Abstract
Meta-analyses evaluating the association between the serotonin transporter polymorphism (5-HTTLPR) with neuroticism and depression diagnosis as phenotypes have been inconclusive. We examined a gene–environment interaction on a cognitive vulnerability marker of depression, cognitive reactivity (CR) to sad mood. A total of 250 university students of European ancestry were genotyped for the 5-HTTLPR, including SNP rs25531, a polymorphism of the long allele. Association analysis was performed for neuroticism, CR and depression diagnosis (using a self-report measure). As an environmental pathogen, self-reported history of childhood emotional abuse was measured because of its strong relationship with depression. Participants with the homozygous low expressing genotype had high CR if they had experienced childhood emotional maltreatment but low CR if they did not have such experience. This interaction was strongest on the Rumination subscale of the CR measure. The interaction was not significant with neuroticism or depression diagnosis as outcome measures. Our results show that 5-HTTLPR is related to cognitive vulnerability to depression. Our findings provide evidence for a differential susceptibility genotype rather than a vulnerability genotype, possibly because of the relatively low levels of abuse in our sample. The selection of phenotype and environmental contributor is pivotal in investigating gene–environment interactions in psychiatric disorders.
Keywords: childhood emotional abuse, cognitive reactivity, depression, neuroticism, serotonin transporter gene
One of the most studied polymorphisms in psychiatry is in the promoter region of the serotonin transporter gene (5-HTTLPR). A recent meta-analysis concluded that there is no association between this polymorphism and depression, and also no interaction effect with stressful life events (Risch et al. 2009). This meta-analysis has major methodological limitations (Rutter et al. 2009), and an updated, comprehensive review provides further evidence of the association (Uher & McGuffin 2010). The inconsistencies in findings may be as a result of the heterogeneity of both the phenotype (depression) and the environment (life events) (Lotrich & Lenze 2009). Some of the largest studies may have been atypical because these did not show the well-documented association between stressful life events and depression (Gillespie et al. 2005; Surtees et al. 2006). Furthermore, depression as defined by DSM-IV is a heterogeneous concept and therefore may not be the most promising outcome for genetic research (Jacobs et al. 2006). Continued investigation of the relationship between 5-HTTLPR and depression is also warranted because of its association with emotion regulation and social cognition (Canli et al. 2007) and with amygdala activity (Munafo et al. 2008).
Studies on the association of 5-HTTLPR with the personality trait of neuroticism have also produced inconsistent results (Munafo et al. 2009). Most studies have failed to take the moderating effects of childhood trauma or life events into consideration. Moreover, longitudinal research has shown that neuroticism scores may simply reflect the average level of distress over a protracted period (Ormel et al. 2004), and thus may lack specificity for depression.
Cognitive reactivity (CR) to sad mood may be a more promising vulnerability measure of depression than neuroticism, and does not have the limitations of a categorical diagnosis of depression. The concept of CR is based on the differential activation theory, which states that conditioned associations that are formed between depressed mood states and dysfunctional cognitions remain intact during periods of remission (Teasdale 1988). These cognitions may then be re-activated by relatively small changes in mood, and may even be present in vulnerable samples before the onset of a first episode of depression. The extent to which maladaptive cognitions are triggered by (non-pathological) low mood is referred to as CR (Scher et al. 2005). Experimental studies using sad mood inductions have shown that formerly depressed patients have higher CR than never-depressed individuals (Segal et al. 1999). High CR increases the risk of depressive relapse, independently from prior treatment (Segal et al. 2006). A self-report measure of CR [the Leiden Index of Depression Sensitivity-Revised (LEIDS-R)] contains several subscales that cover several dimensions of maladaptive cognitions that characterize the vulnerable depressive mind (see Methods). It reliably distinguishes between previously depressed and never-depressed groups and also correlates highly with CR as measured with a mood induction procedure (Van der Does 2002). The LEIDS-R, but not neuroticism, predicts response to serotonin depletion (Booij & Van der Does 2007), which is a potential endophenotype of depression (Hasler et al. 2004). LEIDS-R scores were also found to have a unique contribution to the prediction of depression, over and above trait level of depressive rumination (Moulds et al. 2008). Furthermore, LEIDS-R scores mediate the relationship between neuroticism and depressive symptomatology in both never-depressed and previously depressed groups (Barnhofer & Chittka 2010).
We investigated whether the association of 5-HTTLPR with CR would be stronger than with neuroticism or with depression diagnosis. We hypothesized that this relationship would be moderated by childhood abuse, in particular childhood emotional abuse (CEA). Rose and Abramson (1992) suggested that emotional abuse is more likely to contribute to a depressogenic cognitive style because the child receives negative cognitions by the abuser in a direct manner: ‘you are worthless'. In this way, the child tends to adopt a general negative attributional style, which contributes to the development of depression. For other types of maltreatment, the child has to make his or her own attributions, which may allow more room for less global and more external attributions. Empirical studies support this theory and show that CEA is differentially associated with depression compared with other types of maltreatment (Chapman et al. 2004; Gibb et al. 2001, 2003, 2007; Hovens et al. in press). Prospective studies also indicate that CEA predicts symptoms or diagnosis of depression (Gibb et al. 2001; Hankin 2005; Liu et al. 2009). Furthermore, there is evidence that the relationship between CEA and depressive symptoms is mediated by cognitive factors (Gibb et al. 2001; Hankin 2005; Raes & Hermans 2008; Wright et al. 2009).
Methods
Participants and procedure
We tested the Gene × Environment (G × E) hypothesis in 250 university students of European ancestry. The research was approved by the Ethics Committee of the Leiden University Medical Center in the Netherlands and all participants gave written informed consent before participating in the study.
DNA was obtained using the Oragene Self-Collection Kit—DISC format (DNA Genotek Inc, Ottawa, ON, Canada); 200 µl of saliva was collected in lysis buffer (100 mm NaCl, 10 mm EDTA, 10 mm Tris pH 8, 0.1 mg/ml proteinase K and 0.5% w/v SDS) until further processing.
DNA isolation
Genomic DNA was isolated from the samples using the Chemagic kit on a Chemagen Module I workstation (Chemagen Biopolymer-Technologie AG, Baesweiler, Germany). DNA concentrations were quantified by OD260 measurement and by agarose gel electrophoresis. The average yield was approximately 4 µg of genomic DNA per sample.
Polymerase chain reaction amplification
The region of interest from the serotonin transporter (5-HTT) gene was amplified by triplex polymerase chain reaction (PCR) using the following primers: a FAM-labelled primer HTTLPR-FWFAM 5′-TCCTCCGCTTTGGCGCCTCTTCC-3′, and a reverse primer HTTLPR-RV 5′-TGGGGGTTGCAGGGGAGATCCTG-3′. Typical PCR reactions contained between 10 and 100 ng of genomic DNA template, and 10 pmol of forward and reverse primer. PCR was carried out in the presence of 5% DMSO with 0.5 U of BioThermAB polymerase (GeneCraft, Munster, Germany) in a total volume of 30 µl using the following cycling conditions: initial denaturation step of 5 min at 95°C, followed by 40 cycles of 30 seconds at 96°C, 30 seconds at 61°C, 60 seconds 72°C and a final extension step of 10 min at 72°C. After PCR, 5 µl of the sample was subjected to restriction digestion with the enzyme HpaII in a total volume of 20 µl. Restriction was incubated for 3 h at 37°C.
Analysis of PCR products
One microlitre of PCR product before and after restriction digestion was mixed with LIZ-500 size standard and formamide and run in two separate lanes on an AB 3100 genetic analyser set up for genotyping with 50 cm capillaries. Results were analysed using Genescan software version 3.7 (Applied Biosystems, Carlsbad, CA, USA), and alleles were scored visually according to the following scheme: Uncut: S, 469 bp; L, 512 bp. Cut: Sg, 402 + 67 bp; Lg, 402 + 110 bp.
Genotype analysis failed for two participants, yielding 248 samples for association analysis. Genotype frequencies were as follows: SS, 16.9%; SLg, 5.2%; LgLg, 0.8%; LaLg, 8.9%; SL, 37.1%; LaLa, 31.1%. Participants were divided on the basis of the triallelic classification (Lg alleles were collapsed with ‘s’ variants according to evidence of similar functionality) into three genotype groups: S′S′ (n = 57); L′S′ (n = 114); L′L′ (n = 77). Genotype frequencies were consistent with Hardy–Weinberg Equilibrium χ2(1) = 1.55, P = 0.21.
Measures
CR was measured using the LEIDS-R (Van der Does 2002, 2005; Williams et al. 2008). Participants are asked to indicate whether and how their thinking patterns change when they experience mild dysphoria, by scoring each item on a 5-point Likert scale ranging from 0 ‘not at all’ to 4 ‘very strongly’ applicable. The LEIDS-R has 34 items and covers six subscales: Hopelessness/Suicidality (‘When I feel down, I more often feel hopeless about everything’); Acceptance/ Coping (‘When I am sad, I feel more like myself’); Aggression (‘When I feel down, I lose my temper more easily’); Control/Perfectionism (‘When in a sad mood, I become more bothered by perfectionism’); Risk Aversion (‘When I feel down, I take fewer risks'); Rumination (‘When I feel sad, I spend more time thinking about the possible causes of my moods'). Internal consistency (Cronbach's α) for the LEIDS-R total was 0.89, and ranged between 0.62 and 0.83 for the subscales.
Neuroticism was assessed with the Dutch/Flemish authorized translation of the 60-item NEO PI-R (Hoekstra et al. 2007); Cronbach's α was 0.88. The presence of current and past depression was assessed with the Major Depression Questionnaire. The measure covers all DSM-IV diagnostic criteria for current and past major depression. Consistency of this questionnaire with diagnoses based on structured clinical interview for DSM disorders (SCID) has been examined in a sample of 39 individuals: Sensitivity = 100%; Specificity = 75%; Positive Predictive Value = 79%; Negative Predictive Value = 100%; overall κ = 0.75) (Williams et al. 2008).
CEA was measured with the Childhood Trauma Questionnaire (28-item version), which is a screening measure for maltreatment histories in both clinical and non-referred groups (Bernstein & Fink 1998). The emotional abuse subscale refers to verbal assaults on a child's sense of worth or well-being, or any humiliating and demeaning behaviour directed towards a child by an older person (Bernstein & Fink 1998). Total CEA has a range of 5 (no abuse) to 25, and internal consistency in this sample was α = 0.80. An example item from the CEA scale is ‘People in my family said hurtful or insulting things to me’.
Current symptoms of anxiety and depression were used as a covariate. An authorized Dutch translation of the Hospital Anxiety and Depression Scale (HADS), a 14-item self-report screening scale for anxiety and depression, was used (Spinhoven et al. 1997). Internal consistency for the total score was α = 0.87.
Statistical analysis
SPSS 16.0 was used for data analysis. Data were screened for accuracy and normal distribution assumptions. Square root transformations corrected outlying data (z > 3) and heterogeneity of variance on the LEIDS-R total and subscales. Figures report untransformed values. We used analysis of variance (ANOVA) to detect main effects and interactions on continuous outcomes—Univariate ANOVA was used for the LEIDS-R and neuroticism total scores and a separate multivariate ANOVA for the LEIDS-R subscales, because of high correlations with the total score. Partial eta squared (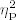 ) is reported as an estimate of effect size. We used logistic regression for depression diagnosis (none vs. lifetime) as an outcome. Independent variables were the 5-HTTLPR genotype (coded 1 for S′S′, coded 2 for S′L′, coded 3 for L′L′) and the CEA (median split: above score 6 coded as 1, below or equal to score 6 coded as 0). Additional analyses were conducted with current depression and anxiety (HADS total score) as covariates, as current levels of symptomatology can affect CR (Williams et al. 2008). We re-ran the analyses with physical abuse for purposes of adversity specificity, and also with the biallelic genotype classification.
) is reported as an estimate of effect size. We used logistic regression for depression diagnosis (none vs. lifetime) as an outcome. Independent variables were the 5-HTTLPR genotype (coded 1 for S′S′, coded 2 for S′L′, coded 3 for L′L′) and the CEA (median split: above score 6 coded as 1, below or equal to score 6 coded as 0). Additional analyses were conducted with current depression and anxiety (HADS total score) as covariates, as current levels of symptomatology can affect CR (Williams et al. 2008). We re-ran the analyses with physical abuse for purposes of adversity specificity, and also with the biallelic genotype classification.
Results
Table 1 displays the sample characteristics. Among genotype groups, no differences were found with respect to age (F2,245 = 1.2, P = 0.30) and gender distribution [χ2(2) = 1.11, P = 0.58]. No between-group differences were found on depression diagnoses [χ2(4) = 1.68, P = 0.80]. There were also no differences between genotypes in current levels of anxiety (HADS anxiety subscale) (P = 0.94), or depression (HADS depression subscale) (P = 0.61), or total symptomatology (HADS total) (F2,245 = 0.20, P = 0.82). Genotype groups also did not differ on CEA scores (F2,245 = 2.44, P = 0.09) (post hoc tests also non-significant).
Table 1.
Participant characteristics by 5-HTTLPR genotype (N = 248)
| Genotype | S′S′ (N = 57) | S′L′ (N = 114) | L′L′ (N = 77) |
|---|---|---|---|
| Age (mean ± SD) | 23.3 ± 6.1 | 22.5 ± 4.2 | 22.0 ± 4.5 |
| Females | 77.2% | 72.8% | 79.2% |
| Depression diagnosis (%) | |||
| No lifetime MDD | 56.1 | 61.4 | 63.6 |
| Past MDD | 38.6 | 31.6 | 28.6 |
| Current MDD | 5.3 | 7.0 | 7.8 |
| Current symptoms (mean ± SD) | |||
| HADS total | 9.4 ± 5.8 | 8.8 ± 5.9 | 9.1 ± 5.3 |
| HADS depression | 2.9 ± 3.1 | 2.5 ± 2.8 | 2.8 ± 3.0 |
| HADS anxiety | 6.4 ± 3.3 | 6.3 ± 3.7 | 6.2 ± 3.0 |
| Childhood emotional abuse (mean ± SD) | 8.2 ± 3.1 | 7.5 ± 3.4 | 7.0 ± 2.9 |
HADS, Hospital Anxiety Depression Scale; MDD, major depressive disorder.
ANOVA yielded a significant interaction between genotype and emotional abuse on CR (F2,242 = 3.21, P = 0.04) (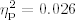 ). The S′S′ genotype scored the lowest in a low abuse environment (Fig. 1a) (F2,129 = 3.4; P = 0.035). Post hoc Tukey test showed that when CEA was low, the S′S′ genotype had significantly lower scores than the L′L′ genotype (P = 0.03). When CEA was high, differences between genotype groups failed to reach significance. Furthermore, CEA was associated with higher CR scores (F1,242 = 6.56, P = 0.01) (
). The S′S′ genotype scored the lowest in a low abuse environment (Fig. 1a) (F2,129 = 3.4; P = 0.035). Post hoc Tukey test showed that when CEA was low, the S′S′ genotype had significantly lower scores than the L′L′ genotype (P = 0.03). When CEA was high, differences between genotype groups failed to reach significance. Furthermore, CEA was associated with higher CR scores (F1,242 = 6.56, P = 0.01) (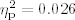 ), but there was no direct association between genotype and CR (F2,242 = 0.492, P = 0.61) (
), but there was no direct association between genotype and CR (F2,242 = 0.492, P = 0.61) (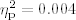 ). The same interaction was also significant for the Rumination subscale of the LEIDS-R (F2,242 = 4.143, P = 0.017) (
). The same interaction was also significant for the Rumination subscale of the LEIDS-R (F2,242 = 4.143, P = 0.017) (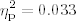 ). The S′S′ genotype again showed the highest scores under high childhood emotional maltreatment, but the lowest scores under low maltreatment. Post hoc tests for each maltreatment group again showed that when CEA was low, the S′S′ genotype had significantly lower scores than the L′L′ (P = 0.02); no other significant differences were found. A similar interaction was marginally significant for the Risk Aversion subscale (F2,242 = 2.920, P = 0.056) (
). The S′S′ genotype again showed the highest scores under high childhood emotional maltreatment, but the lowest scores under low maltreatment. Post hoc tests for each maltreatment group again showed that when CEA was low, the S′S′ genotype had significantly lower scores than the L′L′ (P = 0.02); no other significant differences were found. A similar interaction was marginally significant for the Risk Aversion subscale (F2,242 = 2.920, P = 0.056) (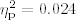 ). After entering current symptoms of anxiety and depression as a covariate, the interactions became stronger and were all significant (P < 0.05). We examined correlations between CEA and CR for each genotype separately. Within the S′S′ genotype, we found significant correlations between CEA and the LEIDS-R total score (r = 0.28, P = 0.04), and with the Rumination subscale (r = 0.30, P = 0.03). Within the S′L′ genotype, we found a significant correlation only with the total score (r = 0.20, P = 0.03). There were no significant correlations within the L′L′ genotype group.
). After entering current symptoms of anxiety and depression as a covariate, the interactions became stronger and were all significant (P < 0.05). We examined correlations between CEA and CR for each genotype separately. Within the S′S′ genotype, we found significant correlations between CEA and the LEIDS-R total score (r = 0.28, P = 0.04), and with the Rumination subscale (r = 0.30, P = 0.03). Within the S′L′ genotype, we found a significant correlation only with the total score (r = 0.20, P = 0.03). There were no significant correlations within the L′L′ genotype group.
Figure 1.
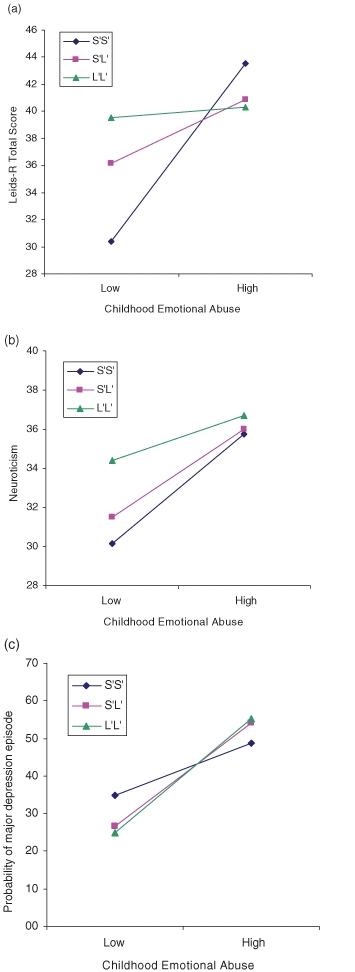
Gene–environment (G × E) interactions on the three depression-related outcomes. (a) G × E interaction on cognitive reactivity (LEIDS-R total score), P = 0.04; (b) G × E interaction on neuroticism, P = 0.54; (c) G × E interaction on depression diagnosis (proportion), P = 0.59.
We found no significant G × E interaction on neuroticism (F2,242 = 0.62, P = 0.54) (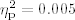 ) (Fig. 1b) with or without covariates. There was a main effect of emotional abuse (F1,242 = 12.39, P = 0.001) (
) (Fig. 1b) with or without covariates. There was a main effect of emotional abuse (F1,242 = 12.39, P = 0.001) (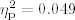 ), but no main effect of genotype (F2,242 = 1,62, P = 0.20) (
), but no main effect of genotype (F2,242 = 1,62, P = 0.20) (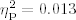 ). Using probability of depression diagnosis (current and/or past) as outcome, we also found a main effect of emotional abuse (P = 0.009) but no main effect of genotype and no interaction (P > 0.58) (Nagelkerke R2 = 0.094) (Fig. 1c). To determine specificity of adversity, main analyses were run with physical abuse, rather than emotional abuse, as the environmental contributor. All G × E interactions were non-significant for all outcome measures.
). Using probability of depression diagnosis (current and/or past) as outcome, we also found a main effect of emotional abuse (P = 0.009) but no main effect of genotype and no interaction (P > 0.58) (Nagelkerke R2 = 0.094) (Fig. 1c). To determine specificity of adversity, main analyses were run with physical abuse, rather than emotional abuse, as the environmental contributor. All G × E interactions were non-significant for all outcome measures.
Biallelic analyses
Genotype groups were re-classified as SS vs. SL vs. LL, not taking into account the rs25531 SNP. Using the biallelic classification, observations were consistent with HWE: χ2(1) = 3.29, P = 0.07. After controlling for current symptoms of depression and anxiety (HADS total), the same results were found as with the triallelic classification [G × E interaction: RUM: (F2,241 = 3.95, P = 0.02) LEIDS-R total: (F2,241 = 4,03, P = 0.02)]. G × E interactions fell short of statistical significance for the uncorrected analyses (RUM: P = .13; LEIDS-R: P = 0.33). However, correlations between emotional abuse and the LEIDS-R outcomes for each genotype separately remained significant, consistent with the results for the triallelic classification. G × E interactions were non-significant for the other outcomes.
Discussion
Our results show that 5-HTTLPR moderates the effect of CEA on CR, a cognitive vulnerability factor of depression. Participants with the SS (including Lg) genotype with a low emotional abuse history had significantly lower CR scores than the other genotype groups. They also had (non-significantly) higher CR than other genotypes when emotional abuse in childhood was above threshold. After controlling for current levels of depression and anxiety symptoms, the G × E interactions become stronger and were replicated in the analysis using the biallelic classification. The LEIDS-R is designed to capture negative cognitive patterns of reactivity, which are habitual in nature. In vulnerable individuals, LEIDS-R scores remain high when symptoms are low, but the scores do tend to get higher with increasing levels of depression. In other words, the scores are partly mood-dependent, which makes it important to rule out variance explained by current state. The gene–environment interaction on the Rumination subscale of the LEIDS-R is noteworthy, as rumination is a robust vulnerability factor for (and characteristic of) depression (Nolen-Hoeksema 2000). The same subscale was also found to mediate the relationship between neuroticism and depressive symptoms in never-depressed individuals (Barnhofer & Chittka 2010). Furthermore, a similar crossover interaction of the 5-HTTLPR and life stress has been previously found with another measure of rumination (Canli et al. 2006).
This pattern of a crossover interaction on CR supports the ‘differential susceptibility’ theory that states that individuals of a certain genetic make-up are not merely vulnerable to an adverse outcome but rather ‘susceptible’: they are more likely to suffer from an adverse environment but also benefit more from a supportive one (Belsky et al. 2009). The same differential susceptibility pattern of the SS genotype across childhood experiences may be observed in a number of previous reports (Eley et al. 2004; Kaufman et al. 2004; Taylor et al. 2006; Wilhelm et al. 2006). In our study, the positive effect of the SS genotype in low childhood maltreatment environments seems to be higher than the adverse effect of the same genotype when having experienced high maltreatment. Similar magnitude of differential effects has been found in previous research (Eley et al. 2004; re-evaluated in Belsky et al. 2009). A possible explanation for this pattern is that in our sample CEA was within the lower quartiles, allowing less variability for detecting higher vulnerability to adversity.
Limitations of the present study include the retrospective assessment of childhood abuse using a self-report measure, which may have led to a biased reporting. However, the study design may actually be rather conservative for detecting G × E interactions because emotionally abused individuals are underrepresented in our young student sample (i.e. restricted range on E variable), and abused individuals may have failed to recollect their maltreatment (i.e. mis-specification of E variable). Another limitation is that we were unable to use the established cut-off scores for dichotomizing CEA because of the relatively low mean scores in our sample. Future studies examining samples with higher exposure to maltreatment may detect genotype vulnerability effects of greater magnitude, especially in the high emotional abuse quartiles. Moreover, our assessment of depression diagnosis by self-report is questionable. Although validity data for all self-report measures are available, these results should be interpreted with caution.
Our sample was comprised of young adults, which is consistent with previous studies that successfully detected a gene–environment interaction on depression outcomes (Caspi et al. 2003; Eley et al. 2004; Taylor et al. 2006) vs. those who did not (Gillespie et al. 2005; Power et al. in press; Surtees et al. 2006). The advantage of using CR as an outcome measure—as opposed to depression history—is that CR can also be assessed in people who are vulnerable to depression but have not yet experienced an episode of depression. Many genetic associations to brain-based endophenotypes are also observed in healthy individuals (Meyer-Lindenberg & Weinberger 2006). Finally, it should be noted that our sample size is small for the detection of gene–environment interactions. Our sample is also not representative of the general population; hence, the generalizability of our findings remains to be investigated. If replicated, our results show that the strength of the association between 5-HTTLPR and depression partly depends on the selection of the environmental pathogen and the marker of depression vulnerability.
Acknowledgments
The authors thank D.P. de Beurs, F.E.A. Verhoeven, H. Cerit and A.W. Kruijt for help with data collection. Funding for this study came from a VICI grant (# 453-06-005) from the Netherlands Organization of Science (N.W.O.-MaGW) to A.J.W.V.D.
References
- Barnhofer T, Chittka T. Cognitive reactivity mediates the relationship between neuroticism and depression. Behav Res Ther. 2010;48:275–281. doi: 10.1016/j.brat.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J, Jonassaint C, Pluess M, Stanton M, Brummett B, Williams R. Vulnerability genes or plasticity genes? Mol Psychiatry. 2009;14:746–754. doi: 10.1038/mp.2009.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein DP, Fink L. Childhood Trauma Questionnaire Manual. San Antonio, TX, USA: Harcourt, The Psychological Corporation; 1998. [Google Scholar]
- Booij L, Van der Does AJW. Cognitive and serotonergic vulnerability to depression: convergent findings. J Abnorm Psychol. 2007;116:86–94. doi: 10.1037/0021-843X.116.1.86. [DOI] [PubMed] [Google Scholar]
- Canli T, Lesch KP. Long story short: the serotonin transporter in emotion regulation and social cognition. Nat Neurosci. 2007;109:1103–1109. doi: 10.1038/nn1964. [DOI] [PubMed] [Google Scholar]
- Canli T, Qiu M, Omura K, Congdon E, Haas BW, Amin Z, Herrmann MJ, Constable RT, Lesch KP. Neural correlates of epigenesis. Proc Natl Acad Sci U S A. 2006;103:16033–16038. doi: 10.1073/pnas.0601674103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffit TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Chapman DP, Whitfield CL, Felitti VJ, Dube SR, Edwards VJ, Anda RF. Adverse childhood experiences and the risk of depressive disorders in adulthood. J Affect Disord. 2004;82:217–225. doi: 10.1016/j.jad.2003.12.013. [DOI] [PubMed] [Google Scholar]
- Eley TC, Sugden K, Corsico A, Gregory AM, Sham P, McGuffin P, Plomin R, Craig IW. Gene-environment interaction analysis of serotonin markers with adolescent depression. Mol Psychiatry. 2004;9:908–915. doi: 10.1038/sj.mp.4001546. [DOI] [PubMed] [Google Scholar]
- Gibb BE, Alloy LB, Abramson LY, Rose DT, Whitehouse NG, Donovan P, Hogan ME, Cronholm J, Tierney S. History of childhood maltreatment, negative cognitive styles and episodes of depression in adulthood. Cogn Ther Res. 2001;25:425–446. [Google Scholar]
- Gibb BE, Butler AC, Beck JS. Childhood abuse, depression, and anxiety in adult psychiatric outpatients. Depress Anxiety. 2003;17:226–228. doi: 10.1002/da.10111. [DOI] [PubMed] [Google Scholar]
- Gibb BE, Chelminski I, Zimmerman M. Childhood emotional, physical, and sexual abuse, and diagnoses of depressive and anxiety disorders in adult psychiatric outpatients. Depress Anxiety. 2007;24:256–263. doi: 10.1002/da.20238. [DOI] [PubMed] [Google Scholar]
- Gillespie NA, Whitfield JB, Williams B, Heath AC, Martin NG. The relationship between stressful life events, the serotonin transporter (5-HTTLPR) genotype and major depression. Psychol Med. 2005;35:101–111. doi: 10.1017/s0033291704002727. [DOI] [PubMed] [Google Scholar]
- Hankin BL. Childhood maltreatment and psychopathology: prospective tests of attachment, cognitive vulnerability, and stress as mediating processes. Cogn Ther Res. 2005;29:645–671. [Google Scholar]
- Hasler G, Drevets WC, Manji HK, Charney DS. Discovering endophenotypes for major depression. Neuropsychopharmacology. 2004;29:1765–1781. doi: 10.1038/sj.npp.1300506. [DOI] [PubMed] [Google Scholar]
- Hoekstra HA, Ormel J, de Fruyt F. NEO-PI-R Handleiding. Amsterdam: Hogrefe Uitgevers B.V.; 2007. [Google Scholar]
- Hovens JGFM, Wiersma JE, Giltay EJ, van Oppen P, Spinhoven P, Penninx BWJH, Zitman FG. Childhood life events and childhood trauma in adult patients with depressive, anxiety and comorbid disorders vs. controls. Acta Psychiatr Scand. doi: 10.1111/j.1600-0447.2009.01491.x. in press. [DOI] [PubMed] [Google Scholar]
- Jacobs N, Kenis G, Peeters F, Derom C, Vlietinck R, van Os J. Stress-related negative affectivity and genetically altered serotonin transporter function. Arch Gen Psychiatry. 2006;63:989–996. doi: 10.1001/archpsyc.63.9.989. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Yang BZ, Douglas-Palumberi H, Houshyar S, Lipschitz D, Krystal JH, Gelernter J. Social supports and serotonin transporter gene moderate depression in maltreated children. Proc Natl Acad Sci U S A. 2004;101:17316–17321. doi: 10.1073/pnas.0404376101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RT, Alloy LB, Abramson LY, Iacoviello BM, Whitehouse WG. Emotional maltreatment and depression: prospective prediction of depressive episodes. Depress Anxiety. 2009;26:174–181. doi: 10.1002/da.20545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotrich FE, Lenze E. Gene-environment interactions and depression. JAMA. 2009;302:1859–1862. doi: 10.1001/jama.2009.1576. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Weinberger DR. Intermediate phenotypes and genetic mechanisms of psychiatric disorders. Nat Rev Neurosci. 2006;7:818–827. doi: 10.1038/nrn1993. [DOI] [PubMed] [Google Scholar]
- Moulds ML, Kandris E, Williams AD, Lang T, Yap C, Hoffmeister K. An investigation of the relationship between cognitive reactivity and rumination. Behav Ther. 2008;391:65–71. doi: 10.1016/j.beth.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Munafo MR, Brown SM, Hariri AR. Serotonin transporter (5-HTTLPR) genotype and amygdala activation: a meta-analysis. Biol Psychiatry. 2008;63:852–857. doi: 10.1016/j.biopsych.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munafo MR, Freimer NB, Ng W, Ophoff R, Veijola J, Miettunen J, Järvelin MR, Taanila A, Flint J. 5-HTTLPR genotype and anxiety-related personality traits: a meta-analysis and new data. Am J Med Genet B Neuropsychiatr Genet. 2009;150B2:271–281. doi: 10.1002/ajmg.b.30808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolen-Hoeksema S. The role of rumination in depressive disorders and mixed anxiety/depressive symptoms. J Abnorm Psychol. 2000;109:504–511. [PubMed] [Google Scholar]
- Ormel J, Rosmalen J, Farmer A. Neuroticism: a non-informative marker of vulnerability to psychopathology. Soc Psychiatry Psychiatr Epidemiol. 2004;39:906–912. doi: 10.1007/s00127-004-0873-y. [DOI] [PubMed] [Google Scholar]
- Power T, Stewart R, Ancelin ML, Jaussent I, Malafosse A, Ritchie K. 5-HTTLPR genotype, stressful life events and late-life depression: no evidence of interaction in a French population. Neurobiol Aging. 31:886–887. doi: 10.1016/j.neurobiolaging.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Raes F, Hermans D. On the mediating role of subtypes of rumination in the relationship between childhood emotional abuse and depressed mood: brooding versus reflection. Depress Anxiety. 2008;25:1067–1070. doi: 10.1002/da.20447. [DOI] [PubMed] [Google Scholar]
- Risch N, Herell R, Lehner T, Liang KY, Eaves L, Hoh J, Griem A, Kovacs M, Ott J, Merikangas KR. Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression: a meta-analysis. JAMA. 2009;30123:2462–2471. doi: 10.1001/jama.2009.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose DT, Abramson LY. Developmental predictors of depressive cognitive style: research and theory. In: Cicchetti D, Toth S, editors. Rochester Symposium of Developmental Psychopathology. Rochester, NY: University of Rochester Press; 1992. pp. 324–349. Vol. IV. [Google Scholar]
- Rutter M, Thapar A, Pickles A. Gene-environment interactions: biologically valid pathway or artifact? Arch Gen Psych. 2009;66:1287–1289. doi: 10.1001/archgenpsychiatry.2009.167. [DOI] [PubMed] [Google Scholar]
- Scher CD, Ingram RE, Segal ZV. Cognitive reactivity and vulnerability: Empirical evaluation of construct activation and cognitive diatheses in unipolar depression. Clin Psychol Rev. 2005;254:487–510. doi: 10.1016/j.cpr.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Segal ZV, Gemar M, Williams S. Differential cognitive response to a mood challenge following successful cognitive therapy or pharmacotherapy for unipolar depression. J Abnorm Psychology. 1999;1081:3–10. doi: 10.1037//0021-843x.108.1.3. [DOI] [PubMed] [Google Scholar]
- Segal ZV, Kennedy S, Gemar M, Hood K, Pedersen R, Buis T. Cognitive reactivity to sad mood provocation and the prediction of depressive relapse. Arch Gen Psychiatry. 2006;637:749–755. doi: 10.1001/archpsyc.63.7.749. [DOI] [PubMed] [Google Scholar]
- Spinhoven P, Ormel J, Sloekers PPA, Kempen GIJM, Speckens AEM, van Hemert AM. A validation study of the Hospital Anxiety and Depression Scale (HADS) in different groups of Dutch subjects. Psychol Med. 1997;272:363–370. doi: 10.1017/s0033291796004382. [DOI] [PubMed] [Google Scholar]
- Surtees PG, Wainwright NWJ, Willis-Owen SAG, Luben R, Day NE, Flint J. Social adversity, the serotonin transporter (5-HTTLPR) polymorphism and major depressive disorder. Biol Psychiatry. 2006;59:224–229. doi: 10.1016/j.biopsych.2005.07.014. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Way BM, Welch WT, Hilmert CJ, Lehman BJ, Eisenberger NI. Early family environment, current adversity, the serotonin transporter promoter polymorphism, and depressive symptomatology. Biol Psychiatry. 2006;607:671–676. doi: 10.1016/j.biopsych.2006.04.019. [DOI] [PubMed] [Google Scholar]
- Teasdale J. Cognitive vulnerability to persistent depression. Cogn & Emot. 1988;2:247–274. [Google Scholar]
- Uher R, McGuffin P. The moderation by the serotonin transporter gene of environmental adversity in the etiology of depression: 2009 update. Mol Psychiatry. 2010;15:18–22. doi: 10.1038/mp.2009.123. [DOI] [PubMed] [Google Scholar]
- Van der Does W. Cognitive reactivity to sad mood: structure and validity of a new measure. Behav Res Ther. 2002;401:105–120. doi: 10.1016/s0005-7967(00)00111-x. [DOI] [PubMed] [Google Scholar]
- Van der Does W. Thought suppression and cognitive vulnerability to depression. Br J Clin Psychol. 2005;44:1–14. doi: 10.1348/014466504x19442. [DOI] [PubMed] [Google Scholar]
- Wilhelm K, Mitchell PB, Niven H, Finch A, Wedgwood L, Scimone A, Blair IP, Parker G, Schofield PR. Life events, first depression onset and the serotonin transporter gene. Br J Psychiatry. 2006;188:210–215. doi: 10.1192/bjp.bp.105.009522. [DOI] [PubMed] [Google Scholar]
- Williams JMG, Van der Does AJW, Barnhofer T, Crane C, Segal ZS. Cognitive reactivity, suicidal ideation and future fluency: preliminary investigation of a differential activation theory of hopelessness/suicidality. Cogn Ther Res. 2008;32:83–104. [Google Scholar]
- Wright MO, Crawford E, Del Castillo D. Childhood emotional maltreatment and later psychological distress among college students: the mediating role of maladaptive schemas. Child Abuse Negl. 2009;33:59–68. doi: 10.1016/j.chiabu.2008.12.007. [DOI] [PubMed] [Google Scholar]


