Abstract
Glucocorticoids regulate glucose concentrations and responses to unpredictable events, while also modulating cognition. Juvenile Belding's ground squirrels (Urocitellus beldingi) learn to respond to whistle and trill alarm calls, warning of aerial and terrestrial predators, respectively, shortly after emerging from natal burrows at one month of age. Alarm calls can cause physiological reactions and arousal, and this arousal, coupled with watching adult responses, might help juveniles learn associations between calls and behavioural responses. I studied whether young show differential cortisol responses to alarm and non-alarm calls, using playbacks of U. beldingi whistles, trills, squeals (a conspecific control vocalization) and silent controls. Trills elicited very high cortisol responses, and, using an individual's response to the silent control as baseline, only their response to a trill was significantly higher than baseline. This cortisol increase would provide glucose for extended vigilance and escape efforts, which is appropriate for evading terrestrial predators which hunt for long periods. Although whistles do not elicit a cortisol response, previous research has shown that they do result in bradycardia, which enhances attention and information processing. This is a novel demonstration of two physiological responses to two alarm calls, each appropriate to the threats represented by the calls.
Keywords: cortisol, learning, alarm calls, development, ground squirrel, Urocitellus beldingi
1. Introduction
In many species of group-living birds and mammals, the threat of a predator elicits vocalizations that alert other animals of danger. These alarm calls are useful to individuals who have not yet detected the predator (Klump & Shalter 1984), particularly immature young who are especially vulnerable to predation (Mateo 1996a). This raises the question of when and how young develop adaptive behavioural responses to alarm calls (Hollén & Radford 2009).
Exposure to a predator, predator vocalization or predator odour leads to changes in oxygen consumption or glucocorticoid release in a variety of species (reviewed by Apfelbach et al. 2005). These glucocorticoid responses can serve several functions that could be beneficial in predator contexts, such as improving cardiovascular tone, inhibiting gastrointestinal, reproductive and immune systems, and, importantly, increasing available glucose. These effects would allow animals to sharpen their focus (e.g. to detect or track predators, or observe behavioural responses of others), maintain vigilance, flee rapidly or suppress activity to avoid detection (Sapolsky et al. 2000; Romero 2002). Glucocorticoids can also negatively or positively influence learning about anti-predator contexts (e.g. Park et al. 2008; Thaker et al. 2010; reviewed in Lupien & McEwen 1997; Sauro et al. 2003).
Here, I examined the potential role of glucocorticoids in the acquisition of anti-predator behaviour in a wild, outbred species. Belding's ground squirrels (Urocitellus beldingi) are group-living, burrowing rodents found at high elevation in the western United States. They are socially active above ground for 3–4 summer months and hibernate the remainder of the year. Adult females raise a litter of 5–8 pups in an underground burrow (the natal burrow) until young emerge above ground at 25–27 days of age (Jenkins & Eshelman 1984). Urocitellus beldingi are hunted by both aerial and terrestrial predators, which elicit whistle and trill alarm calls, respectively. Listeners respond to whistles by running to or entering a burrow, whereas they typically adopt a bipedal stance (‘post’) in response to trills. When juveniles first emerge above ground from natal burrows, they do not discriminate behaviourally among the calls, and if they do respond, they typically just freeze. However, they learn appropriate responses quickly, within about five days. Rapid learning is advantageous because up to 30 per cent of juveniles disappear during their first two weeks above ground, probably owing to predation. In addition, at this time young are becoming nutritionally independent and are vulnerable to infanticide (Mateo 1996a; Mateo & Holmes 1997).
Development of these survival behaviours therefore coincides with considerable potential stressors, and juvenile basal cortisol is moderately elevated during the first five days after natal emergence compared with pre-emergence concentrations, and higher than in the weeks following emergence (J. M. Mateo 2005, unpublished data). The aim of this study was to determine whether juveniles show differential acute cortisol responses to alarm and non-alarm calls. Such responses could enhance detection of predators and facilitate avoidance of discovery or subsequent escape, or perhaps influence acquisition of anti-predator behaviours through sharpened perception (Mateo 1996a; Lupien & McEwen 1997; Sapolsky et al. 2000).
2. Material and methods
I conducted research at the Sierra Nevada Aquatic Research Laboratory (SNARL) near Mammoth Lakes, CA. I live-trapped pregnant females from three sites near SNARL and housed them in a laboratory building where they gave birth and reared their young. Mothers were housed singly in stainless steel cages (61 × 45 × 35 cm) that included a 28 × 20 × 20 cm nest-box. I gave animals LabDiet mouse chow (no. 5015) and water ad libitum.
Starting around the age they would emerge in the field, I placed juveniles in a large, dark wooden box (0.61 m tall × 0.61 m deep × 0.91 m wide) and presented them with an acoustic stimulus in the middle of an otherwise silent 10-min period, with blood collected immediately after. I presented four categories of auditory stimuli: U. beldingi whistles and trill alarm calls, U. beldingi squeals (vocalizations made by juveniles during play) and a silent control (the latter two were used to record responses to common auditory stimuli that are not associated with predators). Digitized calls were played from a Sony TCM-5000 tape player connected to a Mineroff SME-AFS speaker suspended approximately 0.5 m high inside the box. All calls were presented at equal amplitudes. Additional details on the stimuli are in Mateo & Holmes (1997).
Littermates were tested in pairs, with one stimulus per day in a randomized order. Animals were lightly anaesthetized (10–20 s exposure) with isoflurane inhalant before drawing a blood sample (300–400 µl) from a saphenous vein within 3 min of removal from the box. Tests were conducted between the hours of 15.00 and 19.00 to minimize diurnal fluctuations in cortisol. Blood was refrigerated for an hour before being centrifuged for 10 min, and serum was stored at –15° C for four to six weeks, after which it was stored at –80°C until assayed.
Testing ended when I had one blood sample for each of the four stimuli or when juveniles reached 35 days of age. In some cases I was unable to collect enough blood in 3 min, or there was insufficient serum to assay. In 2006, I tested 26 juveniles from seven mothers (13 males, 13 females; obtaining at least one sample from eight males and seven females); I collected complete samples (trill, whistle, squeal and silent) for five males and three females. In 2008, I tested 18 juveniles from six different mothers (10 males, eight females), although no samples were obtained from one male; complete samples were collected from four males and five females.
I used 125I-cortisol Corticote radioimmunoassay kits (MP Biomedicals, CA, USA) and assayed samples in duplicate. The sensitivity of the assay is 0.07 µg dl−1. Two control samples, each made by pooling faecal extracts from five animals, were analysed in every assay (the ‘low’ pool, approx. 60–70% binding and the ‘high’ pool, approx. 20–30% binding; Mateo & Cavigelli 2005). Across eight assays, mean intra-assay coefficients of variation for the assays were 11.72 per cent for the low pool and 5.98 per cent for the high pool. Mean inter-assay coefficients of variation were 6.48 per cent for the low pool and 6.72 per cent for the high pool.
Cortisol values were normally distributed. Using an individual's cortisol concentration following the silent stimulus as its baseline, I calculated the per cent change in its response to the whistle, trill and squeal, relative to the baseline, to standardize responses within individuals and to facilitate comparison across individuals. Because multiple individuals from a litter were used, I then averaged the cortisol responses by juveniles to each stimulus type for each litter. I next tested the null hypothesis that the per cent difference (also normally distributed) was not significantly different from zero using a one-sample t-test; one-tailed tests were used because responses to sounds were a priori expected to be higher than responses to silence. Finally, I analysed the data with a repeated-measures ANOVA using data from 17 individuals with serum samples for all four stimuli, after averaging the data for each litter (seven mothers contributing 1–4 juveniles each; average 2.42).
3. Results
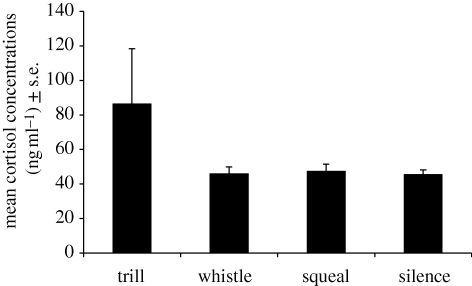
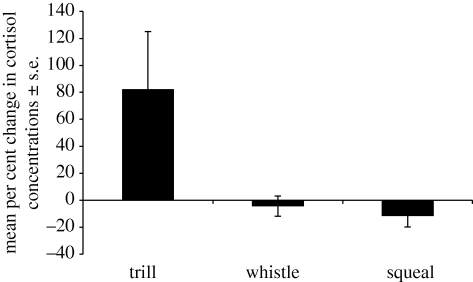
Descriptively, for all litters, cortisol concentrations were higher following playbacks of trills compared with playbacks of whistles, squeals or silent controls (figure 1). Statistically, when examining the per cent change in cortisol relative to an individual's response to the silent playback, only responses to trills were significantly different from zero (per cent-change responses averaged within litters; t9 = 1.928, p = 0.043; whistle: t8 = −0.327, p = 0.375; squeal: t7 = 0.450, p = 0.333). Next, I carried out a repeated-measures ANOVA using juveniles which had adequate blood samples for all four playback categories. Using their response to the silent playback as baseline, juveniles’ cortisol responses (averaged within litters) to trill playbacks were significantly higher than their own responses to whistles or squeals (F2,12 = 4.21, p = 0.041; CMATRIX post hoc test, ps < 0.049; figure 2). There were no differences in acute responses to stimuli between the 2 years (independent t-test for each stimulus; all ps > 0.36).
Figure 1.
Mean serum cortisol concentrations (±s.e.) in ng ml−1 following playback of U. beldingi trill alarm call (n = 10 litters), whistle alarm call (n = 9), U. beldingi squeal (conspecific control vocalization; n = 10) and silent control (n = 10). Data from multiple juveniles in a litter were first averaged. See text for details.
Figure 2.
Mean per cent changes in serum cortisol (±s.e.) to three playbacks by 17 juvenile U. beldingi, using their response to silent playback as a baseline. Data from multiple juveniles in a litter were averaged, resulting in a sample size of seven mothers. See text for details.
4. Discussion
When juvenile Belding's ground squirrels first emerge above ground from their natal burrows at one month of age, they fail to show behavioural discrimination of alarm and non-alarm calls (Mateo 1996a). At this age, they show large cortisol responses to trill alarm calls, but not whistle alarm calls or squeals or silent controls (figure 1). This response to trills is in the absence of any direct threat from a predator, and without seeing conspecifics in danger or escaping. Given the relationship between glucocorticoids and cognition, this response to trills might enhance orientation and attention to subsequent events, and facilitate detection of and escape from predators (Sapolsky et al. 2000). It might also facilitate acquisition of behavioural responses to those calls, although this remains to be tested empirically (Lupien & McEwen 1997). It could be that blood was collected prior to the peak in the cortisol response, in which case very high concentrations of cortisol would impair learning and memory. If so, then such responses to trills might simply facilitate subsequent behavioural responses to the calls (see below). Whistle alarm calls do not raise cortisol, but they do elicit bradycardia in young juveniles, which is associated with decreased motor activity and enhanced information processing, and can improve learning and memory (Mateo 1996b). It is unknown whether the adrenal response to trills and the cardiac response to whistles persist later in development, after alarm-call responses are learned.
It could be adaptive for trills, and not whistles, to elicit acute cortisol responses in U. beldingi of all ages. Avian predator attacks are over within seconds, and most birds do not repeat an attack attempt if unsuccessful (J. M. Mateo 1992–2010, personal observation). Activating the hypothalamic–pituitary–adrenal (HPA) axis in response to whistles would not be adaptive, as the attack would be over long before circulating cortisol increased (up to 3 min later; Sapolsky et al. 2000), and could be maladaptive if it came at the cost of energy storage, particularly during early development or preparation for hibernation (Sapolsky et al. 2000; Reeder & Kramer 2005). By contrast, terrestrial predators that elicit trills typically spend time either moving through the meadow or remaining in one spot to ambush a ground squirrel, hunting for up to 30 min. Trills are usually repeated, and thus responses such as posting are repeated and/or prolonged (J. M. Mateo 1992–2010, personal observation). Increased cortisol would mobilize glucose for sustained vigilance and running during periods of reduced foraging and gastrointestinal activity (Sapolsky et al. 2000; Wingfield 2004).
In summary, the results show a unique adrenal response by developing animals to an alarm call. In U. beldingi, there are two different physiological responses to two different alarm calls warning of different predator classes and requiring different behavioural responses. Furthermore, each physiological response is optimally suited to each type of threat, as aerial predator attacks are brief whereas terrestrial predator hunts can be lengthy. Both of these physiological reactions are known to increase arousal and attention in a variety of species, so in U. beldingi juveniles they might call attention to the responses of nearby adults, and thus facilitate faster learning of an association between alarm calls and appropriate behavioural responses during a developmental period of increased predation risk. Natural selection has shaped proximate mechanisms, the HPA axis and cardiovascular system, appropriate for each threat type signalled by U. beldingi's two alarm calls.
Acknowledgements
All research was carried out under permission of University of Chicago's and University of California-Santa Barbara's IACUCs.
I thank Andrew Dosmann, Krista Gooderham, Trina Hancock, Karen Hurner, Amy Kuczynski and Andrew Valand for data-collection assistance, Erin Loeding and Tina Whitney for conducting hormone assays, and Mateo Laboratory for comments on the manuscript.
References
- Apfelbach R., Blanchard C. D., Blanchard R. J., Hayes R. A., McGregor I. S.2005The effects of predator odors in mammalian prey species: a review of field and laboratory studies. Neurosci. Biobehav. Rev. 29, 1123–1144 (doi:10.1016/j.neubiorev.2005.05.005) [DOI] [PubMed] [Google Scholar]
- Hollén L. I., Radford A. N.2009The development of alarm call behaviour in mammals and birds. Anim. Behav. 78, 791–800 (doi:10.1016/j.anbehav2009.07.021) [Google Scholar]
- Jenkins S. H., Eshelman B. D.1984Spermophilus beldingi. Mamm. Species 221, 1–8 (doi:10.2307/3503911) [Google Scholar]
- Klump G. M., Shalter M. D.1984Acoustic behaviour of birds and mammals in the predator context. II. The functional significance and evolution of alarm signals. Z. Tierpsychol. 66, 206–226 [Google Scholar]
- Lupien S. J., McEwen B. S.1997The acute effects of corticosteroids on cognition: integration of animal and human model studies. Brain Res. Rev. 24, 1–27 (doi:10.1016/S0165-0173(97)00004-0) [DOI] [PubMed] [Google Scholar]
- Mateo J. M.1996aThe development of alarm-call response behaviour in free-living juvenile Belding's ground squirrels. Anim. Behav. 52, 489–505 (doi:10.1006/anbe.1996.0192) [DOI] [PubMed] [Google Scholar]
- Mateo J. M.1996bEarly auditory experience and the ontogeny of alarm-call discrimination in Belding's ground squirrels (Spermophilus beldingi). J. Comp. Psychol. 110, 115–124 (doi:10.1037/0735-7036.110.2.115) [DOI] [PubMed] [Google Scholar]
- Mateo J. M.2006Developmental and geographic variation in stress hormones in wild Belding's ground squirrels (Spermophilus beldingi). Horm. Behav. 50, 718–725 (doi:10.1016/j.yhbeh.2006.05.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateo J. M., Cavigelli S. A.2005A validation of extraction methods for non-invasive sampling of glucocorticoids in free-living ground squirrels. Physiol. Biochem. Zool. 78, 1069–1084 (doi:10.1086/432855) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateo J. M., Holmes W. G.1997Development of alarm-call responses in Belding's ground squirrels: the role of dams. Anim. Behav. 54, 509–524 (doi:10.1006/anbe.1996.0446) [DOI] [PubMed] [Google Scholar]
- Park C. R., Zoladz P. R., Conrad C. D., Fleshner M., Diamond D. M.2008Acute predator stress impairs the consolidation and retrieval of hippocampus-dependent memory in male and female rats. Learn. Mem. 15, 271–280 (doi:10.1101/lm.721108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeder D. M., Kramer K. M.2005Stress in free-ranging mammals: integrating physiology, ecology, and natural history. J. Mammal. 86, 225–235 (doi:10.1644/BHE-003.1) [Google Scholar]
- Romero L. M.2002Seasonal changes in plasma glucocorticoid concentrations in free-living vertebrates. Gen. Comp. Endocrinol. 128, 1–24 (doi:10.1016/S0016-6480(02)00064-3) [DOI] [PubMed] [Google Scholar]
- Sapolsky R. M., Romero L. M., Munck A. U.2000How do glucocorticoids influence stress-responses? Integrating permissive, suppressive, stimulatory, and adaptive actions. Endocr. Rev. 21, 55–89 (doi:10.1210/er.21.1.55) [DOI] [PubMed] [Google Scholar]
- Sauro M. D., Jorgensen R. S., Pedlow C. T.2003Stress, glucocorticoids, and memory: a meta-analytic review. Stress 6, 235–245 (doi:10.1080/10253890310001616482) [DOI] [PubMed] [Google Scholar]
- Thaker M., Vanak A. T., Lima S. L., Hews D. K.2010Stress and aversive learning in a wild vertebrate: the role of corticosterone in mediating escape from a novel stressor. Am. Nat. 175, 50–60 (doi:10.1086/648558) [DOI] [PubMed] [Google Scholar]
- Wingfield J. C.2004Control of behavioural strategies for capricious environments. Anim. Behav. 66, 807–816 (doi:10.1006/anbe.2003.2298) [Google Scholar]