Abstract
It has been proposed that intraspecific ultrasonic communication observed in some moths evolved, through sexual selection, subsequent to the development of ears sensitive to echolocation calls of insectivorous bats. Given this scenario, the receiver bias model of signal evolution argues that acoustic communication in moths should have evolved through the exploitation of receivers' sensory bias towards bat ultrasound. We tested this model using a noctuid moth Spodoptera litura, males of which were recently found to produce courtship ultrasound. We first investigated the mechanism of sound production in the male moth, and subsequently the role of the sound with reference to the female's ability to discriminate male courtship songs from bat calls. We found that males have sex-specific tymbals for ultrasound emission, and that the broadcast of either male songs or simulated bat calls equally increased the acceptance of muted males by the female. It was concluded that females of this moth do not distinguish between male songs and bat calls, supporting the idea that acoustic communication in this moth evolved through a sensory exploitation process.
Keywords: bat predation, courtship ultrasound, receiver bias, tymbal
1. Introduction
Moths have evolved ears that detect the echolocation calls of insectivorous bats (Roeder 1962; Minet & Surlykke 2003). Subsequently, some groups of moths have evolved sound-producing organs for intraspecific acoustic communication and/or defensive responses against predators (Spangler 1988; Conner 1999; Greenfield 2002). In wax moths, males produce ultrasonic clicks with tegular tymbal organs to attract females, while they show a freeze response to bat echolocation calls (Spangler 1988; Greenfield & Weber 2000). In tiger moths, both sexes emit ultrasonic clicks with metathoracic tymbals in response to bat calls to jam the echolocation or to deter bats from attacking (Minet & Surlykke 2003; Barber & Conner 2006). Some tiger moths produce ultrasonic clicks for sexual communication as well (Conner 1999). These examples indicate that acoustically communicating moths have gained the ability to distinguish the ultrasounds of bats from those of conspecifics based on characteristics of the sounds, pulse structure for instance (Greenfield & Weber 2000; Fullard et al. 2007).
The receiver's sensory bias for detecting a predator and/or prey drives the evolution of signals in animal communications (e.g. vibratory displays in water mites, sword ornaments in swordtail fish and calling song frequency in túngara frogs: see ‘receiver bias model’ in Ryan 1998; Greenfield 2002). Ultrasonic communication in moths has also been supposed to have developed through exploitation of the ability to detect ultrasound for avoiding predation by bats (Endler & Basolo 1998; Conner 1999; Greenfield & Weber 2000). However, the receiver bias model has not been experimentally verified in acoustic communication in moths, because the signal receivers in the moths examined had already evolved the ability to distinguish singing mates from echolocating bats, as described above (Surlykke & Fullard 1989; Greenfield & Weber 2000; Skals & Surlykke 2000; Fullard et al. 2007). If we can find a case where the receivers do not discriminate between the sounds of bats and those of a mate, it would present direct evidence for the process of sensory exploitation.
We recently reported acoustic sexual communication in the common cutworm Spodoptera litura (Nakano et al. 2009). However, the mechanism of sound production and the ability of receivers to discriminate sounds have not been studied. Here, we present the mechanism of ultrasound production, and the receivers' responses to male ultrasound playbacks and simulated bat calls in S. litura.
2. Material and methods
Using 1–2-day-old S. litura moths, we conducted two behavioural tests in the last 2 h of the scotophase, in which this moth shows high mating activity (methods in the electronic supplementary material; Nakano et al. 2009). First, to verify the occurrence of acoustic communication, we examined the effect of the muting of males on mating success. For muting, we punctured the tymbals on the male metathorax using fine forceps (methods in the electronic supplementary material). A sham operation was conducted by puncturing the mesothoracic coxae. For all surgical operations, male moths were anaesthetized by CO2 and treated under a stereomicroscope 1 day before the tests.
Next, to examine the ability of the female to discriminate between male songs and bat calls, the mating success of sham-operated males, which generate ultrasound, was compared with that of muted males with the broadcast of (i) male songs, (ii) background noise, or (iii) simulated bat calls from a loudspeaker set 20 cm from the female (methods and figure S1 in the electronic supplementary material). The male songs and bat calls were broadcast at the natural levels, 70 dB peSPL and 100 dB SPL, respectively, at the point of female moth (Surlykke & Kalko 2008; Nakano et al. 2009). Two different types of echolocation calls of moth-eating bats were simulated; one was ultrasonic calls of short frequency-modulation (FM) pulses (e.g. calls of Eptesicus fuscus), and the other was those of long constant-frequency (CF) pulses (e.g. calls of Rhinolophus ferrumequinum) in the search-approach phase, which elicit evasive responses in moths including S. littoralis, a congener of S. litura (Skals et al. 2005) (see methods in the electronic supplementary material).
Males used in this experiment were deafened to eliminate effects of broadcast sound stimuli on their behaviour. In all behavioural tests, a single unmated male was introduced into a cubic mesh cage (18 × 18 × 18 cm) housing 5–10 intact virgin females. Multiple females were housed in the cage so that always at least one female would be releasing sex pheromones during the experiment. The male usually readily flew up to one of the females, and started courting. We defined the female mate acceptance as completion of copulation, which occurred subsequent to the cessation of her locomotion. The observation of mating behaviour was continued until the female accepted the male or rejected him by flying away. The pair observed was removed from the cage, and the experiment was continued with a new male. The ultrasound emitted from males and the loudspeaker was continuously monitored with an ultrasound detector (model D240x, Pettersson Elektronik AB, Uppsala, Sweden).
3. Results
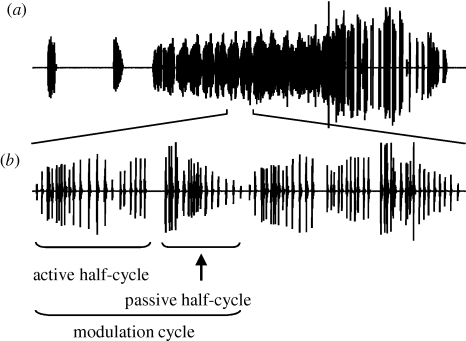
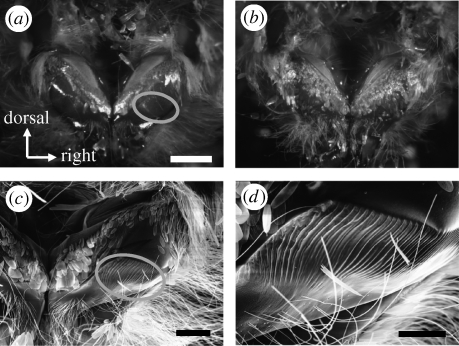
Male S. litura emitted ultrasounds during courtship in the proximity of a female (figure 1; electronic supplementary material, movie S1). The male moth possesses a striated cuticular membrane, i.e. tymbal organ, on the ventral side of the metathorax (figure 2). Destruction of this organ deprived the male of the ability to produce ultrasound. The ultrasonic song emitted from the tymbal consisted of clicks of short duration (0.1–0.2 ms), and showed active/passive modulation cycles divided by a silent gap (figure 1). Females do not have tymbals on the thorax, and did not emit ultrasound during mating behaviour (the electronic supplementary material, movie S1).
Figure 1.
Courtship ultrasounds of male S. litura. (a) Oscillograms of a single song train and (b) two modulation cycles are shown for 500 and 30 ms, respectively. The half-cycles putatively include ultrasonic clicks produced by tymbals ipsilateral and contralateral to the microphone.
Figure 2.
Metathoracic tymbals of S. litura. (a,b) Stereomicrographs of metathoracic coxae of (a) male and (b) female moths from a posterior view. All wings, all legs and the abdomen were removed from the body. (c,d) Scanning electron micrographs of the right tymbal. (d) Magnified image of (c). Grey circles in (a,c) indicate the position of the right tymbal membrane. Females do not possess the tymbals. Scales on the metathorax are not removed. Scale bars, (a) 2 mm; (c) 1 mm; (d) 500 µm.
Behavioural experiments using muted males demonstrated the significance of courtship ultrasound for mating success. Most of the females, 95–100%, accepted singing males for mating, whereas only 40 per cent accepted muted males (Fisher's exact probability test for male treatments, p < 0.0001) (table 1).
Table 1.
Effect of male ultrasound on mating success. (Mating success of muted males was significantly lower than that of intact or sham-operated males.)
| male treatmenta | n | % matingb (no. of pairs mated) |
|---|---|---|
| intact | 21 | 100 (21) |
| tymbal punctured | 17 | 41 ( 7) |
| sham-operatedc | 21 | 95 (20) |
aAll females were intact;
bFisher's exact probability test, p < 0.0001;
cmesothoracic coxae were punctured instead of tymbals.
Sham-operated males producing natural ultrasound had 100 per cent mating success (table 2). Playback of male ultrasounds completely restored the muted males' mating success to 100 per cent in comparison with playback of noise (56% acceptance) (table 2; Fisher's exact probability test for sound stimuli, p < 0.0001). Bat calls were also effective; females showed high mate acceptance (91 and 100%) when simulated FM or CF bat echolocation calls were broadcast (table 2).
Table 2.
Effect of synthesized ultrasound on mating success. (Broadcasts of male ultrasound, FM (frequency-modulation) bat calls, and CF (constant-frequency) bat calls significantly increased the mating success of muted males.)
| sound stimulia | sound level (dB SPL) | n | % matingb (no. of pairs mated) |
|---|---|---|---|
| natural male ultrasoundc | 70 | 13 | 100 (13) |
| playback of male ultrasound | 70 | 20 | 100 (20) |
| playback of background noise | 22 | 18 | 56 (10) |
| simulated FM bat calls | 100 | 22 | 91 (20) |
| simulated CF bat calls | 100 | 18 | 100 (18) |
aAll males tested here were deafened to eliminate the effect of sound stimuli on their behaviour;
bFisher's exact probability test, p < 0.0001;
cmesothoracic coxae were punctured as a sham operation.
4. Discussion
Males of S. litura have evolved a tymbal organ (figure 2), which looks similar to the tymbals found in many arctiid tiger moths (Barber & Conner 2006). The tymbal of S. litura, however, differs in its location, the ventral side of the metathorax, versus the lateral side in arctiids. Also, the tymbals are found in both sexes in arctiids but only in males in S. litura. Both sexes of tiger moths show phonoresponses to bat calls, i.e. they react to bat calls by producing sound (Barber & Conner 2006), but neither sex of intact S. litura shows a phonoresponse (data not shown). These findings suggest that male S. litura have developed a sound-producing mechanism not for defence, but for sexual communication.
The male ultrasound may be a signal used for mate recognition by receptive females, because muted males were accepted at a higher rate when the male song was played back. Hence, we considered the possibility that the female distinguishes male ultrasound from bat calls, as reported for females of wax moths, Achroia grisella and Galleria mellonella, which communicate with males using ultrasonic clicks (Greenfield & Weber 2000; Skals & Surlykke 2000). In playback experiments, however, simulated bat calls had the same effect as the male courtship song (table 2). These results suggest that females of S. litura do not discriminate between male songs and bat calls.
Eared moths freeze to evade echolocating bats because stationary moths have a better chance to be overlooked by the bats (Roeder 1962; Spangler 1988; Greenfield & Weber 2000; Minet & Surlykke 2003). We recently showed that the ultrasonic songs of the male Ostrinia furnacalis render the females motionless, and argued that this is the same response as that to ultrasonic bat calls (Nakano et al. 2010). Therefore, the finding presented here supports the idea that by emitting ultrasounds, males deceive the female into making a freeze response during the male's courting. Subsequently, the singing male can copulate with the stationary female with much less difficulty (Nakano et al. 2008, 2010). Consequently, the female may incidentally select ultrasound-producing males over silent males by accepting a significantly higher proportion of singing males for mating. These reactions by both genders would have contributed to the establishment of sexual communication in moths. In conclusion, we have demonstrated that females of S. litura do not distinguish between male songs and bat calls, consistent with the idea that acoustic communication in this moth evolved through a sensory exploitation process.
Acknowledgements
We thank T. Honda for collecting insects. This study was supported by Grants-in-aid from the Japan Society for the Promotion of Science (R.N. and Y.I.), by Inoue Research Aid for Young Scientists (R.N.), and by the Danish Natural Science Research Council (N.S. and A.S.).
References
- Barber J. R., Conner W. E.2006Tiger moth responses to a simulated bat attack: timing and duty cycle. J. Exp. Biol. 209, 2637–2650 (doi:10.1242/jeb.02295) [DOI] [PubMed] [Google Scholar]
- Conner W. E.1999‘Un chant d'appel amoureux’: acoustic communication in moths. J. Exp. Biol. 202, 1711–1723 [DOI] [PubMed] [Google Scholar]
- Endler J. A., Basolo A. L.1998Sensory ecology, receiver biases and sexual selection. Trend. Ecol. Evol. 13, 415–420 (doi:10.1016/S0169-5347(98)01471-2) [DOI] [PubMed] [Google Scholar]
- Fullard J. H., Ratcliffe J. M., Christie C. G.2007Acoustic feature recognition in the dogbane tiger moth, Cycnia tenera. J. Exp. Biol. 210, 2481–2488 (doi:10.1242/jeb.001909) [DOI] [PubMed] [Google Scholar]
- Greenfield M. D.2002Signalers and receivers. Oxford, UK: Oxford University Press [Google Scholar]
- Greenfield M. D., Weber T.2000Evolution of ultrasonic signalling in wax moths: discrimination of ultrasonic mating calls from bat echolocation signals and the exploitation of an antipredator receiver bias by sexual advertisement. Ethol. Ecol. Evol. 12, 259–279 [Google Scholar]
- Minet J., Surlykke A.2003Auditory and sound producing organs. In Lepidoptera, moths and butterflies. Vol. 2: morphology and physiology (ed. Kristensen N. P.), pp. 289–323 Berlin, New York: Walter de Gruyter [Google Scholar]
- Nakano R., Skals N., Takanashi T., Surlykke A., Koike T., Yoshida K., Maruyama H., Tatsuki S., Ishikawa Y.2008Moths produce extremely quiet ultrasonic courtship songs by rubbing specialized scales. Proc. Natl Acad. Sci. USA 105, 11 812–11 817 (doi:10.1073/pnas.0804056105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano R., Takanashi T., Fujii T., Skals N., Surlykke A., Ishikawa Y.2009Moths are not silent, but whisper ultrasonic courtship songs. J. Exp. Biol. 212, 4072–4078 (doi:10.1242/jeb.032466) [DOI] [PubMed] [Google Scholar]
- Nakano R., Takanashi T., Skals N., Surlykke A., Ishikawa Y.2010Ultrasonic courtship songs of male Asian corn borer moths assist copulation attempts by making the females motionless. Physiol. Entomol. 35, 76–81 (doi:10.1111/j.1365-3032.2009.00712.x) [Google Scholar]
- Roeder K. D.1962The behaviour of free flying moths in the presence of artificial ultrasonic pulses. Anim. Behav. 10, 300–304 (doi:10.1016/0003-3472(62)90053-2) [Google Scholar]
- Ryan M. J.1998Sexual selection, receiver biases, and the evolution of sex differences. Science 281, 1999–2003 (doi:10.1126/science.281.5385.1999) [DOI] [PubMed] [Google Scholar]
- Skals N., Surlykke A.2000Hearing and evasive behaviour in the greater wax moth, Galleria mellonella (Pyralidae). Physiol. Entomol. 25, 354–362 (doi:10.1111/j.1365-3032.2000.00204.x) [Google Scholar]
- Skals N., Anderson P., Kanneworff M., Löfstedt C., Surlykke A.2005Her odours make him deaf: crossmodal modulation of olfaction and hearing in a male moth. J. Exp. Biol. 208, 595–601 (doi:10.1242/jeb.01400) [DOI] [PubMed] [Google Scholar]
- Spangler H. G.1988Moth hearing, defense and communication. Annu. Rev. Entomol. 33, 59–81 (doi:10.1146/annurev.en.33.010188.000423) [Google Scholar]
- Surlykke A., Fullard J. H.1989Hearing of the Australian whistling moth, Hecatesia thyridion. Naturwissenschaften 76, 132–134 (doi:10.1007/BF00366610) [Google Scholar]
- Surlykke A., Kalko E. K. V.2008Echolocating bats cry out loud to detect their prey. PLoS ONE 3, e2036 (doi:10.1371/journal.pone.0002036) [DOI] [PMC free article] [PubMed] [Google Scholar]