Abstract
Building on our previous neurocomputational models of basal ganglia and hippocampal-region function (and their modulation by dopamine and acetylcholine, respectively), we show here how an integration of these models can inform our understanding of the interaction between the basal ganglia and hippocampal region in associative learning and transfer generalization across various patient populations. As a common test bed for exploring interactions between these brain regions and neuromodulators, we focus on the acquired equivalence task, an associative learning paradigm in which stimuli that have been associated with the same outcome acquire a functional similarity such that subsequent generalization between these stimuli increases. This task has been used to test cognitive dysfunction in various patient populations with damages to the hippocampal region and basal ganglia, including studies of patients with Parkinson’s disease (PD), schizophrenia, basal forebrain amnesia, and hippocampal atrophy. Simulation results show that damage to the hippocampal region—as in patients with hippocampal atrophy (HA), hypoxia, mild Alzheimer’s (AD), or schizophrenia—leads to intact associative learning but impaired transfer generalization performance. Moreover, the model demonstrates how PD and anterior communicating artery (ACoA) aneurysm—two very different brain disorders that affect different neural mechanisms—can have similar effects on acquired equivalence performance. In particular, the model shows that simulating a loss of dopamine function in the basal ganglia module (as in PD) leads to slow acquisition learning but intact transfer generalization. Similarly, the model shows that simulating the loss of acetylcholine in the hippocampal region (as in ACoA aneurysm) also results in slower acquisition learning. We argue from this that changes in associative learning of stimulus-action pathways (in the basal ganglia) or changes in the learning of stimulus representations (in the hippocampal region) can have similar functional effects.
Keywords: Hippocampal region, basal ganglia, associative learning, acquired equivalence, Parkinson’s disease, schizophrenia, basal forebrain, amnesia, hippocampal atrophy, Alzheimer’s, dopamine, acetylcholine
Introduction
As a common test bed for neurocomputational exploration of the interactions between the basal ganglia (and dopamine) and the hippocampal region (and acetylcholine), we focus on the acquired equivalence task, an associative learning paradigm in which stimuli that have been associated with the same outcome acquire a functional similarity such that subsequent generalization between these stimuli increases (Bondi et al., 1993; Grice & Davis, 1960). Both human neuropsychological (Bodi, Csibri, Myers, Gluck, & Keri, 2009; Keri, Nagy, Kelemen, Myers, & Gluck, 2005; Myers et al., 2008; Myers et al., 2003; Weiler, Bellebaum, Brune, Juckel, & Daum, 2009) and animal lesion (Coutureau et al., 2002) studies show that both the hippocampal region and the basal ganglia are important for acquired equivalence.
The acquired equivalence task has two phases: acquisition (learning to associate two stimuli) and transfer generalization (learning that cues become equivalent when they were previously associated with the same response). Several neuropsychological studies from our lab have argued that the associative learning and transfer generalization processes rely on different neural structures (Myers et al., 2008; Myers et al., 2003): initial associative learning relies on the integrity of the basal ganglia, whereas transfer generalization relies on the integrity of the hippocampal region.
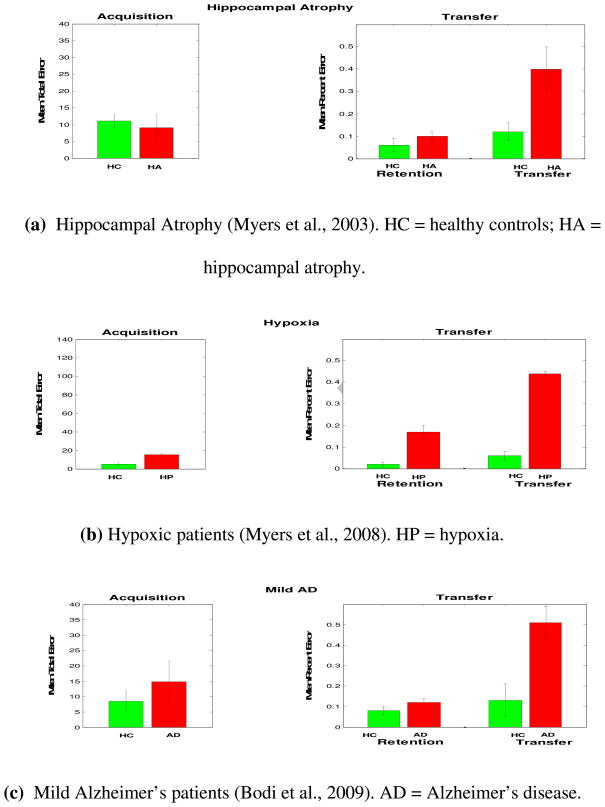
For example, patients with mild Alzheimer’s disease, hippocampal atrophy (HA), and hypoxia are impaired at the transfer generalization phase of the task (Bodi et al., 2009; Myers et al., 2008; Myers et al., 2003) (See Table 1). Mild Alzheimer’s disease is associated with dysfunction to the medial temporal lobe and hippocampal region (de Leon, George, Stylopoulos, Smith, & Miller, 1989). Similarly, hypoxic brain injury causes bilateral neuropathology of the hippocampus and associated medial temporal areas (Kesner & Hopkins, 2001). Recently, Di Paola (Di Paola et al., 2008) reported hippocampal dysfunction in hypoxic patients. These results suggest that the hippocampal region plays an important role in transfer generalization performance.
Table 1.
Acquired equivalence performance in various patient populations. “X” means impairment while “-“signifies performance comparable to healthy controls.
| Acquisition | Transfer | |
|---|---|---|
| Hippocampal atrophy/Hypoxic/mild Alzheimer’s (Bodi et al., 2009; Myers et al. 2003; 2008) |
- | X |
| Parkinson’s patients (medicated) (Myers et al. 2003) |
X | - |
| ACoA aneurysm (Basal forebrain amnesia) (Myers et al. 2008) |
X | - |
| Schizophrenia (Keri et al., 2005) |
- | X |
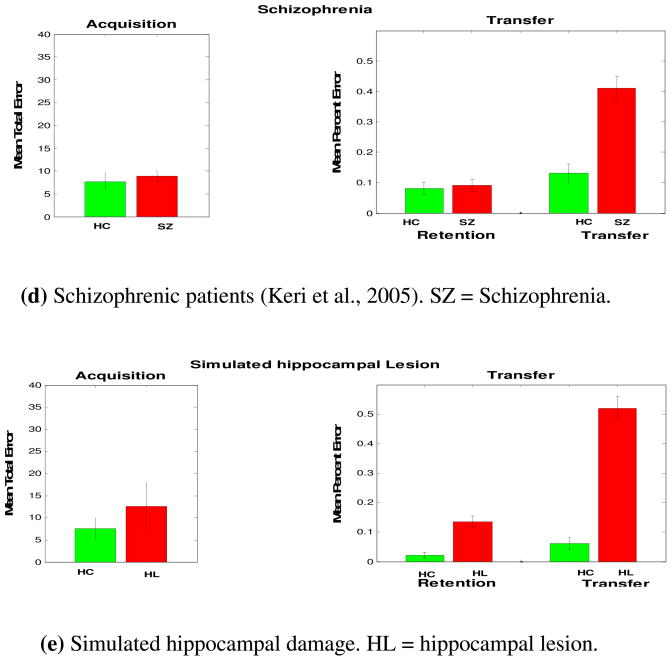
Keri et al. (2005) also found that schizophrenic patients are impaired at transfer generalization in the acquired equivalence task. These results were also confirmed in a recent study (Weiler et al., 2009). It is likely that impaired transfer generalization performance in schizophrenic patients is due to hippocampal dysfunction, which has been reported in the literature (Goldman & Mitchell, 2004; Goldman et al., 2007; Lodge & Grace, 2008; Spoletini et al., 2009). Schizophrenia is a psychiatric disorder which is mainly associated with positive symptoms (e.g., delusions and hallucinations) and negative symptoms (e.g., apathy). It has been shown that schizophrenic patients have mediotemporal lobe and hippocampal dysfunction (Bogerts, Meertz, & Schonfeldt-Bausch, 1985; Boyer et al., 2007; Goldman & Mitchell, 2004; Heckers, 2001; Keri, 2008; Weinberger, 1999). Bogerts et al. (1985) also reported a decrease in hippocampal size but an intact basal ganglia structure in schizophrenic patients as seen in structural brain imaging. Schizophrenic patients also show declarative memory deficits, which suggest hippocampal-region dysfunction (Aleman, Hijman, de Haan, & Kahn, 1999; Cirillo & Seidman, 2003). Lesioning the hippocampus in animals is also used as a model of schizophrenia (Tseng, Chambers, & Lipska, 2009). In addition, Rametti et al. (2009) reported decreased hippocampal activity in schizophrenic patients performing declarative memory tasks. Our model argues that hippocampal dysfunction is responsible for transfer generalization deficits in schizophrenic patients.
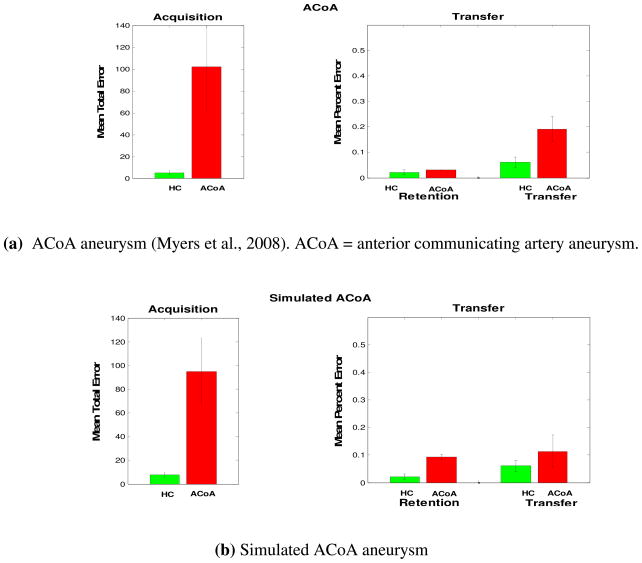
Unlike mild Alzheimer’s disease, schizophrenia, and hypoxia, patients with Parkinson’s disease and ACoA (anterior communicating artery) aneurysm are impaired at the acquisition phase of the acquired equivalence task. Parkinson’s disease is a neurodegenerative disorder associated with reduced dopamine levels in the basal ganglia (Jellinger, 1999; Kish, Shannak, & Hornykiewicz, 1988). On the other hand, the ACoA is one of the most common sites of aneurysm in the brain. The ACoA sends projection to the prefrontal cortex and the basal forebrain (Wright, Boeve, & Malec, 1999), and an aneurysm to ACoA is found to affect basal forebrain functioning (Wright et al., 1999). Patients with ACoA aneurysm have basal forebrain damage, which affects cholinergic input to the hippocampus. Patients with ACoA aneurysm have amnesia (O’Connor & Lafleche, 2004), executive dysfunction (Simard, Rouleau, Brosseau, Laframboise, & Bojanowsky, 2003), and other cognitive deficits (Bondi, Kaszniak, Rapcsak, & Butters, 1993; DeLuca, 1993; Diamond, DeLuca, & Kelley, 1997; Mavaddat, Sahakian, Hutchinson, & Kirkpatrick, 1999). We argue that ACoA amnesia results from basal forebrain damage that disrupts learning in the hippocampal region (Myers et al., 2001; Myers et al., 2002). Cholinergic treatments are used to treat patients with ACoA aneurysm (Benke, Koylu, Delazer, Trinka, & Kemmler, 2005), which is consistent with a dysfunction to the cholinergic system in these patients. See Table 1 for summary of patient populations’ performance on the acquired equivalence task.
Our model seeks to explain how various brain disorders affect acquisition and transfer generalization performance by simulating the interactions between changes in dopamine and acetylcholine in the basal ganglia and hippocampal-region, respectively, as well as from damage to either or both regions. The model integrates features from our existing models of the basal ganglia (Moustafa & Gluck, 2010) and hippocampal region (Myers et al., 1995; Moustafa et al., 2009). In addition, the model explains how disruption to the dopaminergic (as in Parkinson’s disease) or cholinergic (as in ACoA aneurysm) systems affects acquisition performance. Dopamine is produced in the midbrain and is projected to the basal ganglia and prefrontal cortex. Several studies show that phasic dopamine is important for stimulus-response learning (Schultz, Dayan, & Montague, 1997; Wickens, Begg, & Arbuthnott, 1996). Our model assumes that phasic dopamine is key for stimulus-response learning through synaptic modification in the basal ganglia, as we have done in our past models of the basal ganglia (Moustafa & Gluck, 2010; Guthrie et al., 2009; Moustafa & Maida, 2007).
The basal forebrain is an important source of the neurotransmitter acetylcholine throughout the cortex, with the medial septum in particular sending acetylcholine to the hippocampal region (Hasselmo, 1995; Nolte, 1993; Nauta & Feirtag, 1986). In a recent study, Kukolja (Kukolja, Thiel, & Fink, 2009) found that human subjects taking cholinergic medications show enhanced hippocampal functions such as encoding. Septal lesions disrupt hippocampal function and impair acquisition of conditioned eyeblinking in rabbits (Berry & Thompson, 1979; Salvatierra & Berry, 1989; Powell, Milligan, & Buchanan, 1976). Similarly, studies show that scopolamine (an acetylcholine antagonist) impairs encoding of new information in humans and animals, a behavioral task that relies on the integrity of the hippocampal region (Carli, Luschi, & Samanin, 1997; Mewaldt & Ghoneim, 1979). Furthermore, rodent studies have shown that acetylcholine is important for synaptic modification in the hippocampal region (Huerta & Lisman, 1993). In agreement with these experimental studies, our model assumes that acetylcholine plays a critical role in learning in the hippocampal region, much as in our earlier models of septo-hippocampal function in associative learning (Rokers et al., 2002; Myers et al., 1996).
Our integrated model of basal ganglia and hippocampal-region function also attempts to explain how patients with ACoA aneurysm or hippocampal atrophy —brain disorders that affect the hippocampal region —show different performance in the acquired equivalence task. The model assumes that hippocampal atrophy (and also mild Alzheimer’s disease and hypoxia) impairs hippocampal function, while ACoA aneurysm slows down learning in the hippocampal region. The model also shows how patients with ACoA aneurysm or Parkinson’s disease —brain disorders that affect different brain systems—show similar performance in the acquired equivalence task. The model shows that decreasing learning rate parameter values either in the hippocampal region or basal ganglia modules slows down acquisition but does not affect transfer generalization performance. See Table 1 for summary of various patient groups’ performance in the acquired equivalence task.
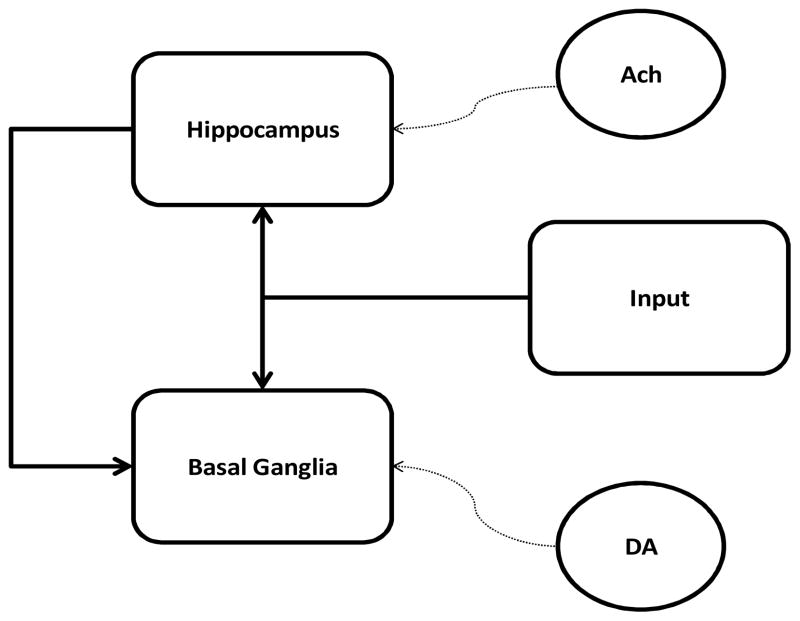
The model has two modules: basal ganglia and hippocampal region (Figure 1). In agreement with most models (Frank, 2005; Moustafa & Gluck, 2010), the basal ganglia is key for stimulus-response learning. Also in agreement with computational models and experimental data (Dusek & Eichenbaum, 1997; Gluck & Myers, 1993), we assume that the hippocampal region is important for stimulus-stimulus representational processes. In the model, dopamine is key for learning in the basal ganglia, while acetylcholine is key for learning in the hippocampal region (see Appendix for more details on model simulations).
Figure 1.
Model architecture. Acetylcholine (Ach) projected from basal forebrain is key for learning in the hippocampal module as in Myers et al. (Myers et al., 1996), while dopamine (DA) is key for learning in the basal ganglia module. Weight update in the hippocampal module is based on Hebbian learning. The basal ganglia and dopamine are modeled using the TD model and actor-critic architecture (also see Moustafa & Gluck, 2010). Dotted lines represent neuromodulatory effects. Abbreviations: DA, dopamine; Ach, acetylcholine.
Results
Below, we present simulation results of healthy control subjects, subjects with hippocampal damage, and lastly Parkinson’s disease and ACoA aneurysm patients. As in all experimental studies with the acquired equivalence task, we present simulation results in terms of number of errors in the acquisition and transfer (including retention and transfer trials) phases.
Healthy controls
As with experimental results from healthy controls in various studies, simulation results show that simulated controls make more errors in retention than in transfer trials of the transfer phase (see healthy control data in Figures 3–5): In the model, hippocampal representations of antecedents and consequents of the “retention” pairs are more overlapped than the representations of “transfer” pair. Accordingly, the model makes more errors during the performance of transfer trials. In other words, the model suggests that the strength of acquired equivalence effect is related to the degree of overlap of representations of stimuli in the hippocampal region: The greater the overlap, the stronger the acquired equivalence effect. Simulation results also show that increasing the number of training trials in the acquisition phase leads to a stronger acquired equivalence effect (not shown).
Figure 3.
Performance of various patient groups with hippocampal damage in the acquired equivalence task. (a–d) Patients with hippocampal atrophy, hypoxia, or schizophrenia, were impaired at transfer generalization performance, particularly during the performance of transfer trials (Keri, Juhasz et al., 2005; Myers et al., 2008; Myers et al., 2003). (e) Simulated hippocampal damage. Simulation results are quantitatively similar to all other groups with hippocampal damage.
Figure 5.
(a) As similar to Parkinson’s disease, Patients with ACoA aneurysm are impaired at acquisition performance. (b) Simulated patients with ACoA aneurysm show similar results.
Hippocampal lesion
We simulated lesioning of the hippocampal region by running the simulations without the hippocampal module (see Appendix). This results in stimulus-response learning in the absence of dynamic modification of stimulus representations. Simulation results are very similar to the performance of patients with hippocampal damage, including patients with hippocampal atrophy, hypoxia, schizophrenia, and mild Alzheimer’s disease (Figure 3). Without a hippocampal module, the model only uses the basal ganglia for stimulus-response learning. Accordingly, model’s performance in the transfer trials is at chance level.
Dopaminergic and cholinergic lesions
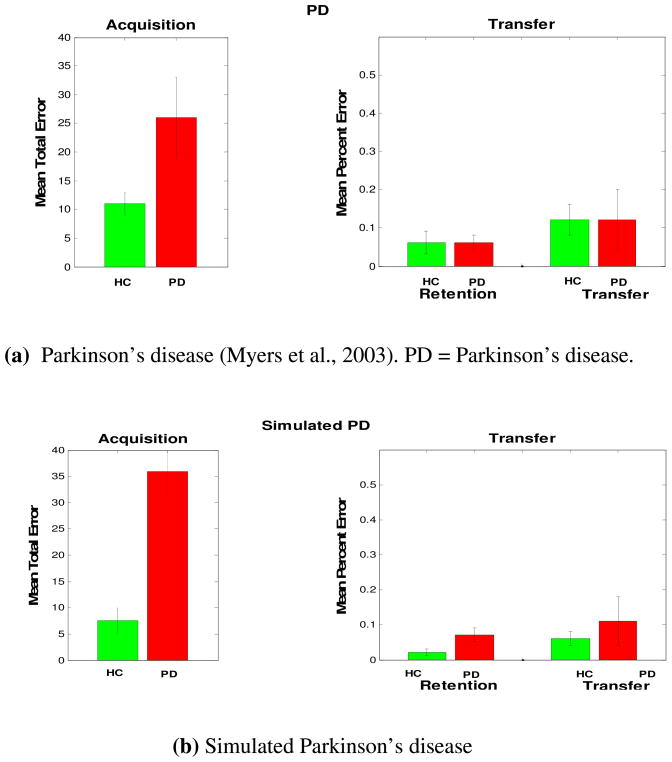
As noted earlier, our model argues that dopamine is important for synaptic modification in the corticostriatal pathway, and acetylcholine is important for learning in the hippocampal region module. We simulated Parkinson’s disease by decreasing the value of the learning rate parameter in the basal ganglia module, as we had previously done in Shohamy et al. (2008). Simulation results show that decreasing the learning rate parameter value in the basal ganglia leads to slow acquisition learning and intact transfer generalization performance (Figure 4). Similarly, we simulated ACoA aneurysm by decreasing the value of the learning rate in the hippocampal module capturing the functional effect of reducing cholinergic inputs to the hippocampal region. As in simulated Parkinson’s disease (with loss of dopamine inputs), our modeling results show that decreasing the learning rate in the hippocampal region leads to slow acquisition learning, as in ACoA aneurysm patients (Figure 5).
Figure 4.
Parkinson’s disease patients’ performance in the acquired equivalence task. (a) Patients with Parkinson’s disease are impaired at acquisition performance but showed similar performance to healthy controls on the transfer phase. (b) Simulated Parkinson’s disease show similar results.
Discussion
We present a new integrated neural network model that simulates functional roles of the basal ganglia and hippocampal region in associative learning and transfer generalization performance. This model integrates various features from our past models of the basal ganglia (Moustafa & Gluck, 2010; Guthrie et al., 2009) and hippocampal region (Gluck & Myers, 1993; Myers et al., 1995). The model simulates performance in the acquired equivalence task in various patient groups, including patients with Parkinson’s disease, mild Alzheimer’s disease, hypoxia, ACoA aneurysm, and schizophrenia. Simulation results show that lesioning the hippocampal region or disrupting the dopaminergic or cholinergic signal interferes with behavioral performance of the acquired equivalence task. First, the model simulates behavioral results in healthy controls. In the transfer phase, the model makes more errors during the performance of transfer than retention trials, as found in all experimental studies using the acquired equivalence task (Bodi et al., 2009; Keri, Nagy et al., 2005; Myers et al., 2008; Myers et al., 2003).
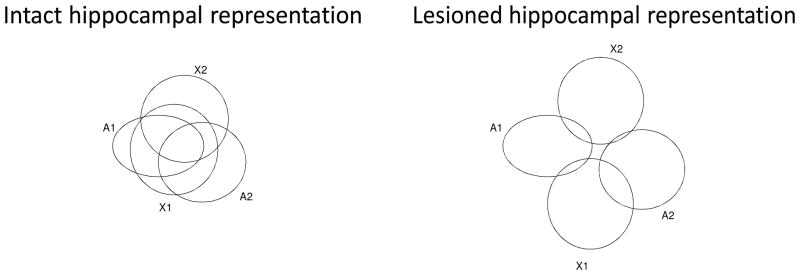
The basal ganglia module in the model is important for stimulus-response learning, in agreement with many modeling and experimental studies (Frank, 2005; Houk, 1995a; Houk, 2005; Jog, Kubota, Connolly, Hillegaart, & Graybiel, 1999; Shohamy, Myers, Geghman, Sage, & Gluck, 2006). The hippocampal module in the model is important for stimulus-stimulus representational learning and responds to the presentation of antecedents and consequents, as in our past models (Gluck & Myers, 1993). This is in agreement with existing neurophysiological studies reporting that hippocampal neurons respond to the presentation of conditioned and unconditioned stimuli (McEchron & Disterhoft, 1997, 1999). Specifically, the simulated hippocampal region in our model is responsible for compressing stimulus inputs that repeatedly co-occur, as we have done in our past models (Myers et al., 1995; Moustafa et al. 2009). Supporting evidence that subjects compress the representation of stimuli in the acquired equivalence paradigm, Meeter, Shohamy, and Myers (2009) found that subjects tend to confuse stimuli that were previously associated with the same response in the acquired equivalence paradigm. This study suggests that subjects’ confusion of stimuli is likely due to overlapped representations of these stimuli, as proposed in our model. In addition, research shows that hippocampal lesioned rats are impaired at performing the sensory preconditioning paradigm (Port & Patterson, 1984), which also relies on compression processes. Lesioning the hippocampal region in the model weakens stimulus-stimulus representational learning (see Figure 2), and leads to making more errors during the performance of transfer than retention trials in the transfer phase of the acquired equivalence task.
Figure 2.
Representational (and compression) processes of the hippocampal region. (a) Compression in intact hippocampal region. During associative learning, the hippocampal region forms compressed representations of antecedents and consequents. In this example, the hippocampal region compresses the representations of A1, A2, X1, X2 when trained on A1→ X1, A2→X1, and A1→X2. This allows the model to associate A2 with X2 during transfer test (and thus acquired equivalence effect). (b) Lesioning the hippocampal region interferes with compression processes. Using the same example in (a), the model here does not properly compress the representations of input stimuli, and thus does not show acquired equivalence effect (i.e., it does not learn to associate A2 with X2).
Furthermore, our modeling results show that different manipulations of different components within the hippocampal module lead to different behavioral performance, which is in agreement with existing experimental studies. Our simulations showed that lesioning the hippocampal-region leads to weak compression (and thus impaired transfer performance). However, our modeling results show that decreasing the learning rate parameter value in the hippocampal module (which simulates the decreased acetylcholine levels in the hippocampal region) leads to slower compression of stimuli. This consequently leads to slow learning in the basal ganglia module as well, thus slow stimulus-response learning. Similarly, decreasing the learning rate in the basal ganglia module, capturing a loss of dopamine inputs, leads to impaired acquisition learning, which models Parkinson’s disease patients’ performance in the acquired equivalence task.
Unlike our model, most existing computational models focus on simulating the role of one neuromodulator to performance (Frank, 2005; Hasselmo, Wyble, & Wallenstein, 1996; Moustafa & Gluck, 2010; Myers et al., 1996), but for some exceptions see Cox and Krickmar (2009), Frank et al. (2007), and Doya (2002, 2008). Our model captures the interaction between two neuromodulators in two brain regions, arguing that dopamine is key for stimulus-response learning in the basal ganglia, while acetylcholine is key for stimulus-stimulus learning in the hippocampal region.
Acetylcholine, dopamine, and learning
A wide range of experimental studies show that acetylcholine is important for learning in the hippocampal region, whereas dopamine is important for learning in the basal ganglia.
Orsetti, Casamenti, and Pepeu ( 1996) found that stimulus-response learning performance is correlated with acetylcholine levels in the hippocampal region. These data argue against the view that basal ganglia alone is key for stimulus-response learning (Houk, 1995b). In our model, the hippocampal region forms representations of stimuli and send these signals to the basal ganglia for further stimulus-response learning, as in the earlier Gluck and Myers (1993) model of cortico-hippocampal function.
Studies also show that galantamine (a cholinesterase inhibitor) enhances eye-blink conditioning learning performance in both young (Simon, Knuckley, & Powell, 2004) and old (Weible, Oh, Lee, & Disterhoft, 2004) rabbits, a behavioral task that has been argued to rely on the integrity of the hippocampal region. Interestingly, using rabbits, Woodruff-Pak, Lehr, Li, and Liu-Chen (2008) found that enhanced eye-blink conditioning learning correlates with nicotinic acetylcholine receptors binding in the hippocampal region. These results suggest that increase in acetylcholine levels in the hippocampal region is important for enhanced eye blink conditioning learning. These experimental data also are consistent with model assumption that acetylcholine is key for learning in the hippocampal region.
As for nigrostriatal dopamine, synaptic plasticity was found in the corticostriatal pathway (Reynolds & Wickens, 2002; Reynolds, Hyland, & Wickens, 2001; Wickens, 1997; Wilson, 2004). It has been reported that dopamine projected to the striatum is necessary for modifying synaptic strength in the corticostriatal pathway (Cepeda et al., 1993; Wickens, Begg, & Arbuthnott, 1996; Wilson, 2004). In an in vitro study on spiny neurons, Wickens et al. (1996) found that dopamine plays an important role in inducing long-term potentiation (LTP) in the corticostriatal pathway. We simulated the effects of dopamine on learning using the 3-factor rule of learning, as we have done in our earlier models (Guthrie, Myers, & Gluck, 2009; Moustafa & Gluck, 2010; Reynolds & Wickens, 2002).
Brain disorders and behavior
Below, we discuss behavioral performance in the acquired equivalence and other related tasks in various patient groups.
Parkinson’s disease
Our simulation results show that Parkinson’s disease patients are impaired at acquisition but show intact performance at the transfer phase of the acquired equivalence task, as reported in Myers et al. (2003). This is consistent with experimental findings of Shohamy et al. (2006) showing similar results albeit using a different behavioral task. In addition, Parkinson’s disease patients’ impaired performance in stimulus-response learning tasks has been shown in various experimental studies (Frank, Seeberger, & O’Reilly R, 2004; Jahanshahi, Wilkinson, Gahir, Dharminda, & Lagnado, 2009; Knowlton, Mangels, & Squire, 1996; Moustafa, Sherman, & Frank, 2008; Shohamy et al., 2006).
ACoA aneurysm
Consistent with experimental results (Myers et al., 2008), our simulation results show that patients with ACoA aneurysm are impaired at the acquisition phase of the acquired equivalence task. This is consistent with existing data showing that patients with ACoA aneurysm also show impairment learning other behavioral tasks, including eye blink conditioning (Myer, Bryant, DeLuca, & Gluck, 2002; Myers, Deluca, Hopkins, & Gluck, 2006; Myers et al., 2001): Performing both of the acquisition phase of the acquired equivalence task and the eye blink conditioning task relies on associative learning processes.
Schizophrenia
Consistent with experimental studies (Keri, Nagy et al., 2005), our simulation results show that schizophrenic patients are impaired at transfer generalization but show intact acquisition performance. The neural dysfunction in schizophrenia is heterogonous, with various studies reporting hippocampal, prefrontal, and striatal dysfunction. Some argue that positive symptoms are due to basal ganglia dysfunction (Keri, 2008), while others argue that positive (and negative and cognitive) symptoms are due to prefrontal (Rolls, Loh, Deco, & Winterer, 2008) or hippocampal (Chen, 1995; Lisman & Otmakhova, 2001; Oertel et al., 2007) dysfunction. The model presented here argues that impaired transfer performance in the acquired equivalence task in schizophrenic patients is due to a dysfunction to the hippocampal region. Hippocampal dysfunction has been reported in schizophrenic patients (Goldman & Mitchell, 2004; Keri, 2008). Given that schizophrenic patients’ performance is very similar to patients with mild Alzheimer’s disease, hippocampal atrophy, and hypoxia, it is very likely that transfer generalization impairment in schizophrenic patients is due to hippocampal dysfunction.
With regard to the acquisition phase of the acquired equivalence task, it is debatable whether schizophrenic patients show feedback learning deficits. Waltz, Frank, Robinson, and Gold (2007) found that patients are impaired at learning from reward but not from punishment. In our own prior studies (Polgar et al., 2008; Farkas et al., 2008), we found that deficit patients (i.e., schizophrenic patients with negative symptoms) show feedback learning impairments, arguing that the basal ganglia might be responsible for negative symptoms in schizophrenia. In Keri et al. (Keri, Juhasz et al., 2005; Keri et al., 2000) and Wickert et al. (2002), we found intact performance in schizophrenic patients on a multi-cue probabilistic learning task (the “weather prediction” task). Overall, consistent with many existing experimental studies, our model assumes that schizophrenic patients show intact feedback learning.
Alzheimer’s disease
Hippocampal dysfunction has been consistently reported in Alzheimer’s disease patients. For example, several studies report dysfunction to different segments of the hippocampus in Alzheimer’s disease patients (Apostolova et al., 2006; de Leon et al., 1989; Jack et al., 2000). In addition, fMRI studies report decreased hippocampal activity in Alzheimer’s disease patients (Allen et al., 2007; Wang et al., 2006). Our model argues that transfer generalization impairment in Alzheimer’s disease patients may be due to hippocampal dysfunction.
Relation to existing models
Below we compare our model to existing models that simulate performance in similar behavioral tasks and/or model of brain disorders that we have simulated in this project.
Our model is novel in that it simulates performance in the acquired equivalence task through integration of our past modeling of the basal ganglia and hippocampal region. To our knowledge, no existing neural network model simulates performance in this task. Our previous models (Gluck, Myers, & Meeter, 2005; Gluck & Myers, 1993; Myers, Gluck, & Granger, 1995; Rokers, Mercado, Allen, Myers, & Gluck, 2002) simulated performance in other associative learning paradigms, such as latent inhibition and sensory preconditioning, but did not simulate performance in the acquired equivalence task, because these past models did not integrate the functionality of both brain regions. Turnock and Becker (2007) proposed a model that assumes that the hippocampus plays a role in contextual processes and is important for gating prefrontal cortex information into the basal ganglia. This model did not simulate functional roles of dopamine and acetylcholine in learning and performance.
As noted earlier, our current integrated model, builds on our earlier models of septo-hippocampal function in associative learning. In particular, as in Rokers et al. (2002), we showed that cholinergic input to the hippocampal region is key for learning classical conditioning tasks. The Rokers et al. model builds on our earlier model (Hasselmo et al., 1996), which argues that acetylcholine is key for encoding of new information in the hippocampus. Unlike existing models, Rokers et al. simulate a role for the hippocamposeptal pathway in learning. Rokers et al. argue that the hippocampus is key for novelty detection, and that the hippocampus projects this novelty detection signal to the septum. In this model, septal acetylcholine increases for novel stimuli, and thus learning to encode information is faster for novel than familiar stimuli. The model presented here does not simulate the function of the hippocamposeptal pathway. However, the Hasselmo et al. and Rokers et al. models do not simulate performance in acquired equivalence and do not incorporate a basal ganglia module. Another novel feature of our newer integrated model is that it simulates functional roles of both dopamine and acetylcholine in behavioral performance and learning.
Unlike most existing models, the model presented here also simulates performance in various neurological and psychiatric patient groups. Most existing models focus on simulating behavioral performance in various tasks in one patient group (Cohen, Braver, & O’Reilly, 1996; Frank, 2005; Moustafa & Gluck, 2010). One exception, however, is a computational model by Amos (2000) which simulates performance in the Wisconsin Card Sorting Task in Parkinson’s disease, Huntington’s disease, and schizophrenic patients. We believe that both classes of models are important. Accounting for performance in various tasks in one patient group explains similar neural mechanisms for seemingly different behavioral tasks. An example is a basal ganglia model proposed by Frank (Frank, 2005; Frank, Loughry, & O’Reilly, 2001) which shows how the basal ganglia and prefrontal cortex interact during the performance of both working memory and decision making tasks (also see Moustafa & Maida, 2007). Simulating performance in one behavioral task in various patient groups helps explain how Parkinson’s disease and ACoA aneurysm—which affect different brain structures—are associated with similar behavioral performance (as we show in our simulation results).
Models of brain disorders
Below, we present a summary of existing models of Parkinson’s disease, schizophrenia, and Alzheimer’s disease, and discuss the similarities and differences between these models and ours.
Parkinson’s disease
Most, if not all, existing models of Parkinson’s disease focus on simulating the functional contribution of the basal ganglia and/or prefrontal cortex to motor and cognitive processes (Amos, 2000; Frank, 2005; Guthrie et al., 2009; Moustafa & Gluck, 2010; Moustafa, Gluck, & Myers, 2009). These models are consistent with our model in that nigrostriatal dopamine is important for stimulus-response learning. For a review of existing models of Parkinson’s disease, see Discussion section of Moustafa and Gluck (Moustafa & Gluck, 2010).
Schizophrenia
As for schizophrenia, most existing models focus on simulating the contribution of a single brain area to behavioral performance (Cohen & Servan-Schreiber, 1992; Rolls et al., 2008; Talamini & Meeter, 2009). However, it is experimentally known that various brain structures are affected in schizophrenia (Keri, 2008; Perlstein, Carter, Noll, & Cohen, 2001; Snitz et al., 2005), including prefrontal cortex (Perlstein et al., 2001; Snitz et al., 2005), mediotemporal lobe (Keri, 2008), and basal ganglia (Waltz et al., 2007). Most computational models of schizophrenia focus on simulating the role of prefrontal cortex in cognitive performance (Cohen & Servan-Schreiber, 1992; Rolls et al., 2008). Some other models focus on simulating dopaminergic (Schmajuk, 2005) or mediotemporal lobe (Talamini & Meeter, 2009) dysfunction. Chen (1995) proposed a one-layer attractor network which addresses how hippocampal dysfunction in schizophrenia leads to psychotic symptoms. This model assumes that psychosis is related to aberrant retrieval of information from memory. This model shows that an increase of correlation of encoding inputs in the hippocampus does interferes with retrieval processes, such that the model will retrieve wrong information at wrong time (which arguably corresponds to psychotic symptoms).
The Amos model mentioned above simulates performance in Parkinson’s disease and schizophrenia, using mathematical techniques similar to those used by Moustafa and Gluck (2010). The Amos model showed that decreasing activity of the prefrontal cortex explains working memory deficits in schizophrenia, and it also shows that decreasing activity in basal ganglia and prefrontal cortex modules simulates impaired performance in Parkinson’s disease patients. Unlike our model, the Amos model is not a learning model and does not simulate stimulus-stimulus or stimulus-response learning processes.
Alzheimer’s disease
Unlike Parkinson’s disease and schizophrenia, there are fewer models of Alzheimer’s disease in the literature. For example, Hasselmo (Hasselmo, 1994; Hasselmo, 1997) provided a model of Alzheimer’s disease which shows how damage to the hippocampus can affect functional processing in efferent cortical structures. This model argues that memory symptoms in Alzheimer’s disease are related to impaired encoding of new information in the hippocampus, such that new and existing information share similar representations. This consequently makes it difficult to retrieve information from memory, which arguably equivalent to amnesic symptoms. McAuley et al. (2009) built a computational model that addresses how changes in cortisol levels affects hippocampal functioning, which consequently leads to Alzheimer’s disease symptoms. The model shows that increase in cortisol levels inhibits hippocampal function and leads to amnesia, as found with Alzheimer’s disease patients. Meeter and Murre (2005) built a model showing that damage to the hippocampus can lead to anterograde amnesia, a main symptom of Alzheimer’s disease. As in our current model, the Meeter and Murre model simulates amnesia by disabling hippocampal neurons. This model argues that consolidation of declarative memories takes place in the cortex, and thus damaging the hippocampus leads to anterograde amnesia. It is not clear if these models can simulate performance in the acquired equivalence task. In Gluck, Myers, Nicolle, and Johnson (2006), we provided a computational analysis (though not a simulation model) of how Alzheimer’s disease might affect hippocampal functioning and behavioral performance, especially in learning and transfer. The model presented here in this paper partially builds on ideas proposed by Gluck et al., including how damaging the hippocampal region might affect transfer generalization performance. For a recent review of models of Alzheimer’s disease, see Duch (2007).
Model limitations
Even though our current integrated model simulates functional roles of the hippocampal region and basal ganglia in various patient populations, it has some limitations.
For example, the model does not simulate the different symptoms in patients with hippocampal damage (e.g., hypoxia, mild Alzheimer’s disease, and hippocampal atrophy patients). The model simply assumes that these disorders interfere with computational processes of the hippocampal region, and we simulated these disorders by running the simulations without the hippocampal module. It is likely that these disorders affect the hippocampal structure in different ways, such that hippocampal structural changes in these brain disorders lead to different behavioral changes that were not captured by the acquired equivalence task. Such speculations are beyond the scope of our model. Future work should explain how damage to the hippocampal region can lead to either amnesia or psychosis in one integrated model, as reported in different models of these symptoms (Chen et al., 1995; Meeter & Murre, 2005; Hoffman et al., 2001, 2006).
Another limitation of the current model are the findings that in addition to the hippocampal region and basal ganglia, other brain structures may be key for acquired equivalence including the prefrontal cortex (Iordanova, Killcross, & Honey, 2007). Further, Shohamy and Wagner (2008) found that dopaminergic projections to the hippocampus are involved in acquired equivalence. They found that correlated hippocampal and ventral tegmental area activity during the acquisition phase of the acquired equivalence task predicts subject’s performance in the transfer phase. Shohamy and Wagner argue that the correlated activity between these structures suggests that dopamine projected to the hippocampus is key for learning the acquired equivalence task. Similarly, Lisman and Grace (2005) argued that dopamine is key for learning in the hippocampus. As in the Rokers et al. model, Lisman and Grace argue that the hippocampus is key for novelty detection. However, unlike Rokers et al, Lisman and Grace assume that hippocampal projection to the ventral tegmental area is important for driving dopamine responses for novel stimuli, and dopamine projection to the hippocampus is important for encoding of novel stimuli. The model presented here does not include this dopamine-hippocampus pathway. It is possibly the case that both dopamine and acetylcholine interact during learning in the hippocampal region. Similarly, acetylcholine may be key for attentional processes (Cox & Krichmar, 2009) where the role of the acetylcholine in attention may be mediated by cholinergic projections to the cortex (Bucci, Holland, & Gallagher, 1998). Further modeling work to address these data needs to include a cholinergic projection to the cortex, and to address how attention affects cognitive performance in the acquired equivalence task.
In a recent study from our lab, we have shown that subjects with cocaine addiction exhibit a specific deficit on performing the new consequents phase (the third phase of acquisition, see Table 2) of the acquired equivalence task (Vadhan et al., 2008). It is possible that they are specifically impaired in this phase due to increased memory load. The new consequents phase has more trial types than those of the previous phases (see Table 2), and it is possibly the case that subjects with cocaine addiction are impaired at maintaining the various trials types in that phase in working memory. Our model does not account for this finding because it does not have a working memory mechanism. Further modeling to address these data needs to include a working memory mechanism that maintains representation of trial types, and also should explain how cocaine addiction impairs working memory, as reported in experimental studies (George et al., 2008).
Table 2.
Acquired Equivalence task. The task has 2 phases: acquisition and transfer. In the transfer phase, trials that have”?” are trials that were not previously presented to the subjects (Transfer Trials); the rest are trials that were previously presented to the subjects (Retention Trials). In data presented, we will look at performance in each type separately. A’s and B’s are called stimuli (cues), while X’s and Y’s are outcomes (or consequents).
| Acquisition: Shaping | Acquisition: Equivalence Training | Acquisition: New Consequents | Transfer |
|---|---|---|---|
| A1→X1 | A1→X1 | A1→X1 | A1→X1 |
| A2→X1 | A2→X1 | A2→X1 | |
| A1→X2 | A1→X2 | ||
| A2→X2? | |||
| B1→Y1 | B1→Y1 | B1→Y1 | B1→Y1 |
| B2→Y1 | B2→Y1 | B2→Y1 | |
| B1→Y2 | B1→Y2 | ||
| B2→Y2? | |||
Furthermore, experimental data show that the hippocampal region also receives dopaminergic projections, and that the basal ganglia receives cholinergic input. Experimental data show that dopamine is also key for learning in the hippocampus (Rossato et al., 2009; Lisman & Grace, 2005), but this was shown to be related to long-term memory and novelty detection processes. Our model did not simulate performance in these tasks. Further, it was found that acetylcholine in the basal ganglia is key for controlling motor processes (Graybiel, 1998). In our model, we incorporate a simple module of motor learning. A more complex model of initiating and sequencing motor plans will definitely requires the simulating of both acetylcholine an dopamine in the basal ganglia. This is beyond the scope of our current model.
Our model makes several testable predictions. For example, our model predicts that decreasing acetylcholine (using scopolamine) or dopamine (using 6-OHDA) will equally impair acquisition of the acquired equivalence task. Lesioning the hippocampal region should lead to impaired transfer performance (for similar ideas, see Coutureau et al., 2002). As mentioned above, the model also predicts that the acquired equivalence effect is related to the number of training trials of the acquisition phase: The larger the number of trials, the greater the acquired equivalence effect. Future experimental studies are needed to test these predictions in either rodents or humans.
Overall, our model provides a mechanistic account for behavioral performance in the acquired equivalence task in various patient populations, including Alzheimer’s disease, Parkinson’s disease, hypoxia, ACoA aneurysm, and schizophrenia, and through this suggests how the basal ganglia (and dopamine) interact with the hippocampal region (and acetylcholine) in both learning and transfer generalization.
Appendix
Below, we describe details of the acquired equivalence task used in the simulation. We then describe details of the model simulation.
Acquired equivalence task
The acquired equivalence task has two phases: acquisition and transfer (see Table 2). Phase 1 has three sub-phases, in which subjects learn to associate different stimuli with consequents (responses). In this task, four drawings of faces (man, woman, girl, boy) served as the antecedent stimuli. In the model, we represent stimuli using an input of binary values. In the original task, the consequents were four drawings of colored fish, which we also represent using binary values. Subjects (and the model) learn to associate antecedents with consequents, as explained in Table 1. For more details on task description see Myers et al. (2003). The number of trials in the acquisition phase in both the original and simulated task is as follows: 32 (Shaping phase), 64 (Equivalence Training phase), 96 (New Consequents phase). The transfer phase has 16 trials.
Simulation details
As described above, the model has two modules: basal ganglia and hippocampal modules (Figure 1). Below, we describe simulations details for each module.
Basal ganglia module
We model the basal ganglia using the actor-critic architecture, as previously proposed in various models (Berns & Sejnowski, 1995; Houk, 1995b; Moustafa & Gluck, 2010; Moustafa & Maida, 2007; Suri & Schultz, 1998, 1999). The critic is key for reward and feedback-based learning and the actor is key for action selection (motor) learning.
Learning in the striatal module relies on phasic dopamine signals projected from the midbrain (for similar ideas see Suri & Schultz, 1998, 1999). In this model, phasic dopamine signals are important for motor learning. Learning in the basal ganglia module is based on the TD algorithm, which simulates various characteristics of phasic dopamine firing (Schultz et al., 1997; Sutton & Barto, 1987; Sutton & Barto, 1990). Let TD(t) be the temporal difference error signal at time t (also known as the effective reinforcement); R(t) be the reward presented at time t (reward is 1 when reward is presented after correct feedback and is 0 otherwise); P(t) be the reward prediction at time t; γbe the discount factor (which determines how future reward affect reward predictions; is set to 0.99 in all simulation runs presented here). The TD error is computed as follows:
Let wi be the weight connecting unit i to the critic node; n be the number of Input nodes; and xi be activation of input units (which take binary (0,1) values). Reward prediction P(t) is computed by the critic node as follows:
Now, we describe the equations of the actor module. Let wji be the weight connecting unit i to unit j; δ ji (t) be the Gaussian noise associated with the weight wji. All weights are perturbed using Gaussian noise, which is included to induce exploration in the system. Let u ji be the perturbed weight connecting unit i to unit j. Perturbed weight values are computed as follows:
Activations of all units in the network are computed using a sigmoidal function:
where g is the gain parameter. Let n be the number of Input units. The input units take binary (0,1) values. The activation of a unit j is computed as:
In the model, a winner-take all network computes the unit with the highest activation in the basal ganglia module. In other words, we assume that winner-take all competition among striatal neurons is assumed to be the mechanism underlying the choice of motor responses..
where β is a threshold; Aj is the activation of unit j; is the activation of unit j resulting from winner-take all computations (for similar ideas see Barto, 1995; Berns & Sejnowski, 1995; Schultz et al., 1997; Suri & Schultz, 1998, 1999).
Learning in the basal ganglia model is based on the three-factor rule of learning—also known as the dopamine-based Hebbian learning rule (for similar ideas, see Guthrie et al., 2009). According to this rule, the phasic dopamine signal is important for strengthening weights linking active nodes. Dopamine phasic signal is also important for weakening weights linking an active node and another inactive node. Also, different computational models incorporate this learning rule (Braver & Cohen, 2000; Guthrie et al., 2009; Suri & Schultz, 1998, 1999).
Let LRbg be the learning rate, which we assume to correspond to phasic dopamine levels in the model, as previously proposed by Shohamy et al. (Shohamy, Myers, Kalanithi, & Gluck, 2008). Let xi represents the activation level of the presynaptic node. The weight update rule is,
Hippocampal module
The hippocampal module is a 2-layer network in which the Input layer is fully connected to the hippocampal layer. The input pattern specifies the values of stimuli and outcomes (which corresponds to Faces and Fishes in the acquired equivalence task). As in the basal ganglia module, all weights are perturbed using Gaussian noise. Activation levels of all units in the model are computed as follows:
where uji is the perturbed weight connecting unit i to unit j, n is the number of units in the input; the input units take binary (0,1) values (for similar simulation details see Barto, 1995; Schultz et al., 1997; Suri & Schultz, 1999); t is time step, f is the logistic sigmoid function.
Weights are updated at every time step. Learning in this module is Hebbian. The Hebbian learning algorithm is a model of associative learning, a process ascribed to the hippocampal region function (Bunsey & Eichenbaum, 1995; Henke, Buck, Weber, & Wieser, 1997). It is also a simple model for synaptic change through Long Term Potentiation (LTP) in the hippocampal region (Bilkey, 1996; Bliss & Lomo, 1973). The weight update rule here is as follows:
where LRhipp is the learning rate for the hippocampal module, and represents acetylcholine levels in the hippocampal region; xi is the cortical input unit i; y j is the activation of the unit j in the hippocampal layer. The hippocampal layer consists of many patches of neurons (10 patches and each patch has 20 nodes), each form a separate representational code of the input (for details see Moustafa et al., 2009). Winner-take-all networks are used to simulate lateral inhibitory connections among neurons in each patch.
A hippocampal representation is projected to the basal ganglia module. There is one-toone connection from hippocampal layer to medial temporal cortex, with non-adaptive, fixed weights.
Simulations of neurological and psychiatric disorders
We simulated Parkinson’s disease in the model by decreasing learning rate parameter value in the basal ganglia module ( LRbg ). Similarly, we simulated ACoA aneurysm by decreasing learning rate value in the hippocampal module ( LRhipp ). The model shows that decreasing the learning rate value in the hippocampal module affects motor learning in the basal ganglia. This explains how ACoA aneurysm patients show impaired performance in acquisition (stimulus-response) performance, a process that has been ascribed to basal ganglia function. See Table 3 for a summary of parameters manipulated to simulate various brain disorders presented here.
Table 3.
Simulation of all disorders in the model. Lesion means taking out all structure. “Yes” signifies corresponding area is lesioned in the corresponding group. “↓”signifies learning rate (LR) is lower than learning rate used to simulate healthy controls. “-“signifies parameter values used is not different from controls.
| Hippocampal Learning rate | Basal ganglia Learning rate | Hippocampal Lesion | Basal ganglia Lesion | |
|---|---|---|---|---|
| Hippocampal atrophy/Hypoxic/mild Alzheimer’s | - | - | Yes | - |
| Parkinson’s patients (medicated) | - | ↓ | - | - |
| ACoA aneurysm (Basal forebrain amnesia) | ↓ | - | - | - |
| Schizophrenia | - | - | Yes | - |
We simulated lesioning of the hippocampal region in the model running the simulations without the hippocampal module. Learning in the model in this case depends only on weight update in the basal ganglia module. We assume that lesioning the hippocampal region simulates hippocampal dysfunction in hippocampal atrophy, hypoxic, mild Alzheimer’s disease, and schizophrenic patients. Lesioning the hippocampal region in the model interferes with compression processes (see Figure 2).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aleman A, Hijman R, de Haan EH, Kahn RS. Memory impairment in schizophrenia: a meta-analysis. Am J Psychiatry. 1999;156(9):1358–1366. doi: 10.1176/ajp.156.9.1358. [DOI] [PubMed] [Google Scholar]
- Allen G, Barnard H, McColl R, Hester AL, Fields JA, Weiner MF, et al. Reduced hippocampal functional connectivity in Alzheimer disease. Arch Neurol. 2007;64(10):1482–1487. doi: 10.1001/archneur.64.10.1482. [DOI] [PubMed] [Google Scholar]
- Amos A. A computational model of information processing in the frontal cortex and basal ganglia. J Cogn Neurosci. 2000;12(3):505–519. doi: 10.1162/089892900562174. [DOI] [PubMed] [Google Scholar]
- Apostolova LG, Dutton RA, Dinov ID, Hayashi KM, Toga AW, Cummings JL, et al. Conversion of mild cognitive impairment to Alzheimer disease predicted by hippocampal atrophy maps. Arch Neurol. 2006;63(5):693–699. doi: 10.1001/archneur.63.5.693. [DOI] [PubMed] [Google Scholar]
- Barto AG. Adaptive critics and the basal ganglia. In: Houk JC, Davis JL, Beiser DG, editors. Models of information processing in the basal ganglia. Cambridge, Mass: MIT Press; 1995. p. xii.p. 382. [Google Scholar]
- Benke T, Koylu B, Delazer M, Trinka E, Kemmler G. Cholinergic treatment of amnesia following basal forebrain lesion due to aneurysm rupture--an open-label pilot study. Eur J Neurol. 2005;12(10):791–796. doi: 10.1111/j.1468-1331.2005.01063.x. [DOI] [PubMed] [Google Scholar]
- Berns GS, Sejnowski TJ. How the Basal Ganglia Make Decisions. In: Damasio A, Damasio H, Christen Y, editors. The Neurobiology of Decision Making. Springer-Verlag; 1995. [Google Scholar]
- Bilkey DK. Long-term potentiation in the in vitro perirhinal cortex displays associative properties. Brain Res. 1996;733(2):297–300. doi: 10.1016/0006-8993(96)00789-5. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973;232(2):331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodi N, Csibri E, Myers CE, Gluck MA, Keri S. Associative learning, acquired equivalence, and flexible generalization of knowledge in mild Alzheimer disease. Cogn Behav Neurol. 2009;22(2):89–94. doi: 10.1097/WNN.0b013e318192ccf0. [DOI] [PubMed] [Google Scholar]
- Bogerts B, Meertz E, Schonfeldt-Bausch R. Basal ganglia and limbic system pathology in schizophrenia. A morphometric study of brain volume and shrinkage. Arch Gen Psychiatry. 1985;42(8):784–791. doi: 10.1001/archpsyc.1985.01790310046006. [DOI] [PubMed] [Google Scholar]
- Bonardi C, Rey V, Richmond M, Hall G. Acquired equivalence of cues in pigeon autoshaping: Effects of training with common consequences and common antecedents. Animal Learning and Behavior. 1993;21:369–376. [Google Scholar]
- Bondi MW, Kaszniak AW, Rapcsak SZ, Butters MA. Implicit and explicit memory following anterior communicating artery aneurysm rupture. Brain Cogn. 1993;22(2):213–229. doi: 10.1006/brcg.1993.1035. [DOI] [PubMed] [Google Scholar]
- Boyer P, Phillips JL, Rousseau FL, Ilivitsky S. Hippocampal abnormalities and memory deficits: new evidence of a strong pathophysiological link in schizophrenia. Brain Res Rev. 2007;54(1):92–112. doi: 10.1016/j.brainresrev.2006.12.008. [DOI] [PubMed] [Google Scholar]
- Braver TS, Cohen JD. On the control of control: The role of dopamine in regulating prefrontal function and working memory. In: Monsell S, Driver J, editors. Control of cognitive processes: attention and performance XVIII. Cambridge, Mass: MIT Press; 2000. p. xvi.p. 779. [Google Scholar]
- Bucci DJ, Holland PC, Gallagher M. Removal of cholinergic input to rat posterior parietal cortex disrupts incremental processing of conditioned stimuli. J Neurosci. 1998;18(19):8038–8046. doi: 10.1523/JNEUROSCI.18-19-08038.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunsey M, Eichenbaum H. Selective damage to the hippocampal region blocks long-term retention of a natural and nonspatial stimulus-stimulus association. Hippocampus. 1995;5(6):546–556. doi: 10.1002/hipo.450050606. [DOI] [PubMed] [Google Scholar]
- Carli M, Luschi R, Samanin R. Dose-related impairment of spatial learning by intrahippocampal scopolamine: antagonism by ondansetron, a 5-HT3 receptor antagonist. Behav Brain Res. 1997;82(2):185–194. doi: 10.1016/s0166-4328(97)80988-6. [DOI] [PubMed] [Google Scholar]
- Chen EY. A neural network model of cortical information processing in schizophrenia. II--Role of hippocampal-cortical interaction: a review and a model. Can J Psychiatry. 1995;40(1):21–26. doi: 10.1177/070674379504000107. [DOI] [PubMed] [Google Scholar]
- Cirillo MA, Seidman LJ. Verbal declarative memory dysfunction in schizophrenia: from clinical assessment to genetics and brain mechanisms. Neuropsychol Rev. 2003;13(2):43–77. doi: 10.1023/a:1023870821631. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Braver TS, O’Reilly RC. A computational approach to prefrontal cortex, cognitive control and schizophrenia: recent developments and current challenges. Philos Trans R Soc Lond B Biol Sci. 1996;351(1346):1515–1527. doi: 10.1098/rstb.1996.0138. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Servan-Schreiber D. Context, cortex, and dopamine: a connectionist approach to behavior and biology in schizophrenia. Psychol Rev. 1992;99(1):45–77. doi: 10.1037/0033-295x.99.1.45. [DOI] [PubMed] [Google Scholar]
- Cools R, Barker RA, Sahakian BJ, Robbins TW. Enhanced or impaired cognitive function in Parkinson’s disease as a function of dopaminergic medication and task demands. Cereb Cortex. 2001;11(12):1136–1143. doi: 10.1093/cercor/11.12.1136. [DOI] [PubMed] [Google Scholar]
- Coutureau E, Killcross AS, Good M, Marshall VJ, Ward-Robinson J, Honey RC. Acquired equivalence and distinctiveness of cues: II. Neural manipulations and their implications. J Exp Psychol Anim Behav Process. 2002;28(4):388–396. [PubMed] [Google Scholar]
- Cox BR, Krichmar JL. Neuromodulation as a robot controller. [Google Scholar]
- de Leon MJ, George AE, Stylopoulos LA, Smith G, Miller DC. Early marker for Alzheimer’s disease: the atrophic hippocampus. Lancet. 1989;2(8664):672–673. doi: 10.1016/s0140-6736(89)90911-2. [DOI] [PubMed] [Google Scholar]
- DeLuca J. Predicting neurobehavioral patterns following anterior communicating artery aneurysm. Cortex. 1993;29(4):639–647. doi: 10.1016/s0010-9452(13)80287-0. [DOI] [PubMed] [Google Scholar]
- Di Paola M, Caltagirone C, Fadda L, Sabatini U, Serra L, Carlesimo GA. Hippocampal atrophy is the critical brain change in patients with hypoxic amnesia. Hippocampus. 2008;18(7):719–728. doi: 10.1002/hipo.20432. [DOI] [PubMed] [Google Scholar]
- Diamond BJ, DeLuca J, Kelley SM. Memory and executive functions in amnesic and non-amnesic patients with aneurysms of the anterior communicating artery. Brain. 1997;120(Pt 6):1015–1025. doi: 10.1093/brain/120.6.1015. [DOI] [PubMed] [Google Scholar]
- Doya K. Metalearning and neuromodulation. Neural Netw. 2002;15(4–6):495–506. doi: 10.1016/s0893-6080(02)00044-8. [DOI] [PubMed] [Google Scholar]
- Doya K. Modulators of decision making. Nat Neurosci. 2008;11(4):410–416. doi: 10.1038/nn2077. [DOI] [PubMed] [Google Scholar]
- Duch W. Computational models of dementia and neurological problems. Methods Mol Biol. 2007;401:305–336. doi: 10.1007/978-1-59745-520-6_17. [DOI] [PubMed] [Google Scholar]
- Dusek JA, Eichenbaum H. The hippocampus and memory for orderly stimulus relations. Proc Natl Acad Sci U S A. 1997;94(13):7109–7114. doi: 10.1073/pnas.94.13.7109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas M, Polgar P, Kelemen O, Rethelyi J, Bitter I, Myers CE, et al. Associative learning in deficit and nondeficit schizophrenia. Neuroreport. 2008;19(1):55–58. doi: 10.1097/WNR.0b013e3282f2dff6. [DOI] [PubMed] [Google Scholar]
- Frank MJ. Dynamic dopamine modulation in the basal ganglia: a neurocomputational account of cognitive deficits in medicated and nonmedicated Parkinsonism. J Cogn Neurosci. 2005;17(1):51–72. doi: 10.1162/0898929052880093. [DOI] [PubMed] [Google Scholar]
- Frank MJ, Loughry B, O’Reilly RC. Interactions between frontal cortex and basal ganglia in working memory: a computational model. Cogn Affect Behav Neurosci. 2001;1(2):137–160. doi: 10.3758/cabn.1.2.137. [DOI] [PubMed] [Google Scholar]
- Frank MJ, Scheres A, Sherman SJ. Understanding decision-making deficits in neurological conditions: insights from models of natural action selection. Philos Trans R Soc Lond B Biol Sci. 2007;362(1485):1641–1654. doi: 10.1098/rstb.2007.2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MJ, Seeberger LC, O’Reilly RC. By carrot or by stick: cognitive reinforcement learning in parkinsonism. Science. 2004;306(5703):1940–1943. doi: 10.1126/science.1102941. [DOI] [PubMed] [Google Scholar]
- George O, Mandyam CD, et al. Extended access to cocaine self-administration produces long-lasting prefrontal cortex-dependent working memory impairments. Neuropsychopharmacology. 2008;33(10):2474–82. doi: 10.1038/sj.npp.1301626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluck MA, Myers C, Meeter M. Cortico-hippocampal interaction and adaptive stimulus representation: a neurocomputational theory of associative learning and memory. Neural Netw. 2005;18(9):1265–1279. doi: 10.1016/j.neunet.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Gluck MA, Myers CE. Hippocampal mediation of stimulus representation: a computational theory. Hippocampus. 1993;3(4):491–516. doi: 10.1002/hipo.450030410. [DOI] [PubMed] [Google Scholar]
- Gluck MA, Myers CE, Nicolle MM, Johnson S. Computational models of the hippocampal region: implications for prediction of risk for Alzheimer’s disease in non-demented elderly. Curr Alzheimer Res. 2006;3(3):247–257. doi: 10.2174/156720506777632826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman MB, Mitchell CP. What is the functional significance of hippocampal pathology in schizophrenia? Schizophr Bull. 2004;30(2):367–392. doi: 10.1093/oxfordjournals.schbul.a007086. [DOI] [PubMed] [Google Scholar]
- Goldman MB, Torres IJ, Keedy S, Marlow-O’Connor M, Beenken B, Pilla R. Reduced anterior hippocampal formation volume in hyponatremic schizophrenic patients. Hippocampus. 2007;17(7):554–562. doi: 10.1002/hipo.20292. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. The basal ganglia and chunking of action repertoires. Neurobiol Learn Mem. 1998;70(1–2):119–36. doi: 10.1006/nlme.1998.3843. [DOI] [PubMed] [Google Scholar]
- Guthrie M, Myers CE, Gluck MA. A neurocomputational model of tonic and phasic dopamine in action selection: A comparison with cognitive deficits in Parkinson’s disease. Behav Brain Res. 2009 doi: 10.1016/j.bbr.2008.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grice GR, Davis JD. Effect of concurrent responses on the evocation and generalization of the conditioned eyeblink. J Exp Psychol. 1960;59:391–5. doi: 10.1037/h0044981. [DOI] [PubMed] [Google Scholar]
- Hasselmo M. Runaway Synaptic Modification in Models of Cortex: Implications for Alzheimer’s Disease. Neural Netw 1994 [Google Scholar]
- Hasselmo ME. A computational model of the progression of Alzheimer’s disease. MD Comput. 1997;14(3):181–191. [PubMed] [Google Scholar]
- Hasselmo ME, Wyble BP, Wallenstein GV. Encoding and retrieval of episodic memories: role of cholinergic and GABAergic modulation in the hippocampus. Hippocampus. 1996;6(6):693–708. doi: 10.1002/(SICI)1098-1063(1996)6:6<693::AID-HIPO12>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Heckers S. Neuroimaging studies of the hippocampus in schizophrenia. Hippocampus. 2001;11(5):520–528. doi: 10.1002/hipo.1068. [DOI] [PubMed] [Google Scholar]
- Hoffman RE, McGlashan TH. Neural network models of schizophrenia. Neuroscientist. 2001;7(5):441–54. doi: 10.1177/107385840100700513. [DOI] [PubMed] [Google Scholar]
- Hoffman RE, McGlashan TH. Using a speech perception neural network computer simulation to contrast neuroanatomic versus neuromodulatory models of auditory hallucinations. Pharmacopsychiatry. 2006;39:S54–64. doi: 10.1055/s-2006-931496. [DOI] [PubMed] [Google Scholar]
- Henke K, Buck A, Weber B, Wieser HG. Human hippocampus establishes associations in memory. Hippocampus. 1997;7(3):249–256. doi: 10.1002/(SICI)1098-1063(1997)7:3<249::AID-HIPO1>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Honey RC, Hall G. Acquired equivalence and distinctiveness of cues. J Exp Psychol Anim Behav Process. 1989;15(4):338–346. [PubMed] [Google Scholar]
- Honey RC, Hall G. Acquired equivalence and distinctiveness of cues using a sensory-preconditioning procedure. Q J Exp Psychol B. 1991;43(2):121–135. [PubMed] [Google Scholar]
- Honey RC, Ward-Robinson J. Acquired equivalence and distinctiveness of cues: I. Exploring a neural network approach. J Exp Psychol Anim Behav Process. 2002;28(4):378–387. [PubMed] [Google Scholar]
- Houk JC. Information processing in modular circuits linking basal ganglia and cerebral Cortex. In: Houk JC, Davis JL, Beiser DG, editors. Models of information processing in the basal ganglia. Cambridge, Mass: MIT Press; 1995a. p. xii.p. 382. [Google Scholar]
- Houk JC. A model of how the basal ganglia generate and use neural signals that predict reinforcement. In: Houk JC, Davis JL, Beiser DG, editors. Models of information processing in the basal ganglia. Cambridge, Mass: MIT Press; 1995b. p. xii.p. 382. [Google Scholar]
- Houk JC. Agents of the mind. Biol Cybern. 2005;92(6):427–437. doi: 10.1007/s00422-005-0569-8. [DOI] [PubMed] [Google Scholar]
- Huerta PT, Lisman JE. Heightened synaptic plasticity of hippocampal CA1 neurons during a cholinergically induced rhythmic state. Nature. 1993;364(6439):723–725. doi: 10.1038/364723a0. [DOI] [PubMed] [Google Scholar]
- Iordanova MD, Killcross AS, Honey RC. Role of the medial prefrontal cortex in acquired distinctiveness and equivalence of cues. Behav Neurosci. 2007;121(6):1431–1436. doi: 10.1037/0735-7044.121.6.1431. [DOI] [PubMed] [Google Scholar]
- Jack CR, Jr, Petersen RC, Xu Y, O’Brien PC, Smith GE, Ivnik RJ, et al. Rates of hippocampal atrophy correlate with change in clinical status in aging and AD. Neurology. 2000;55(4):484–489. doi: 10.1212/wnl.55.4.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahanshahi M, Wilkinson L, Gahir H, Dharminda A, Lagnado DA. Medication impairs probabilistic classification learning in Parkinson’s disease. Neuropsychologia. 2009 doi: 10.1016/j.neuropsychologia.2009.12.010. [DOI] [PubMed] [Google Scholar]
- Jellinger KA. Post mortem studies in Parkinson’s disease--is it possible to detect brain areas for specific symptoms? J Neural Transm Suppl. 1999;56:1–29. doi: 10.1007/978-3-7091-6360-3_1. [DOI] [PubMed] [Google Scholar]
- Jog MS, Kubota Y, Connolly CI, Hillegaart V, Graybiel AM. Building neural representations of habits. Science. 1999;286(5445):1745–1749. doi: 10.1126/science.286.5445.1745. [DOI] [PubMed] [Google Scholar]
- Keri S. Interactive memory systems and category learning in schizophrenia. Neurosci Biobehav Rev. 2008;32(2):206–218. doi: 10.1016/j.neubiorev.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Keri S, Juhasz A, Rimanoczy A, Szekeres G, Kelemen O, Cimmer C, et al. Habit learning and the genetics of the dopamine D3 receptor: evidence from patients with schizophrenia and healthy controls. Behav Neurosci. 2005;119(3):687–693. doi: 10.1037/0735-7044.119.3.687. [DOI] [PubMed] [Google Scholar]
- Keri S, Kelemen O, Szekeres G, Bagoczky N, Erdelyi R, Antal A, et al. Schizophrenics know more than they can tell: probabilistic classification learning in schizophrenia. Psychol Med. 2000;30(1):149–155. doi: 10.1017/s0033291799001403. [DOI] [PubMed] [Google Scholar]
- Keri S, Nagy O, Kelemen O, Myers CE, Gluck MA. Dissociation between medial temporal lobe and basal ganglia memory systems in schizophrenia. Schizophr Res. 2005;77(2–3):321–328. doi: 10.1016/j.schres.2005.03.024. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Hopkins RO. Short-term memory for duration and distance in humans: role of the hippocampus. Neuropsychology. 2001;15(1):58–68. doi: 10.1037//0894-4105.15.1.58. [DOI] [PubMed] [Google Scholar]
- Kish SJ, Shannak K, Hornykiewicz O. Uneven pattern of dopamine loss in the striatum of patients with idiopathic Parkinson’s disease. Pathophysiologic and clinical implications. N Engl J Med. 1988;318(14):876–880. doi: 10.1056/NEJM198804073181402. [DOI] [PubMed] [Google Scholar]
- Knowlton BJ, Mangels JA, Squire LR. A neostriatal habit learning system in humans. Science. 1996;273(5280):1399–1402. doi: 10.1126/science.273.5280.1399. [DOI] [PubMed] [Google Scholar]
- Kukolja J, Thiel CM, Fink GR. Cholinergic stimulation enhances neural activity associated with encoding but reduces neural activity associated with retrieval in humans. J Neurosci. 2009;29(25):8119–8128. doi: 10.1523/JNEUROSCI.0203-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman JE, Grace AA. The hippocampal-VTA loop: controlling the entry of information into long-term memory. Neuron. 2005;46(5):703–713. doi: 10.1016/j.neuron.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Otmakhova NA. Storage, recall, and novelty detection of sequences by the hippocampus: elaborating on the SOCRATIC model to account for normal and aberrant effects of dopamine. Hippocampus. 2001;11(5):551–568. doi: 10.1002/hipo.1071. [DOI] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. Hippocampal dysfunction and disruption of dopamine system regulation in an animal model of schizophrenia. Neurotox Res. 2008;14(2–3):97–104. doi: 10.1007/BF03033801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavaddat N, Sahakian BJ, Hutchinson PJ, Kirkpatrick PJ. Cognition following subarachnoid hemorrhage from anterior communicating artery aneurysm: relation to timing of surgery. J Neurosurg. 1999;91(3):402–407. doi: 10.3171/jns.1999.91.3.0402. [DOI] [PubMed] [Google Scholar]
- McAuley JD, Stewart AL, Webber ES, Cromwell HC, Servatius RJ, Pang KC. Wistar-Kyoto rats as an animal model of anxiety vulnerability: support for a hypervigilance hypothesis. Behav Brain Res. 2009;204(1):162–168. doi: 10.1016/j.bbr.2009.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEchron MD, Disterhoft JF. Sequence of single neuron changes in CA1 hippocampus of rabbits during acquisition of trace eyeblink conditioned responses. J Neurophysiol. 1997;78(2):1030–1044. doi: 10.1152/jn.1997.78.2.1030. [DOI] [PubMed] [Google Scholar]
- McEchron MD, Disterhoft JF. Hippocampal encoding of non-spatial trace conditioning. Hippocampus. 1999;9(4):385–396. doi: 10.1002/(SICI)1098-1063(1999)9:4<385::AID-HIPO5>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Meeter M, Murre JMM. TraceLink: A model of Consolidation and Amnesia. cognitive neuropsychology. 2005 doi: 10.1080/02643290442000194. [DOI] [PubMed] [Google Scholar]
- Meeter M, Shohamy D, Myers CE. Acquired equivalence changes stimulus representations. J Exp Anal Behav. 2009;91(1):127–141. doi: 10.1901/jeab.2009.91-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mewaldt SP, Ghoneim MM. The effects and interactions of scopolamine, physostigmine and methamphetamine on human memory. Pharmacol Biochem Behav. 1979;10(2):205–210. doi: 10.1016/0091-3057(79)90088-1. [DOI] [PubMed] [Google Scholar]
- Moustafa AA, Gluck MA. A Neurocomputational Model of Dopamine and Prefrontal-Striatal Interactions during Multicue Category Learning by Parkinson’s Patients. J Cogn Neurosci. 2010 doi: 10.1162/jocn.2010.21420. [DOI] [PubMed] [Google Scholar]
- Moustafa AA, Gluck MA, Myers CE. A neurocomputational model of classical conditioning phenomena: A putative role of the parahippocampal region. Brain Research. 2009 doi: 10.1016/j.brainres.2009.04.020. [DOI] [PubMed] [Google Scholar]
- Moustafa AA, Maida AS. Using TD learning to simulate working memory performance in a model of the prefrontal cortex and basal ganglia. Cognitive Systems Research. 2007;8:262–281. [Google Scholar]
- Moustafa AA, Sherman SJ, Frank MJ. A dopaminergic basis for working memory, learning and attentional shifting in Parkinsonism. Neuropsychologia. 2008;46(13):3144–3156. doi: 10.1016/j.neuropsychologia.2008.07.011. [DOI] [PubMed] [Google Scholar]
- Myer CE, Bryant D, DeLuca J, Gluck MA. Dissociating basal forebrain and medial temporal amnesic syndromes: insights from classical conditioning. Integr Physiol Behav Sci. 2002;37(2):85–102. doi: 10.1007/BF02688822. [DOI] [PubMed] [Google Scholar]
- Myers CE, Deluca J, Hopkins RO, Gluck MA. Conditional discrimination and reversal in amnesia subsequent to hypoxic brain injury or anterior communicating artery aneurysm rupture. Neuropsychologia. 2006;44(1):130–139. doi: 10.1016/j.neuropsychologia.2005.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers CE, DeLuca J, Schultheis MT, Schnirman GM, Ermita BR, Diamond B, et al. Impaired delay eyeblink classical conditioning in individuals with anterograde amnesia resulting from anterior communicating artery aneurysm rupture. Behav Neurosci. 2001;115(3):560–570. [PubMed] [Google Scholar]
- Myers CE, Ermita BR, Harris K, Hasselmo M, Solomon P, Gluck MA. A computational model of cholinergic disruption of septohippocampal activity in classical eyeblink conditioning. Neurobiol Learn Mem. 1996;66(1):51–66. doi: 10.1006/nlme.1996.0043. [DOI] [PubMed] [Google Scholar]
- Myers CE, Gluck MA, Granger R. Dissociation of hippocampal and entorhinal function in associative learning: a computational approach. Psychobiology. 1995;23(2):116–138. [Google Scholar]
- Myers CE, Hopkins RO, DeLuca J, Moore NB, Wolansky LJ, Sumner JM, et al. Learning and generalization deficits in patients with memory impairments due to anterior communicating artery aneurysm rupture or hypoxic brain injury. Neuropsychology. 2008;22(5):681–686. doi: 10.1037/0894-4105.22.5.681. [DOI] [PubMed] [Google Scholar]
- Myers CE, Kluger A, Golomb J, Ferris S, de Leon MJ, Schnirman G, et al. Hippocampal atrophy disrupts transfer generalization in nondemented elderly. J Geriatr Psychiatry Neurol. 2002;15(2):82–90. doi: 10.1177/089198870201500206. [DOI] [PubMed] [Google Scholar]
- Myers CE, Shohamy D, Gluck MA, Grossman S, Kluger A, Ferris S, et al. Dissociating hippocampal versus basal ganglia contributions to learning and transfer. J Cogn Neurosci. 2003;15(2):185–193. doi: 10.1162/089892903321208123. [DOI] [PubMed] [Google Scholar]
- Nauta WJH, Feirtag M. Fundamental neuroanatomy. New York: Freeman; 1986. [Google Scholar]
- Nolte J. The human brain: An introduction to its functional anatomy. 3. St. Louis, MO: Mosby-Year Book; 1993. [Google Scholar]
- O’Connor MG, Lafleche GM. Retrograde amnesia in patients with rupture and surgical repair of anterior communicating artery aneurysms. J Int Neuropsychol Soc. 2004;10(2):221–229. doi: 10.1017/S1355617704102087. [DOI] [PubMed] [Google Scholar]
- Oertel V, Rotarska-Jagiela A, van de Ven VG, Haenschel C, Maurer K, Linden DE. Visual hallucinations in schizophrenia investigated with functional magnetic resonance imaging. Psychiatry Res. 2007;156(3):269–273. doi: 10.1016/j.pscychresns.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Orsetti M, Casamenti F, Pepeu G. Enhanced acetylcholine release in the hippocampus and cortex during acquisition of an operant behavior. Brain Res. 1996;724(1):89–96. doi: 10.1016/0006-8993(96)00292-2. [DOI] [PubMed] [Google Scholar]
- Perlstein WM, Carter CS, Noll DC, Cohen JD. Relation of prefrontal cortex dysfunction to working memory and symptoms in schizophrenia. Am J Psychiatry. 2001;158(7):1105–1113. doi: 10.1176/appi.ajp.158.7.1105. [DOI] [PubMed] [Google Scholar]
- Polgar P, Farkas M, Nagy O, Kelemen O, Rethelyi J, Bitter I, et al. How to find the way out from four rooms? The learning of “chaining” associations may shed light on the neuropsychology of the deficit syndrome of schizophrenia. Schizophr Res. 2008;99(1–3):200–207. doi: 10.1016/j.schres.2007.06.027. [DOI] [PubMed] [Google Scholar]
- Port RL, Patterson MM. Fimbrial lesions and sensory preconditioning. Behav Neurosci. 1984;98(4):584–589. doi: 10.1037//0735-7044.98.4.584. [DOI] [PubMed] [Google Scholar]
- Rametti G, Junque C, Vendrell P, Catalan R, Penades R, Bargallo N, et al. Hippocampal underactivation in an fMRI study of word and face memory recognition in schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2009;259(4):203–211. doi: 10.1007/s00406-008-0852-5. [DOI] [PubMed] [Google Scholar]
- Reynolds JN, Wickens JR. Dopamine-dependent plasticity of corticostriatal synapses. Neural Netw. 2002;15(4–6):507–521. doi: 10.1016/s0893-6080(02)00045-x. [DOI] [PubMed] [Google Scholar]
- Rokers B, Mercado E, 3rd, Allen MT, Myers CE, Gluck MA. A connectionist model of septohippocampal dynamics during conditioning: closing the loop. Behav Neurosci. 2002;116(1):48–62. [PubMed] [Google Scholar]
- Rolls ET, Loh M, Deco G, Winterer G. Computational models of schizophrenia and dopamine modulation in the prefrontal cortex. Nat Rev Neurosci. 2008;9(9):696–709. doi: 10.1038/nrn2462. [DOI] [PubMed] [Google Scholar]
- Rossato JI, Bevilaqua LR, et al. Dopamine controls persistence of long-term memory storage. Science. 2009;325(5943):1017–20. doi: 10.1126/science.1172545. [DOI] [PubMed] [Google Scholar]
- Schmajuk N. Brain-behaviour relationships in latent inhibition: a computational model. Neurosci Biobehav Rev. 2005;29(6):1001–1020. doi: 10.1016/j.neubiorev.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275(5306):1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Shohamy D, Myers CE, Geghman KD, Sage J, Gluck MA. L-dopa impairs learning, but spares generalization, in Parkinson’s disease. Neuropsychologia. 2006;44(5):774–784. doi: 10.1016/j.neuropsychologia.2005.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shohamy D, Myers CE, Kalanithi J, Gluck MA. Basal ganglia and dopamine contributions to probabilistic category learning. Neurosci Biobehav Rev. 2008;32(2):219–236. doi: 10.1016/j.neubiorev.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shohamy D, Wagner AD. Integrating memories in the human brain: hippocampal-midbrain encoding of overlapping events. Neuron. 2008;60(2):378–389. doi: 10.1016/j.neuron.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard S, Rouleau I, Brosseau J, Laframboise M, Bojanowsky M. Impact of executive dysfunctions on episodic memory abilities in patients with ruptured aneurysm of the anterior communicating artery. Brain Cogn. 2003;53(2):354–358. doi: 10.1016/s0278-2626(03)00142-8. [DOI] [PubMed] [Google Scholar]
- Simon BB, Knuckley B, Powell DA. Galantamine facilitates acquisition of a trace-conditioned eyeblink response in healthy, young rabbits. Learn Mem. 2004;11(1):116–122. doi: 10.1101/lm.66204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snitz BE, MacDonald A, 3rd, Cohen JD, Cho RY, Becker T, Carter CS. Lateral and medial hypofrontality in first-episode schizophrenia: functional activity in a medication-naive state and effects of short-term atypical antipsychotic treatment. Am J Psychiatry. 2005;162(12):2322–2329. doi: 10.1176/appi.ajp.162.12.2322. [DOI] [PubMed] [Google Scholar]
- Spoletini I, Cherubini A, Banfi G, Rubino IA, Peran P, Caltagirone C, et al. Hippocampi, Thalami, and Accumbens Microstructural Damage in Schizophrenia: A Volumetry, Diffusivity, and Neuropsychological Study. Schizophr Bull. 2009 doi: 10.1093/schbul/sbp058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suri RE, Schultz W. Learning of sequential movements by neural network model with dopamine-like reinforcement signal. Exp Brain Res. 1998;121(3):350–354. doi: 10.1007/s002210050467. [DOI] [PubMed] [Google Scholar]
- Suri RE, Schultz W. A neural network model with dopamine-like reinforcement signal that learns a spatial delayed response task. Neuroscience. 1999;91(3):871–890. doi: 10.1016/s0306-4522(98)00697-6. [DOI] [PubMed] [Google Scholar]
- Sutton RS, Barto AG. A temporal-difference model of classical conditioning. Paper presented at the Proceedings of the Ninth Annual Conference of the Cognitive Science Society.1987. [Google Scholar]
- Sutton RS, Barto AG. Time-derivative models of Pavlovian reinforcement. In: Moore MGaJ., editor. Learning and computational neuroscience: Foundations of adaptive networks. Cambridge, MA: MIT Press; 1990. pp. 497–537. [Google Scholar]
- Talamini LM, Meeter M. Dominance of objects over context in a mediotemporal lobe model of schizophrenia. PLoS One. 2009;4(8):e6505. doi: 10.1371/journal.pone.0006505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng KY, Chambers RA, Lipska BK. The neonatal ventral hippocampal lesion as a heuristic neurodevelopmental model of schizophrenia. Behav Brain Res. 2009;204(2):295–305. doi: 10.1016/j.bbr.2008.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnock M, Becker S. A neural network model of hippocampal-striatal-prefrontal interactions in contextual conditioning. Brain Res. 2007 doi: 10.1016/j.brainres.2007.06.078. [DOI] [PubMed] [Google Scholar]
- Vadhan NP, Myers CE, Rubin E, Shohamy D, Foltin RW, Gluck MA. Stimulus-response learning in long-term cocaine users: acquired equivalence and probabilistic category learning. Drug Alcohol Depend. 2008;93(1–2):155–162. doi: 10.1016/j.drugalcdep.2007.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waltz JA, Frank MJ, Robinson BM, Gold JM. Selective reinforcement learning deficits in schizophrenia support predictions from computational models of striatal-cortical dysfunction. Biol Psychiatry. 2007;62(7):756–764. doi: 10.1016/j.biopsych.2006.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Zang Y, He Y, Liang M, Zhang X, Tian L, et al. Changes in hippocampal connectivity in the early stages of Alzheimer’s disease: evidence from resting state fMRI. Neuroimage. 2006;31(2):496–504. doi: 10.1016/j.neuroimage.2005.12.033. [DOI] [PubMed] [Google Scholar]
- Weible AP, Oh MM, Lee G, Disterhoft JF. Galantamine facilitates acquisition of hippocampus-dependent trace eyeblink conditioning in aged rabbits. Learn Mem. 2004;11(1):108–115. doi: 10.1101/lm.69804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weickert TW, Terrazas A, Bigelow LB, Malley JD, Hyde T, Egan MF, et al. Habit and skill learning in schizophrenia: evidence of normal striatal processing with abnormal cortical input. Learn Mem. 2002;9(6):430–442. doi: 10.1101/lm.49102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiler JA, Bellebaum C, Brune M, Juckel G, Daum I. Impairment of probabilistic reward-based learning in schizophrenia. Neuropsychology. 2009;23(5):571–580. doi: 10.1037/a0016166. [DOI] [PubMed] [Google Scholar]
- Weiler JA, Bellebaum C, Daum I. Aging affects acquisition and reversal of reward-based associative learning. Learn Mem. 2008;15(4):190–197. doi: 10.1101/lm.890408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger DR. Cell biology of the hippocampal formation in schizophrenia. Biol Psychiatry. 1999;45(4):395–402. doi: 10.1016/s0006-3223(98)00331-x. [DOI] [PubMed] [Google Scholar]
- Wickens JR, Begg AJ, Arbuthnott GW. Dopamine reverses the depression of rat corticostriatal synapses which normally follows high-frequency stimulation of cortex in vitro. Neuroscience. 1996;70(1):1–5. doi: 10.1016/0306-4522(95)00436-m. [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Lehr MA, Li JG, Liu-Chen LY. Young and older good learners have higher levels of brain nicotinic receptor binding. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright RA, Boeve BF, Malec JF. Amnesia after basal forebrain damage due to anterior communicating artery aneurysm rupture. J Clin Neurosci. 1999;6(6):511–515. doi: 10.1016/s0967-5868(99)90013-9. [DOI] [PubMed] [Google Scholar]