Abstract
Aims
We have previously shown that preconditioning of stem and progenitor cells promotes their survival post-engraftment in the infarcted heart. The present study was designed to (i) delineate the role of microRNA-21 (miR-21) in interleukin-11 (IL-11) signalling during preconditioning of skeletal myoblasts (MY) and (ii) study the long-term fate of preconditioned MY (PCMY) post-transplantation in the infarcted heart.
Methods and results
We report that pharmacological preconditioning of MY with diazoxide showed robust expression of IL-11 and activation of extracellular signal-regulated kinase 1/2 (Erk1/2) and signal transducers and activators of transcription-3 (Stat3) with concomitantly increased miR-21. These molecular events improved cytoprotection of PCMY under oxidant stress in vitro which was compromised by pre-treatment of PCMY with IL-11-specific siRNA, Erk1/2 blocker, or anti-miR-21. In vivo studies for sry-gene detection in a female rat heart model of acute myocardial infarction showed two-fold higher survival of male donor PCMY 4 and 7 days post-engraftment. Long-term fate of the engrafted cells was determined at 4 months after transplantation. Immunohistological studies revealed that in comparison with non-PCMY, PCMY improved angiogenic response in the heart which was evident from a higher number of blood vessels per surface area (0.155 mm2) and myogenic differentiation of PCMY in the heart. Indices of myocardial contractility including ejection fraction and fractional shortening showed significant improvement in PCMY-treated animals.
Conclusion
miR-21 is a key regulator of Erk1/2–Stat3 signalling downstream of IL-11 during preconditioning of MY. The therapeutic benefits of PCMY were stable and persisted until 4 months of observation.
Keywords: Diazoxide, IL-11, MicroRNA, Myoblast, Myocardial infarction, Survival
1. Introduction
We have already shown that ischaemic preconditioning results in significantly improved survival of stem cells via altered expression of microRNAs (miRs).1 miRs are post-transcriptional regulators of molecular mechanisms and play a crucial role in the determination of cell fate and functionality in both physiological and pathophysiological conditions.2 From the maintenance of pluripotency to survival and differentiation potential, miRs play an important role in controlling the inherent properties of stem and progenitor cell. Reprogramming of stem and progenitor cells by miRs manipulation therefore can be exploited to improve their therapeutic effectiveness in the clinical settings. We report the mechanistic participation of miR-21 in skeletal myoblasts (MY) survival by pharmacological preconditioning using diazoxide (Dz) via interleukin-11 (IL-11)-induced extracellular signal-regulated kinase 1/2 (Erk1/2)–signal transducers and activators of transcription-3 (Stat3) signalling.
miR-21 is a ubiquitous small RNA which is dynamically involved in cellular responsiveness to various stimuli.3,4 In a recent study, miR-21 was reported as one of the member of a select group of miRs which showed robust up-regulation in the infarcted heart and was associated with various cardiac pathologies.5 With little change in its expression in cardiomyocytes in response to cardiac failure, miR-21 had significant role in cardiac fibroblasts survival in an Erk1/2-dependent fashion.5 The pro-survival and anti-apoptotic activity of miR-21 was also linked with multiple transcription factors including signal transducers and Stat3.6 Interleukin-6 (IL6) and its family members are known activators of Stat3-mediated anti-apoptotic signalling in a variety of cells under oxidant stress. Stat3-mediated induction of miR-21 has been implicated in IL-6-dependent survival of multiple myeloma cells.7 In another interesting study which linked Stat3 anti-apoptotic pathway with induction of miR-21, IL-6 treatment of pre-implantation mouse embryo during in vitro development significantly reduced the number of apoptotic cells.8 Nevertheless, the role of Stat3 and miR-21 in stem and progenitor cell survival signalling especially during preconditioning remains less well studied. In accordance with these studies, we hypothesize that IL-11, which is biologically related to IL-6 family and shares a common gp130 as a critical component for signal transduction,9 showed significant up-regulation in preconditioned MY (PCMY) and was responsible for longer cytoprotective effects of preconditioning. We observed mechanistic participation of miR-21 in IL-11-induced Erk1/2–Stat3 signalling in PCMY. Besides, the study was also designed to determine the long-term fate of PCMY in continuation of our previous study.10 We observed that our combinatorial approach of cellular preconditioning and heart cell therapy gave stable therapeutic effects until 4 weeks of observation in a rodent heart model of myocardial infarction.
2. Methods
2.1. Isolation and culture of MY
MY were isolated from the skeletal muscle biopsies taken from the hind-limb of male Fischer-344 rats.10 Briefly, under sedation, the site of skeletal muscle biopsy was stimulated with intramuscular injection of 0.3 mL of a mixture of ketamine (10 mg/mL) and xylazine (2 mg/mL). Three days later, an excisional biopsy (1–2 g) of the stimulated muscle was taken, minced into coarse slurry serially digested with 0.2% collagenase type-XI (Gibco-BRL) for 90 min, 2.4 U/mL of dispase (Gibco-BRL) for 45 min at 37°C, and finally 0.1% trypsin–EDTA (Gibco-BRL) for 15 min. The muscle extract was pre-plated thrice at 1 h time interval and twice additionally at 8 and 16 h to remove the cell debris. After the last pre-plate, 0.1 mM BrdU (BD Pharmingen) was added to the cell culture for 3 days to inhibit fibroblast growth followed by 3 days treatment with 15 ng/mL bFGF (Sigma). The cells were later propagated in Medium-199 supplemented with 20% foetal bovine serum (FBS) at 37°C/5% CO2 atmosphere. The purity of MY was determined by desmin-specific immunostaining.9
The investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1985) and protocol approved by the Institutional Animal Care and Use Committee, University of Cincinnati.
2.2. Preconditioning of MY
The cells were grown in Petri dishes at a density of 3.5 × 104 cells/cm2. One day later, cells were randomly assigned to four experimental groups: (i) non-PCMY, (ii) PCMY treated with 200 µM Dz (US Pharmacopeia) 30 min, (iii) PCMY pre-treated with 40 µM Wortmannin (Sigma) for 1 h before preconditioning, (iv) PCMY pre-treated with 50 µM PD098059 for 1 h with (Calbiochem) before preconditioning, and (v) PCMY pre-transfected with IL-11-specific siRNA. The cells from various treatment groups were later used either for changes in molecular expression of IL-11, Erk1/2, Stat3, and miR-21 or for the assessment of cytoprotective effects of preconditioning under oxidant stress using LDH release as a marker of cellular injury as detailed in the Supplementary methods.
For IL-11 siRNA transfection, cells were seeded a day prior to the start of experiment at a density of 2 × 104 cells/cm2 in DMEM supplemented with 10% FBS. The cells were transfected with 10 nM of ON-TARGET plus SMART pool rat IL-11 siRNA (Dharmacon Cat. # L-092679-01-0010) using Lipofectamine-2000 (Invitrogen) as per the instructions of the manufacturer at 37°C in a CO2 incubator. For control, cells were transfected with scrambled siRNA. The cells were later preconditioned with 200 µM Dz for 30 min at 37°C in a CO2 incubator.
In another set of experiments, we mimicked the effects of preconditioning induced by IL-11 by treatment of the cells with recombinant IL-11 (rIL-11). MY were seeded in 35 mm2 at a cell density of 3.5 × 104 cells/cm2 and were treated with rIL-11. In another group, the cells were pre-treated with IL-11Rα-specific antibody, PD098059, or Wortmannin before treatment with rIL-11. Using non-PCMY as a control, the cell samples were observed for change in miR-21 expression and also to study the effect of miR-21 inhibition on cell survival under oxidant stress.
For cytoprotection studies, cells were treated with 100 µM H2O2 for 2 h. For western blot studies, the cells were lysed to extract proteins as described earlier.8
2.3. Isolation and detection of miR-21
Extraction of miR-21 was carried out by using mirVana™ miR Isolation Kit and detected by using mirVana™ qRT-PCR miRNA Detection Kit (Ambion). Specific miR primers provided by Ambion were used during qRT–PCR as per the instructions of the manufacturer.
2.4. Transfection with miR-21 inhibitor
For inhibition of miR-21, anti-miR-21 (anti-sense) was transfected with siPORT™ NeoFx™ Transfection Agent (NeoFx; Ambion) according to the instructions of the manufacturer. Briefly, 5 µL NeoFx was diluted with 100 µL Opti-MEM for each sample and incubated for 10 min at room temperature. Then, miR-21 inhibitor was diluted with 100 µL Opti-MEM at a final concentration of 30 nM and mixed with diluted NeoFx mixture. After incubation for another 10 min, transfection mixture was added to 2.3 mL of cell suspension (3 × 105 cells). Cell suspension containing transfection mixture was plated into six-well plate and an assay was performed at 72 h after incubation.
2.5. RT–PCR and western immunoblotting
RT–PCR and western immunoblotting was carried out as described earlier11 and in the Supplementary material online.
2.6. In vivo studies
2.6.1. Cell labelling
The cells were labelled using PKH-26 cell tracker dye or nlsLacZ reporter gene for post-transplantation identification as described in Supplementary methods.
2.6.2. Experimental rat model of acute myocardial infarction and cell transplantation
An experimental rat heart model of acute myocardial infarction was developed in young female Fisher-344 rats by permanent coronary artery ligation.12 The animals were grouped to receive intramyocardial injections of 70 µL of basal DMEM without cells (Group 1, n = 14) or containing 1.5 × 106 non-PCMY (Group 2, n = 18) or PCMY (Group 3, n = 18). For detailed methodology, please refer to Supplementary methods.
2.6.2.1. Physiological assessment of the heart function
Echocardiography was performed using Compact Linear Array probe CL10–5 on HDI/5000 SONOS CT (HP) to study change in the heart function.12
2.6.3. Analysis of cell survival
The survival of the male donor cells in the female recipient hearts was assessed by real-time PCR and conventional PCR by quantification of the sry-gene 4 and 7 days cell post-transplantation. The samples of the rat heart were snap frozen in liquid nitrogen and powdered. The DNA was purified using Genomic DNA Isolation kit (Qiagen). Real-time PCR was performed using iQ SYBR-Green supermix (Bio-Rad) in a Bio-Rad iQ5 optical module. A specific sequence of rat sry-gene in between the following sequence was amplified: 5′-tcatcgaagggttaaagtgc-3′ and 5′-gtaggttgttgtcccattgc-3′. The cycling conditions were set as follows: 3 min at 95°C for initial denaturation, 40 cycles of denaturation at 95°C for 30 s, annealing at 55°C for 40 s, and extension at 72°C for 50 s. Real-time data were acquired during the extension step. A melting curve was obtained at the end of the reaction by raising the temperature gradually by 1°C/min from 55–95°C over a time period of 35 min and calculations were performed as described earlier.11
2.6.4. Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labelling
Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labelling (TUNEL) on different treatment groups was performed using an in situ Cell Death Detection Kit (Roche, Indianapolis, IN, USA) as per the manufacturer's instruction and described earlier.11
2.6.5. Immunohistochemical studies
Immunostaining was performed as described previously.10,11 The antibodies used were specific for connexin-43 (1:30 dilution; sc-9059 Santa Cruz Biotechnology), α-sarcomeric actinin (1:50 dilution; Sigma), skeletal MHC fast (pre-diluted; ab909 Abcam), slow muscle MHC (1:100 dilution; MAB1628 Chemicon), smooth muscle actin (1:50 dilution; A2547 Sigma), von Willebrand factor (vWF) (1:50 dilution; A0082 Dako), Ki67 (1:40 dilution; BD Pharmingen), and PCNA (1:200 dilution; M0879 Dako). The primary antigen–antibody reaction was detected with goat anti-mouse IgG secondary antibody conjugated with Alexa Fluor-488 or Alexa Fluor-350 (1:100 dilution) or with goat anti-rabbit IgG secondary antibody conjugated with Alexa Fluor-633 or Alexa Fluor-488 (1:100 dilution) (Molecular Probes). The samples were examined using fluorescent microscope (BX41, Olympus, Japan).
For blood vessel density, the blood vessels positive for vWF were counted in both infarct and peri-infarct regions.12 At least 32 high-power microscopic fields (×400) each in infarct and peri-infarct regions were randomly selected and counted in each treatment group of animals (n = 4 animals/group). Blood vessel density was expressed as the number of vessels per surface area (0.155 mm2).
2.7. Statistical analysis
All data were described as mean ± SEM. To analyse the data statistically, we performed one-way ANOVA with post hoc analysis and considered a value of P < 0.05 as statistically significant.
3. Results
3.1. Role of IL-11 in PCMY survival under oxidant stress
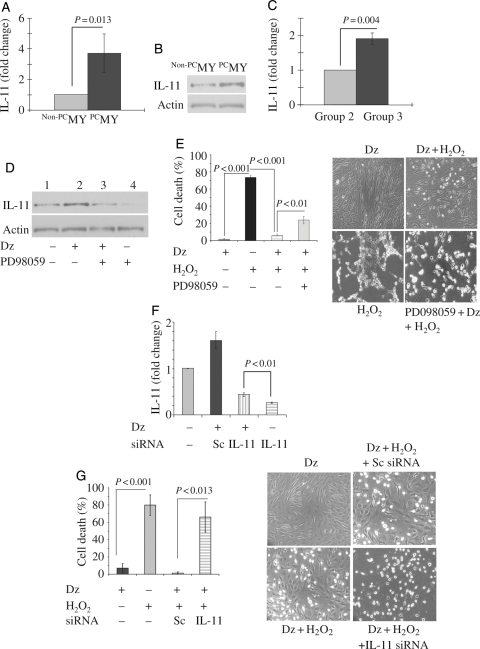
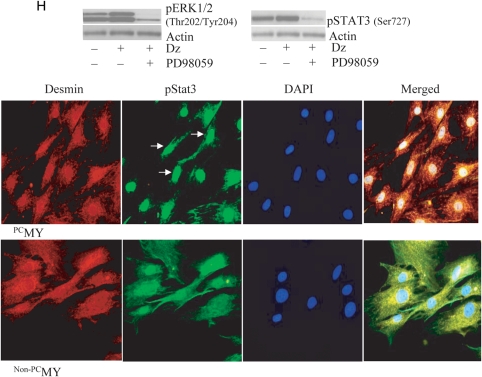
We observed that mRNA expression of IL-11 was increased in PCMY (3.72-fold vs. non-PCMY) (Figure 1A). Immunoblotting for IL-11 confirmed its up-regulated protein expression in PCMY (Figure 1B). Likewise, this trend was observed in vivo in the left ventricle in Group 3 animals at 4 days post-transplantation of PCMY which showed 1.91-fold increase in IL-11 expression when compared with Group 2 (Figure 1C). Western blot analysis showed that up-regulation of IL-11 in PCMY was abrogated by pre-treatment of the cells with PD098059 Erk1/2 inhibitor (Figure 1D). Dz pre-treatment significantly reduced LDH release from PCMY under oxidant stress when compared with non-PCMY (P < 0.001; Figure 1E). Dz treatment alone was innocuous for the cells as was obvious from negligible release of LDH and hence high cell viability. However, cytoprotective effects of preconditioning were abolished by pre-treatment of the cells with Erk1/2 inhibitor PD98059 which was also evident from the obvious morphological distortion in non-PCMSCs under oxidant stress when compared with PCMY (Figure 1E). IL-11-specific RNAi transfection significantly abolished IL-11 expression in PCMY in response to Dz treatment when compared with scramble (Sc) siRNA-treated PCMY (Figure 1F). These molecular events led to abrogation of cytoprotective effects of preconditioning in IL-11-specific RNAi-transfected PCMY upon subsequent exposure to 100 µM H2O2 for 2 h when compared with their counterparts transfected with scrambled siRNA (P = 0.013; Figure 1G). These cells showed shrunken and hypercontracted morphology (rounded or irregular shape) which was similar to non-PCMY under oxidant stress (Figure 1G). Preconditioning significantly up-regulated of IL-11 expression and activation of Erk1/2 (Thr 202/Tyr204) which was blocked by PD98059 (Figure 1H). A significantly higher level of phosphorylation of Stat3 (pStat3; Ser727) was observed in PCMY in comparison to non-PCMY which was abolished by PD98059 pre-treatment of the cells. Fluorescent immunostaining revealed extensive localization of pStat3 in the nuclei of PCMY (Figure 1H). These observations confirmed that activation of Erk1/2 was involved in preconditioning-induced IL-11 expression and for its downstream effect on Stat3 pathway.
Figure 1.
IL-11 expression in PCMY and its role in survival signalling. (A) Real-time PCR showing 3.72-fold higher expression of IL-11 in vitro in PCMY (P = 0.013 vs. non-PCMY; n = 3) which was confirmed by (B) western blot studies. (C) Real-time PCR showing 1.91-fold up-regulation of IL-11 in the LV of Group 3 animal hearts when compared with Group 2 (P = 0.004; n = 3/group) 4 days after their respective treatments. (D) Western blotting revealed up-regulation of IL-11 protein in PCMY which was sensitive to Erk1/2 inhibition by PD098059. Pre-treatment of the cells with PD98059 for 30 min abrogated IL-11 expression with resultant loss of cytoprotection against oxidant stress. (E) Treatment of MY with Dz alone had no cytotoxicity on the cells; however, significantly higher LDH release was observed in non-PCMY upon treatment with H2O2 when compared with PCMY (P = 0.004; n = 5). Pre-treatment of PCMY with PD98059 significantly diminished the cytoprotective effects as well as morphological integrity of the cells. (F) RT–PCR showing successful knock-down of IL-11 expression in MY by IL-11-specific siRNA transfection Dz preconditioning of the cells failed to induce IL-11 expression. Sc siRNA-transfected cells were used as a control. (G) Treatment with H2O2 caused significantly more LDH leakage from IL-11-silenced cells when compared with its preconditioned counterpart (P = 0.013; n = 3) with concomitant morphological distortions. (H) Western blot analysis showed that reconditioning activated Erk1/2 and Stat3, which was evident from higher level of phosphorylation. Pre-treatment of cells with PD98059 abolished their phosphorylation upon subsequent treatment with Dz. Treatment with H2O2 caused significantly more LDH leakage from cells pre-treated with PD98059 when compared with its preconditioned counterpart (P = 0.004). Fluorescent immunostaining for pStat3 expression revealed that preconditioning promoted pStat3 (green fluorescence; indicated by white arrows) localization in the nucleus. The cells were stained for desmin expression (red fluorescence) to show the purity of skeletal myoblasts. The nuclei were visualized by DAPI staining (blue fluorescence). In non-PCMY, pStat3 was missing in the nucleus.
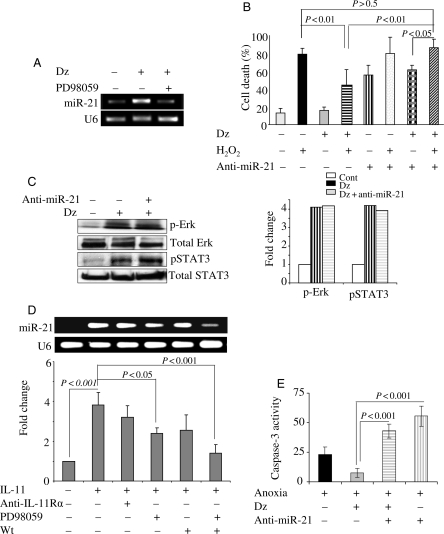
Interestingly, preconditioning with Dz also increased miR-21 in PCMY when compared with the baseline expression in non-PCMY which was abolished by pre-treatment for 30 min with 50 µM PD98059 (Figure 2A). Abrogation of miR-21 with anti-miR-21 abolished the cytoprotective effects of preconditioning which was evident from higher leakage of LDH which was indicative of higher cell death (P < 0.01 vs. PCMY without anti-miR-21 treatment; Figure 2B). On the contrary, anti-miR-21 failed to abolish phosphorylation of Erk42/44 and Stat3 in response to preconditioning (Figure 2C). These data show that altered expression of miR-21 during preconditioning was downstream of Erk1/2 and not vice versa.
Figure 2.
Role of miR-21 in preconditioning-induced cytoprotection. (A) PCMY showed up-regulated expression of miR-21 when compared with non-PCMY which was abolished by pre-treatment of the cells with 50 µM PD98059. (B) LDH release assay from various treatment groups of cells. Prior transfection with anti-miR-21 resulted in significant abrogation of the cytoprotective effects of preconditioning in PCMY under oxidant stress when compared with PCMY without anti-miR-21 transfection (P < 0.01). LDH leakage from anti-miR-21-transfected PCMY under oxidant stress was similar to non-PCMY (P < 0.5; n = 3). (C) Western blots showing higher activation of Erk1/2 and pStat3 in PCMY when compared with non-PCMY and remained unaffected after transfection with anti-miR-21 prior to preconditioning of the cells. (D) Treatment with rIL-11 protein significantly increased miR-21 expression in MY (P < 0.001 vs. non-treated control cells; n = 3). Prior treatment of the cells with IL-11Rα-specific antibody, PD098059, or Wortmannin abolished miR-21 expression in the cells upon subsequent treatment with rIL-11. However, simultaneous treatment with PD098059 and Wortmannin was more effective in abolishing IL-11-induced miR-21. (E) Caspase activity was significantly lower in PCMY under anoxic culture conditions (P < 0.001 vs. non-PCMY; n = 3). However, prior treatment with anti-miR-21 abolished the effects of preconditioning and significantly increased caspase activity was observed in PCMY under anoxic culture conditions.
Next, we showed that miR-21 induction in PCMY could be duplicated by treatment of cells with rIL-11 protein which increased miR-21 expression when compared with non-PCMY (P < 0.001; Figure 2D). Pre-treatment of cells with either IL-11 receptor-α-specific antibody or PD098059 or Wortmannin significantly reduced miR-21 expression in IL-11-treated cells. This reduction in miR-21 expression was, however, partial. On the contrary, combined treatment of cells with PD098059 and Wortmannin almost completely abolished miR-21 expression in IL-11-treated MY (P < 0.001 vs. IL-11 treatment without pre-treatment with inhibitor; Figure 2D).
As we have previously demonstrated that preconditioning activated Akt which was abolished by Wortmannin treatment of cells,10 we looked into the downstream effector molecules in PI3K-Akt signalling in PCMY. Earlier studies have also demonstrated that Stat3 activation could also be mediated by PI3K with potential participation in apoptosis.13,14 Caspase activity was significantly attenuated in PCMY under anoxic culture conditions (P < 0.001 vs. non-PCMY). Additionally, pre-treatment of PCMY with anti-miR-21 significantly abolished the preconditioning-induced cytoprotection (P < 0.001 vs. PCMY without anti-miR-21 treatment; Figure 2E).
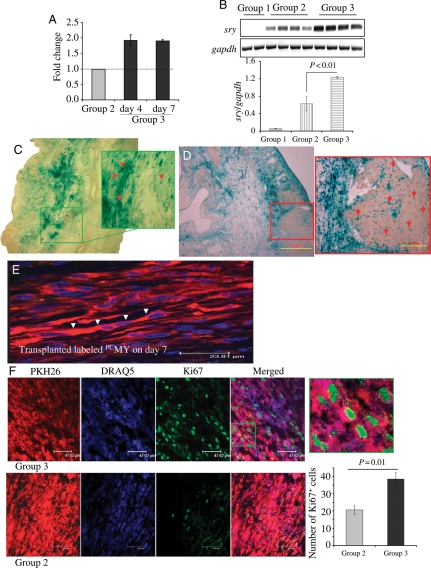
3.2. Survival and myogenic differentiation of PCMY
Intramyocardial transplantation of sex-mismatched male donor cells was carried out in a female rat model of acute myocardial infarction which allowed sry-gene analysis of the recipient hearts by PCR at 4 and 7 days post-engraftment (n = 2 for DMEM without cells injected Group 1 and, n = 4 each for non-PCMY-transplanted Group 2 and PCMY-transplanted Group 3 per group on each time point). Real-time PCR for sry-gene showed that PCMY (Group 3) had two-fold higher survival post-engraftment 4 and 7 days when compared with non-PCMY (Group 2; Figure 3A). Group 1 animal hearts treated with DMEM without cells did not show sry-gene and served as negative controls. Conventional PCR followed by densitometry was also performed for sry-gene studies on 4 days post-engraftment which confirmed our real-time PCR data (Figure 3B). In another set of experiments, MY pre-labelled with nlsLacz gene by adenoviral transduction were transplanted which allowed us to track and confirm their survival by nlsLacz expression. Histochemical staining showed that non-PCMY (Figure 3C) and PCMY (Figure 3D) continued to express nlsLacz post-engraftment which was evident from extensive β-galactosidase activity at the site of the cell graft. Interestingly, we also observed that both non-PCMY- and PCMY-expressing nlsLacz dissipated from the site of the cell injection to the nearby regions (Figure 3C, green box, and D, red box). Fluorescence imaging for PKH26 (red fluorescence) co-localized with DAPI staining (blue fluorescence) showed that the transplanted PCMY had already adopted elongated morphology (indicative of multinucleated myotube formation) and oriented in accordance with the host muscle architecture by 7 days post-engraftment (Figure 3E, white arrowheads). The proliferative capacity of the transplanted cells was higher in PCMY in Group 3 (P = 0.01 vs. Group 2; Figure 3F). Green box in the merged image in Figure 3F has been magnified to show co-localization of PKH26 (red fluorescence)-labelled cells with Ki67 expression (green fluorescence). TUNEL positivity was also reduced in Group 3 (P = 0.01 vs. Group 2; Supplementary material online, Figure S1). Put together, these results clearly demonstrated that preconditioning enhanced cell proliferation and survival at the site of cell graft.
Figure 3.
Enhanced survival and proliferation of PCMY in the infarcted heart. Quantification of cell survival by (A) real-time PCR and (B) conventional PCR for donor-specific sry-gene. A significantly higher survival of PCMY (Group 3) was observed on 4 and 7 days post-transplantation, respectively (n = 4 on each time point) when compared with non-PCMY (Group 2; n = 4 on each time point; P < 0.01 vs. non-PCMY). DMEM without cells injected Group 3 (n = 2 on each time point) did not show sry-gene expression and served as a negative control. (C and D) Tracking of nlsLacz-labelled transplanted cells by β-gal histochemistry at 7 days post-transplantation (C) non-PCMY transplanted and (D) PCMY transplanted tissue sections (original magnification, ×100). Red and green boxes in the respective figures represent the magnified regions with red arrows showing nlsLacz-labelled transplanted cells. (E) Histological tissue section from Group 3 animal heart 7 days after PCMY transplantation showing multinucleated myotubes formed from PKH26-labelled transplanted cells (red). The nuclei were stained blue with DAPI (original magnification, ×1000). (F) Preconditioning increased the proliferative capacity of transplanted cells. Fluorescence immunostaining for Ki67 antigen on heart tissue sections from 7 days post-transplanted animals (red, PKH26-labelled cells; green, Ki67-positive cells; blue, DAPI for nuclear staining).
3.3. Stable angiomyogenic response in the infarcted heart
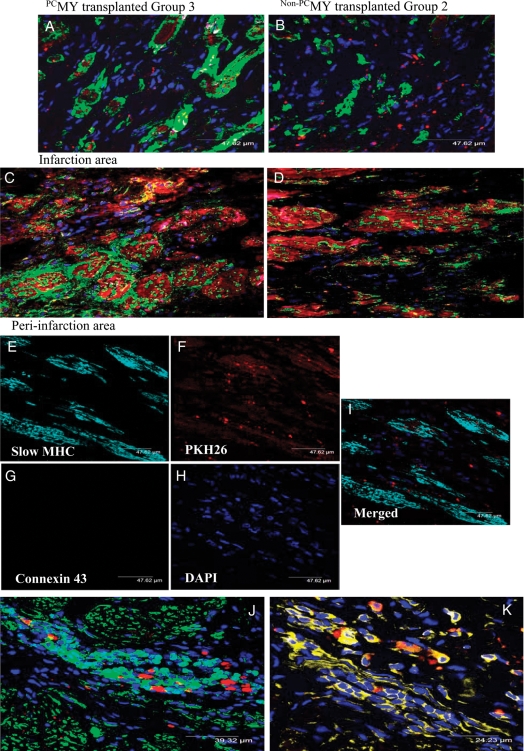
There were no animals deaths related to cell transplantation. All animals survived full length of experiments. Immunohistological studies provided convincing evidence of myogenic differentiation of the transplanted cells at 4 months post-engraftment, also showing their long-term survival in the infarcted myocardium (Figure 4). This was demonstrated in the confocal images of the histological sections immunostained for myogenic marker proteins including α-sarcomeric actinin (Figure 4A–D and J) and myosin heavy chain slow isoform (Figure 4E–I and K). An obvious co-localization of PKH26 (red fluorescence) and myogenic marker protein expression (green fluorescence) was observed in Group 3 animal hearts in both infarct and peri-infarct regions. These observations evidenced the persistence of transplanted cells until 4 months after transplantation. Although preconditioning induced connexin-43 expression in PCMY, lack of coupling between the neofibres and host cardiomyocytes was evident from the absence of connexin-43 positivity on histological sections counter-immunostained for myosin heavy chain slow isoform. Confocal images showed clusters of intrinsic repair cells staining positive for α-sarcomeric actinin (Figure 4J) and myosin heavy chain slow isoform (Figure 4K) in the infarcted heart at 4 months of observation.
Figure 4.
Improved myogenic potential of PCMY. Confocal images of the heart tissue histological sections immunostained for myogenic marker α-sarcomeric actinin (green) showing myogenic differentiation of PKH26-labelled (red) donor (A–C) PCMY and (B–D) non-PCMY in the infarction and peri-infarction areas. The animals were harvested at 16 weeks post-transplantation of cells. Blue fluorescence is indicative of DAPI nuclear staining. Negative staining for connexin-43 (magenta fluorescence) in (A–D) indicates lack of coupling between the neofibres and the host cardiomyocytes. (E–I) Immunostaining for myosin heavy chain (slow isoform) expression to elucidate myogenic differentiation of PCMY (cyan fluorescence) without staining for connexin-43 (magenta fluorescence) in this region. Interestingly, we observed (J) cluster of myosin heavy chain-positive (yellow) and α-sarcomeric actin-positive (green) cells mobilized into the infarcted heart at the site of cell injection indicated by the presence of PKH26-labelled (red) cells.
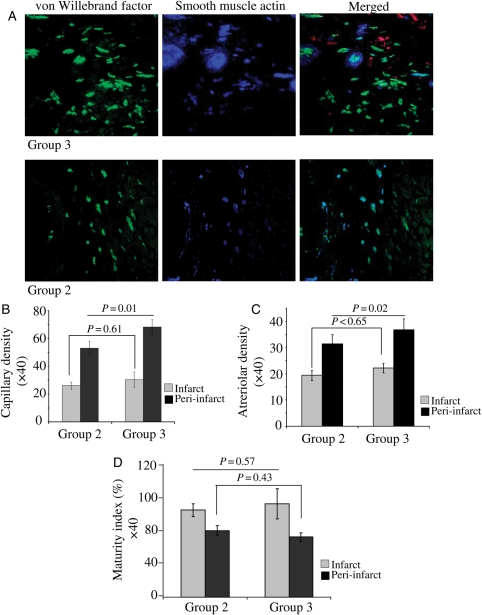
Immunostaining for vWF-VIII revealed significantly higher capillary density per surface area (0.155 mm2) at ×400 magnification in the cell transplanted groups when compared with DMEM-injected Group 1 in both peri-infarct and infarct areas (Figure 5A and B). The highest capillary density was observed in peri-infarct area in Group 3 (68 ± 5) which was higher when compared with Group 2 (53 ± 5; P = 0.01). Although capillary density was higher in the centre of the infarct in Group 3, it was insignificantly different from Group 2 in the same region (P < 0.61). A similar trend for matured blood vessels was observed after immunohistology of the heart section for smooth muscle actin expression. The highest number of arterioles (blood vessels positive for both vWF-VIII and smooth muscle actin) per surface area (0.155 mm2) was observed in Group 3 which was significantly higher than Group 2 (P < 0.02) in the peri-infarct area, whereas arteriolar density was insignificantly different between these two groups in the infarct area (P = 0.65; Figure 5C). However, overall maturity index in Groups 2 and 3 in both infarct (P = 0.57) and peri-infarct (P = 0.43) regions remained insignificantly different from each other (Figure 5D).
Figure 5.
Assessment of blood vessel density in the infarcted heart. (A) Fluorescent immunostaining for vWF-VIII (green) and smooth muscle actin (blue) at 4 months post-engraftment of PCMY in Group 3 and non-PCMY in Group 2. Red fluorescence seen in the background is from the transplanted cells labelled with PKH26. (B) Capillary density (P < 0.01; indicated by green fluorescence = vWF-VIII) and (C) arteriolar density (P < 0.02; indicated by green fluorescence = vWF-VIII and smooth muscle actin = red fluorescence) were significantly increased in the peri-infarct region in Group 3 when compared with Group 2 at 4 weeks post-respective treatment. (D) Maturity index, indicated by co-localization of the green and blue fluorescence (merged images), was insignificantly different between the two groups; however, the total number of mature blood vessels was higher in Group 3.
3.4. The heart function studies
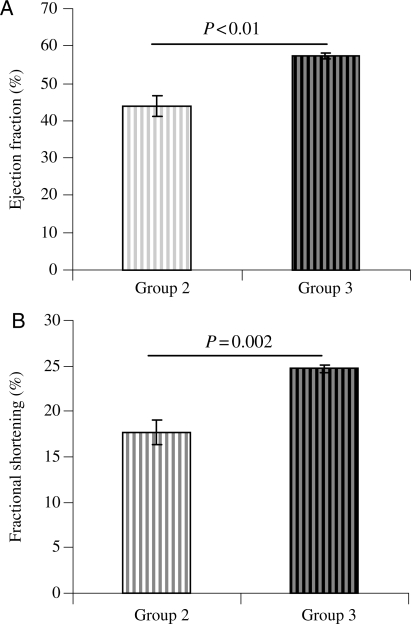
Echocardiography showed well-preserved left ventricular ejection fraction (LVEF) and left ventricular fractional shortening (LVFS) in Group 3 when compared with its control Group 2 which persisted until 4 months (Figure 6A and B). LVEF (57.32 ± 0.67) and LVFS (24.74 ± 0.40) in Group 3 was more preserved as compared with Group 2 (44.02 ± 2.83, P < 0.01 and 17.70 ± 1.39, P < 0.01, respectively). EKG recordings were obtained in three animals per groups before cell transplantation and at 24 and 48 h after cell transplantation. We observed no arrythmogenicity in non-PCMY- as well as PCMY-transplanted hearts until 48 h of observation.
Figure 6.
Heart function studies of animals at 16 weeks post-transplantation. (A) LVEF and (B) LVFS was significantly improved in PCMY-transplanted Group 3 when compared with non-PCMY Group 2 (57.32 ± 0.67 vs. 44.02 ± 2.83 and 24.74 ± 0.40 vs. 17.70 ± 1.39, respectively).
4. Discussion
Despite the presence of resident stem and progenitor cells,15 inadequacy of the intrinsic repair mechanism in the heart warrants stem cell-based intervention to substitute the massive loss of functioning cardiomyocytes in the event of myocardial injury. Unfortunately, the strategy of heart cell therapy suffers from rapid and massive death of the grafted cells during acute phase of engraftment.16 The dynamics of early donor cell death in the hostile microenvironment of the infarcted heart involves several factors.17 Pharmacological manipulation of donor cells with preconditioning mimetics is an effective strategy to prime the cells by initiating survival signalling prior to engraftment to improve their survival, paracrine activity, and differentiation potential.
We have recently reported that ischaemic preconditioning can effectively protect bone marrow-derived mesenchymal stem cells (MSCs) against ischaemic injury.1 The cytoprotective effects of ischaemic preconditioning can be duplicated with preconditioning mimetics.17,18 Indeed, pharmacological preconditioning now matches ischaemic preconditioning as one of the most potent intervention to reduce apoptosis. Dz is one of the classical preconditioning mimetics which opens ATP-sensitive potassium channels (mitoK+ATP channel) to exert therapeutic benefit.19,20 Besides, Dz also works through multiple signalling mechanisms including inhibition of succinate dehydrogenase, mitochondrial depolarization, protein kinase-C activation, and involvement of a potassium conductance-independent pathway for cellular protection.21–24 We have previously reported that cardiac protection from mitoK+ATP channels is Akt-dependent which entails its translocation from the cytosol to the mitochondria.25 The present study elucidates the role of preconditioning-induced miR-21 in protection of PCMY via activation of Erk1/2 and Stat3 signalling.
IL-11 is a non-glycosylated protein of ∼23-kDa and is secreted by various cells including bone marrow stromal cells. Besides IL-11 activity related to cell proliferation and colony formation, IL-11 prevents apoptosis and activates both Erk1/2 and Stat3.9,26 A direct evidence of the cytoprotective effects of IL-11 in vitro was first reported in vascular endothelium.27 The study reported a coordinated signalling of Erk1/2 and Stat3 wherein Erk1/2 phosphorylated Stat3 to promote its transcriptional activity. Although the study focused on immune-mediated injury, its application was also proposed to non-immune models of cellular injury. Subsequently, the same research group reported that pre-treatment of HUVECs with recombinant IL6 and rIL-11 could protect the cells against oxidant stress with the coordinated interaction between Erk1/2 and Stat3 activation.6 In the present study, we observed that up-regulation of IL-11 initiated intracellular signalling circuitry along Erk1/2–Stat3 pathway to promote cytoprotection in PCMY. This was evident from the higher level phosphorylation of Erk1/2 and Stat3 in PCMY which was abrogated in the presence of PD98059 with a concomitant reduction in cell survival. However, the present study observed the effects of IL-11 which was intrinsic to the cells in response to preconditioning rather than transgenic overexpression or the use of rIL-11 protein. Silencing of IL-11 gene with specific siRNA in PCMY reduced Stat3 phosphorylation to affirm that preconditioning-induced IL-11 was as effective in cytoprotection as rIL-11 treatment of the cells.
A novel aspect of our study is the role for miR-21 in preconditioning of MY elicited by IL-11 signalling. This conclusion is supported by significant up-regulation of miR-21 in PCMY in an Erk1/2-dependent manner and its participation in cell survival signalling under oxidant stress. Although there is no evidence in literature regarding the role of miR-21 in preconditioning, there is mounting evidence of its participation in Erk1/2 and Stat3-mediated cell survival.4,7 In the light of these results, it is possible to precondition the cells by manipulation of miR-21 expression prior to engraftment and duplicate the cytoprotective effects of Dz treatment at miR level. As a single miR can influence multiple gene expression, future studies are required to investigate the influence of miR-21 on other properties of stem cells besides cytoprotection such as paracrine effects, differentiation, etc. in general and during preconditioning of stem cells in particular. The expression of miR-21 during preconditioning was sensitive to Erk1/2 inhibition and abrogation of miR-21 in PCMY compromised their survival under oxidant stress. We observed significant cell death upon transfection of the cells with anti-miR-21 without preconditioning which might be indicative of a combined effect of the role of miR-21 in survival together with technical issues and experimental conditions related with transfection of anti-miR-21. PCMSCs which escaped the acute phase of cell death managed to survive for longer time (4 months) and contributed towards myoangiogenesis in the infarcted heart.9 In summary, we propose that Dz preconditioning triggers the PI3K-Akt pathway to activate IL-11 expression which up-regulates miR-21 via Erk1/2–Stat3 signalling to promote stem cell survival.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Conflict of interest: none declared.
Funding
This work was supported by National Institutes of Health (NIH) Grants # R37-HL074272, HL-080686, and HL-087246 (M.A.) and HL-087288 and HL-089535 (K.H.H).
Supplementary Material
References
- 1.Kim HW, Haider HK, Jiang S, Ashraf M. Ischemic preconditioning augments survival of stem cells via MIR-210 expression by targeting caspase-8 associated protein 2. J Biol Chem. 2009;284:33161–33168. doi: 10.1074/jbc.M109.020925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rane S, Sayed D, Abdellatif M. MicroRNA with a MacroFunction. Cell Cycle. 2007;6:1850–1855. doi: 10.4161/cc.6.15.4551. [DOI] [PubMed] [Google Scholar]
- 3.Krichevsky AM, Gabriely G. miR-21: a small multi-faceted RNA. J Cell Mol Med. 2009;13:39–53. doi: 10.1111/j.1582-4934.2008.00556.x. doi:10.1111/j.1582-4934.2008.00556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yan LX, Huang XF, Shao Q, Huang MY, Deng L, Wu QL, et al. MicroRNA miR-21 overexpression in human breast cancer is associated with advanced clinical stage, lymph node metastasis and patient poor prognosis. RNA. 2008;14:2348–2360. doi: 10.1261/rna.1034808. doi:10.1261/rna.1034808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thum T, Gross C, Fiedler J, Fischer T, Kissler S, Bussen M, et al. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature. 2008;456:980–984. doi: 10.1038/nature07511. doi:10.1038/nature07511. [DOI] [PubMed] [Google Scholar]
- 6.Waxman AB, Mahboubi K, Knickelbein RG, Mantell LL, Manzo N, Pober JS, et al. Interleukin-11 and interleukin-6 protect cultured human endothelial cells from H2O2-induced cell death. Am J Respir Cell Mol Biol. 2003;29:513–522. doi: 10.1165/rcmb.2002-0044OC. doi:10.1165/rcmb.2002-0044OC. [DOI] [PubMed] [Google Scholar]
- 7.Loffler D, Brocke-Heidrich K, Pfeifer G, Stocsits C, Hackermuller J, Kretzschmar AK, et al. Interleukin-6 dependent survival of multiple myeloma cells involves the Stat3-mediated induction of microRNA-21 through a highly conserved enhancer. Blood. 2007;110:1330–1333. doi: 10.1182/blood-2007-03-081133. doi:10.1182/blood-2007-03-081133. [DOI] [PubMed] [Google Scholar]
- 8.Shen XH, Han YJ, Zhang DX, Cui XS, Kim NH. A link between the interleukin-6/Stat3 anti-apoptotic pathway and microRNA-21 in preimplantation mouse embryos. Mol Reprod Dev. 2009;76:854–862. doi: 10.1002/mrd.21048. doi:10.1002/mrd.21048. [DOI] [PubMed] [Google Scholar]
- 9.Boulton TG, Zhong Z, Wen Z, Darnell JE, Jr, Stahl N, Yancopoulos GD. STAT3 activation by cytokines utilizing gp130 and related transducers involves a secondary modification requiring an H7-sensitive kinase. Proc Natl Acad Sci USA. 1995;92:6915–6919. doi: 10.1073/pnas.92.15.6915. doi:10.1073/pnas.92.15.6915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Niagara MI, Haider H, Jiang S, Ashraf M. Pharmacologically preconditioned skeletal myoblasts are resistant to oxidative stress and promote angiomyogenesis via release of paracrine factors in the infarcted heart. Circ Res. 2007;100:545–555. doi: 10.1161/01.RES.0000258460.41160.ef. doi:10.1161/01.RES.0000258460.41160.ef. [DOI] [PubMed] [Google Scholar]
- 11.Lu G, Haider HK, Jiang S, Ashraf M. Sca-1+ stem cell survival and engraftment in the infarcted heart: dual role for preconditioning-induced connexin-43. Circulation. 2009;119:2587–2596. doi: 10.1161/CIRCULATIONAHA.108.827691. doi:10.1161/CIRCULATIONAHA.108.827691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang S, Haider H, Idris NM, Salim A, Ashraf M. Supportive interaction between cell survival signaling and angiocompetent factors enhances donor cell survival and promotes angiomyogenesis for cardiac repair. Circ Res. 2006;99:776–784. doi: 10.1161/01.RES.0000244687.97719.4f. doi:10.1161/01.RES.0000244687.97719.4f. [DOI] [PubMed] [Google Scholar]
- 13.Tenney R, Stansfield K, Pekala PH. Interleukin 11 signaling in 3T3-L1 adipocytes. J Cell Physiol. 2005;202:160–166. doi: 10.1002/jcp.20100. doi:10.1002/jcp.20100. [DOI] [PubMed] [Google Scholar]
- 14.Aoki Y, Feldman GM, Tosato G. Inhibition of STAT3 signaling induces apoptosis and decreases survivin expression in primary effusion lymphoma. Blood. 2003;101:1535–1542. doi: 10.1182/blood-2002-07-2130. doi:10.1182/blood-2002-07-2130. [DOI] [PubMed] [Google Scholar]
- 15.Leri A, Kajstura J, Anversa P. Cardiac stem cells and mechanisms of myocardial regeneration. Physiol Rev. 2005;85:1373–1416. doi: 10.1152/physrev.00013.2005. doi:10.1152/physrev.00013.2005. [DOI] [PubMed] [Google Scholar]
- 16.Muller-Ehmsen J, Krausgrill B, Burst V, Schenk K, Neisen UC, Fries JW, et al. Effective engraftment but poor mid-term persistence of mononuclear and mesenchymal bone marrow cells in acute and chronic rat myocardial infarction. J Mol Cell Cardiol. 2006;41:876–884. doi: 10.1016/j.yjmcc.2006.07.023. doi:10.1016/j.yjmcc.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 17.Haider H, Ashraf M. Strategies to promote donor cell survival: combining preconditioning approach with stem cell transplantation. J Mol Cell Cardiol. 2008;45:554–566. doi: 10.1016/j.yjmcc.2008.05.004. doi:10.1016/j.yjmcc.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 19.Rajapakse N, Kis B, Horiguchi T, Snipes J, Busija D. Diazoxide pretreatment induces delayed preconditioning in astrocytes against oxygen glucose deprivation and hydrogen peroxide-induced toxicity. J Neurosci Res. 2003;73:206–214. doi: 10.1002/jnr.10657. doi:10.1002/jnr.10657. [DOI] [PubMed] [Google Scholar]
- 20.Garlid KD, Dos Santos P, Xie ZJ, Costa AD, Paucek P. Mitochondrial potassium transport: the role of the mitochondrial ATP-sensitive K(+) channel in cardiac function and cardioprotection. Biochim Biophys Acta. 2003;1606:1–21. doi: 10.1016/s0005-2728(03)00109-9. [DOI] [PubMed] [Google Scholar]
- 21.Takashi E, Wang Y, Ashraf M. Activation of mitochondrial K(ATP) channel elicits late preconditioning against myocardial infarction via protein kinase C signaling pathway. Circ Res. 1999;85:1146–1153. doi: 10.1161/01.res.85.12.1146. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y, Takashi E, Xu M, Ayub A, Ashraf M. Downregulation of protein kinase C inhibits activation of mitochondrial K(ATP) channels by diazoxide. Circulation. 2001;104:85–90. doi: 10.1161/01.cir.104.1.85. [DOI] [PubMed] [Google Scholar]
- 23.Kudo M, Wang Y, Xu M, Ayub A, Ashraf M. Adenosine A(1) receptor mediates late preconditioning via activation of PKC-delta signaling pathway. Am J Physiol Heart Circ Physiol. 2002;283:H296–H301. doi: 10.1152/ajpheart.01087.2001. [DOI] [PubMed] [Google Scholar]
- 24.Dzeja PP, Bast P, Ozcan C, Valverde A, Holmuhamedov EL, Van Wylen DG, et al. Targeting nucleotide-requiring enzymes: implications for diazoxide-induced cardioprotection. Am J Physiol Heart Circ Physiol. 2003;284:H1048–H1056. doi: 10.1152/ajpheart.00847.2002. [DOI] [PubMed] [Google Scholar]
- 25.Ahmad N, Wang Y, Haider KH, Wang B, Pasha Z, Uzun O, et al. Cardiac protection by mitoKATP channels is dependent on Akt translocation from cytosol to mitochondria during late preconditioning. Am J Physiol Heart Circ Physiol. 2006;290:H2402–H2408. doi: 10.1152/ajpheart.00737.2005. doi:10.1152/ajpheart.00737.2005. [DOI] [PubMed] [Google Scholar]
- 26.Yang YC, Yin T. Interleukin (IL)-11-mediated signal transduction. Ann N Y Acad Sci. 1995;762:31–40. doi: 10.1111/j.1749-6632.1995.tb32312.x. [DOI] [PubMed] [Google Scholar]
- 27.Mahboubi K, Biedermann BC, Carroll JM, Pober JS. IL-11 activates human endothelial cells to resist immune-mediated injury. J Immunol. 2000;164:3837–3846. doi: 10.4049/jimmunol.164.7.3837. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.