Müller W, Palm N, Rüe E. IL-9 production of naive CD4+ T cells depends on IL-2, is synergistically enhanced by a combination of TGF-beta and IL-4, and is inhibited by IFN-gamma. J. Immunol. 1994;153:3989–3996. [PubMed] [Google Scholar]
- 18.Zeller JC, Panoskaltsis-Mortari A, Murphy WJ, Ruscetti FW, Narula S, Roncarolo MG, Blazar BR. Induction of CD4+ T cell alloantigen-specific hyporesponsiveness by IL-10 and TGF-beta. J. Immunol. 1999;163:3684–3691. [PubMed] [Google Scholar]
- 19.Awasthi A, Carrier Y, Peron JP, Bettelli E, Kamanaka M, Flavell RA, Kuchroo VK, Oukka M, Weiner HL. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat. Immunol. 2007;8:1380–1389. doi: 10.1038/ni1541. [DOI] [PubMed] [Google Scholar]
- 20.Levings MK, Sangregorio R, Galbiati F, Squadrone S, de Waal Malefyt R, Roncarolo MG. IFN-alpha and IL-10 induce the differentiation of human type 1 T regulatory cells. J. Immunol. 2001;166:5530–5539. doi: 10.4049/jimmunol.166.9.5530. [DOI] [PubMed] [Google Scholar]
- 21.Ansel KM, Lee DU, Rao A. An epigenetic view of helper T cell differentiation. Nat. Immunol. 2003;4:616–623. doi: 10.1038/ni0703-616. [DOI] [PubMed] [Google Scholar]
- 22.Xu L, Kitani A, Fuss I, Strober W. Cutting edge: regulatory T cells induce CD4+CD25-Foxp3- T cells or are self-induced to become Th17 cells in the absence of exogenous TGF-beta. J. Immunol. 2007;178:6725–6729. doi: 10.4049/jimmunol.178.11.6725. [DOI] [PubMed] [Google Scholar]
- 23.Houssiau FA, Renauld JC, Stevens M, Lehmann F, Lethe B, Coulie PG, Van Snick J. Human T cell lines and clones respond to IL-9. J. Immunol. 1993;150:2634–2640. [PubMed] [Google Scholar]
- 24.Bradshaw EM, Raddassi K, Elyaman W, Orban T, Gottlieb PA, Kent SC, Hafler DA. Monocytes from patients with type 1 diabetes spontaneously secrete proinflammatory cytokines inducing Th17 cells. J. Immunol. 2009;183:4432–4439. doi: 10.4049/jimmunol.0900576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murphy CA, Langrish CL, Chen Y, Blumenschein W, McClanahan T, Kastelein RA, Sedgwick JD, Cua DJ. Divergent pro- and anti-inflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J. Exp. Med. 2003;198:1951–1957. doi: 10.1084/jem.20030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tzartos JS, Friese MA, Craner MJ, Palace J, Newcombe J, Esiri MM, Fugger L. Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am. J. Pathol. 2008;172:146–155. doi: 10.2353/ajpath.2008.070690. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.