Abstract
As humans continue to explore the last uncharted regions of the planet, discoveries of previously unknown species of large vertebrates have become infrequent. Here, we report on the discovery of a spectacular new species of giant, secretive, frugivorous, forest monitor lizard (Genus: Varanus) from the forests of the northern Philippines. Using data from morphology and mitochondrial and nuclear DNA sequences, we demonstrate the taxonomic distinctiveness of this new 2 m long species and provide insight into its historical biogeography and systematic affinities. Our molecular phylogenetic analyses indicate that the new species is closely related to Varanus olivaceus (from southern Luzon and nearby islands), but it differs from this and other varanids with respect to characteristics of scalation, colour pattern, body size, anatomy of the reproductive organs and genetic divergence. The new species appears to be restricted to forests of the central and northern Sierra Madre mountain range; it is separated from the range of V. olivaceus by a more than 150 km stretch that includes at least three low-elevation river valley barriers to dispersal. This discovery identifies a seldom-perceived biogeographic boundary and emphasizes the need for continued biodiversity research in the megadiverse conservation hotspot of the Philippines. It is anticipated that the new species will serve as an important flagship species for conservation efforts aimed at preserving the remaining forests of northern Luzon.
Keywords: biodiversity, conservation hotspots, flagship species, frugivory, Sierra Madre, Varanus
1. Introduction
Although the description of biodiversity is a continual process, discovery of previously undocumented, large-bodied and conspicuous new species of vertebrates is now a rare occurrence. As human populations increase, forested regions of the planet are charted, and large intact tracks of forest continue to shrink or become fragmented. With increasing exploration, fewer large-bodied vertebrates remain to be discovered. Exceptions to the asymptotic decline in the discovery of large new species of vertebrates include the discovery of the Vietnamese forest bovid Pseudoryx nghetinhensis (Dung et al. 1993) and the discovery of a new African primate (Rungwecebus kipunji; Davenport et al. 2006).
Monitor lizards occur throughout the Old World tropics (Bennett 1998; Pianka et al. 2004). They vary greatly in habitat (arid to tropical) and body size (V. brevicauda: less than 25 cm, 8–17 g versus V. komodoensis: greater than 3 m, 70–90 kg) and include the most massive lizards in the world (Komodo dragons). Fewer than 10 new species of monitor lizard have been described in recent years, and most have been small-bodied forms (Harvey & Barker 1998; Pianka et al. 2004; Ziegler et al. 2007). Philippine lizards of the family Varanidae include five species (Taylor 1922; Gaulke 1992): the water monitors (Varanus cumingi, V. marmoratus, and V. nuchalis), and the arboreal, frugivorous monitors (Varanus olivaceus and V. mabitang). Varanus olivaceus and V. mabitang, the world's only two frugivorous species, are rare and difficult to study (Auffenberg 1988; Gaulke et al. 2005). Shy and reclusive, V. olivaceus is known from lowland forests of southeastern Luzon and the smaller eastern islands of Polillo and Catanduanes (figure 1). Varanus mabitang is a poorly known species, endemic to Panay Island (Gaulke & Curio 2001). Both species are considered heavily threatened, primarily due to habitat destruction.
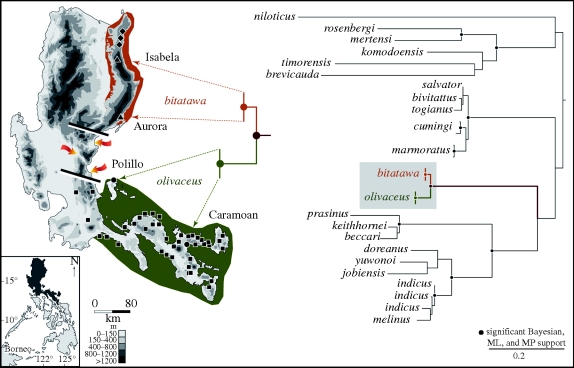
Figure 1.
Distribution and phylogenetic relationships of V. bitatawa, n. sp. and its closest relative, V. olivaceus. Arrows indicate three river valleys constituting the Mid-Sierra Filter Zone (black lines bisecting the Sierra Madre). The phylogenetic tree is derived from a combined, partitioned, RAxML ML analysis of mitochondrial (16S–ND1) and nuclear (PRLR, DNAH3, SNCAIP) genes (−ln L 7336.421498). Filled triangles, bitatawa; filled diamonds, bitatawa (previous photographic records); filled circles, olivaceus (this study); filled squares, olivaceus (Affenberg 1988).
Because large species are conspicuous components of terrestrial faunas, they are usually well known to people. Here we describe a surprising finding from recent biodiversity studies: a giant (2 m body length), strikingly distinct, brightly coloured monitor lizard from the unexplored forests of northern Luzon Island.
2. Material and methods
Data were scored from fluid-preserved specimens deposited in US and Philippine collections and measurements were taken following Auffenberg (1988) and Gaulke & Curio (2001). We sequenced the mitochondrial 16S–ND1 fragments and nuclear PRLR, DNAH3, SNCAIP genes and incorporated these data into a previously published Varanus phylogenetic dataset. We subjected the combined data to phylogenetic analyses using parsimony, maximum likelihood and Bayesian methods (figure 1). The description of the new species is based on diagnostic characters of morphology. Full details of analyses and results are included in the electronic supplementary material.
3. Systematics
Varanus bitatawa sp. nov.
(a). Etymology
The specific epithet is derived from bitatawa, the Agta tribespeoples' common name for the new species.
(b). Holotype and paratypes
Holotype: PNM 9719 (formerly KU 320000; LJW field number 071), adult male, salvaged from hunter at the base of the San Ildefonso Peninsula, Sitio Casapsipan, Barangay Casiguran, Municipality of Casiguran, Aurora Province, Luzon Island, Philippines (16.286667° N, 122.185833° E, WGS-84; 1 m above sea level), 29 June 2009. Paratypes: KU 322188 (ACD 2796), juvenile male, from Sitio Dunoy, Barangay Dibuluan, Municipality of San Mariano, Isabela Province, Luzon Island, Philippines (200 m above sea level), April 2006; collected by A. C. Diesmos; PNM 9008, female, collected from the same locality on 16 July 2005 by R. Dugay.
(c). Diagnosis
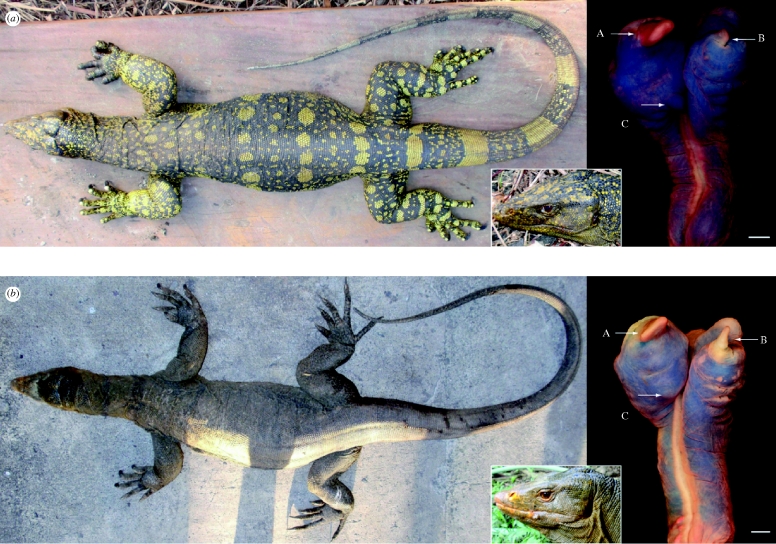
Varanus bitatawa differs from congeners by the combination of (i) large, robust body (and related scalation differences: table 1), (ii) contrasting black and golden yellow dorsal coloration, (iii) dark gular coloration in juveniles, (iv) anteriorly directed, slit-like external nares, and (v) multiple characters of hemipeneal morphology (figure 2; electronic supplementary material). Additionally, the new species is distinguished from congeners by marked divergence in mitochondrial and nuclear gene sequences and differs from its closest relative, V. olivaceus, by genetic divergence in excess of the divergence between other Asian species (figure 1 and table 2; electronic supplementary material, figure S2 and table S3). Detailed comparisons are provided in the electronic supplementary material.
Table 1.
Characters distinguishing V. bitatawa from close relatives. Ranges for snout–vent and tail length of V. olivaceus represent our data combined with Auffenberg (1988; 90 specimens) and D. Bennett (2009, unpublished; 28 specimens); dashes, missing data. See electronic supplementary material for definitions and discussion.
| bitatawa | olivaceus | mabitang | |
|---|---|---|---|
| vouchers | PNM 9719, 9008, KU 322188 | KU 322186, 322187 | PNM 7272, one released specimen |
| geographical range | central and northern Sierra Madres, Luzon | Polillo, Catanduanes, southern Luzon | Panay |
| snout–vent length | 766, 490, 613 | 485–730 | 527, 640 |
| tail length | 1036, 694, 950 | 749–1025 | 741, 1110 |
| hindlimb length | 198, 112, 138 | 181, 124 | 227, — |
| forelimb length | 164, 96, 115 | 127, 92 | 188, — |
| head length | 124.9, 91.5, 122 | 127, 97.4 | 89.9, — |
| head width | 73, 52.7, 64 | 76.9, 54.5 | 43.9, — |
| head depth at eye | 45.4, 34.6, — | 56.7, 40.7 | 32.9, — |
| maximum head depth | 66.8, 41.4, 60 | 68.9, 49.9 | 35, — |
| eye–snout length | 66.8, 47.7, 61 | 65.4, 49.6 | 49.1, — |
| eye–mid-nares length | 39.7, 26.4, — | 36.6, 25.6 | 27.2, — |
| Mid-nares–snout length | 26.4, 18.5, — | 27.9, 21.7 | 21.9, — |
| snout length/head length | 0.54, 0.52, 0.50 | 0.52, 0.52 | 0.55, — |
| tympanum–eye length | 37.8, 29.2, — | 45.1, 30.5 | 42.3, — |
| rictus–rictus scales | 69, 78, — | 84, 69 | 70, — |
| scales around base of tail | 101, 99, 103 | 109, 113 | 113, — |
| scales around tail one-third from base | 59, 62, 49 | 74, 58 | 86, — |
| midbody scales | 193, 185, 175 | 193, 193 | 212, — |
| ventrals (gular fold–hindlimb insertion) | 107, 110, 106 | 117, 110 | 124, — |
| dorsals (paravertebrals) | 127, 113, 116 | 108, 115 | 138, — |
| scales around neck | 145, 151, 133 | 161, 148 | 160, — |
| gulars (chin–gular fold) | 120, 109, 107 | 106, 106 | 117, — |
| supralabials | 58, 54, 62 | 61, 64 | —, — |
| infralabials | 66, 59, 61 | 70, 62 | 68, — |
| mid-nares–snout tip/snout length | 0.40, 0.39, — | 0.43, 0.44 | 0.45, — |
| head length/depth | 2.8, 2.6, — | 2.2, 2.4 | 2.7, — |
Figure 2.
Dorsal views of bodies, lateral views of heads and close-ups of hemipenes of (a) V. bitatawa (PNM 9719) and (b) V. olivaceus (KU 322187). Letters indicate: A, primary apical hemibaculum horn; B, secondary apical hemibaculum horn; and C, presence or absence of an evagination at the base of the primary hemibaculum.
Table 2.
Uncorrected mitochondrial DNA sequence divergence (%) in V. bitatawa, V. olivaceus and three clades of Varanus (figure 1). Percentages on the diagonal represent intraspecific (or within clade) genetic diversity. See electronic supplementary material for nuclear gene divergences.
| bitatawa | olivaceus | clade 1 (prasinus clade) | clade 2 (salvator clade) | clade 3 (niloticus clade) | |
|---|---|---|---|---|---|
| bitatawa | 0 | ||||
| olivaceus | 4.6 | 0 | |||
| clade 1 | 18.4—20.5 | 19.8—21.1 | 14.9–20.7 | ||
| clade 2 | 17.1–18.4 | 17.0–18.4 | 16.1–20.9 | 1.5–5 | |
| clade 3 | 18.4–19.3 | 18.0–20.8 | 17.3–24.1 | 14.4–19.5 | 9.4–20 |
(d). Brief description of holotype
A large-bodied species of Varanus, holotype snout–vent length (SVL) 766 mm, tail 1036; head robust, length 124.9, width 73, maximum depth 66.8, depth at eye 45.4; snout length 65.7; snout rounded anteriorly; narial openings slit-like, surrounded by an elevated protuberance; cranial table squarish, wider than long, with hypertrophied adductor musculature; head scales heterogeneous; supralabials 58; infralabials 66, decreasing in size to rictus; nuchals large, polygonal, decreasing in size laterally; scales arranged in 56 semi-regular rows from the posterior margin of the cranial table to forelimb insertion; scales of the dorsal trunk smaller than those on the head, polygonal; scales in 94 rows in the axilla–groin region; paravertebrals from the gular fold to the anterior edge of hindlimb insertion 127; axilla–groin distance 272 mm; limb scales large, polygonal, slightly convex, decreasing in size distally; forelimb and hindlimb 164 and 198 mm respectively; digits terminating in robust, recurved claws; scales of manus and pes squarish, smaller than those of trunk; supradigitals ovular; caudals rectangular, in semi-regular rows; scales around the base of the tail 101, around the tail one-third from the base, 59; double keel of paired, raised rectangular scales originating 110 mm from the base and extending posteriorly to tail terminus; ventrals less variable than dorsals; ventral nuchals small, increasing in size and becoming squarish through the mid-nuchal region, then decreasing in size anterior to the gular fold; scales around the neck anterior to the gular fold 145; gulars in 68 rows between the gular fold and the margin of the tympanum; ventrals posterior to the gular fold heterogeneous, larger than nuchals; scales in the scapular region polygonal, increasing in size posteriorly; midbody scales 193; ventrals from the gular fold to the anterior margin of hindlimb insertion 107; total ventrals 227; precloacals irregular, round to polygonal, decreasing in size distally through limbs; subcaudals small, heterogeneous, increasing in size posterior to the hemipenal bulge. Dorsal ground coloration black, accentuated with bright golden yellow in life; dorsum covered with golden yellow spots and flecks; head speckled yellow on black; forelimbs more yellow than black; trunk traversed by four distinct rows of yellow oscelli; hindlimbs black with large, distinct, yellow spots; tail barred, contrasting black and yellow. Detailed description of the type series is provided in the electronic supplementary material.
4. Discussion
Discovery of a highly distinctive new species of monitor lizard from heavily populated and highly deforested Luzon Island comes as an unprecedented surprise. How could such a large-bodied (2 m total length) monitor lizard have escaped the notice of the many biologists that have worked in the northern Philippines? We suspect a combination of factors have contributed to this astonishing set of circumstances. Despite being identified as a conservation priority (Mallari & Jensen 1993; van Weerd et al. 2004), surprisingly few surveys have characterized the herpetological diversity of the forests of Sierra Madre (but see Brown et al. 2000a, 2007). Additionally, if the new species is similar in habitat preferences and behaviour to its closest relative (V. olivaceus), V. bitatawa is most likely a highly secretive species that may never leave forests to traverse open areas (D. Bennett 2009, unpublished data). Despite escaping recognition by biologists, the new species is well known to resident Agta and Ilongot tribespeople who rely on it as a major source of protein (M. R. Duya & E. L. B. Rico 2004, unpublished data).
(a). Biogeography
The geographical gap between the new species and its sister taxon V. olivaceus, together with our new phylogenetic data, is key to our interpretation of the biogeographic and evolutionary history of these taxa. In hindsight, it is not surprising that the forested Sierra Madre Range supports an evolutionarily distinct lineage of monitor lizard. The northern and southern portions of the mountain range are geologically isolated (Auffenberg 1988; Hall 2002; Yumul et al. 2009) and are now separated by at least three non-forested river valleys representing barriers to dispersal (figure 1). This series of topological, ecological and atmospheric barriers to dispersal has resulted in a more than 150 km gap, isolating the northern portions of the range of V. olivaceus from the southern portions of the range of V. bitatawa (figure 1). Our hypothesized history of isolation of these new species is entirely consistent with (i) the allopatric distributions of V. bitatawa and V. olivaceus on either side of these three low-elevation valleys (figure 1), (ii) the high levels of genetic divergence and sister relationship between the two taxa (figure 1 and table 2), and (iii) the concomitant morphological differentiation between the two species (figure 2; electronic supplementary material). The discovery of the new species identifies a disjunct distribution pattern that may embody a more general, and under appreciated, biogeographic phenomenon affecting other, unrelated lineages. If low-elevation valleys are barriers to gene flow in montane forest species endemic to the separate geological components of Luzon, we might expect to see additional north–south differentiation in other lineages, sister species pairs or closely related higher taxa. Although available data are few, recent findings suggest that the Mid-Sierra Filter Zone detected here may have similarly affected other lineages (Brown et al. 2000a,b, 2007) and may therefore have a more general biogeographic significance.
(b). Conservation
Our unexpected finding of a highly conspicuous new species of a large vertebrate that has escaped discovery in the forests of the northern Luzon emphasizes the unexplored nature of the Philippines. The discovery of a new Varanus, an anticipated flagship species for conservation, adds to the recognition of the Philippines as a global conservation hotspot and a regional superpower of biodiversity (Brown & Diesmos 2009).
References
- Auffenberg W.1988Gray's monitor lizard .Gainsville, FL: University of Florida Press [Google Scholar]
- Bennett D.1998Monitor lizards, natural history, biology, and husbandry. Frankfurt, Germany: Chimaira [Google Scholar]
- Brown R. M., Diesmos A. C.2009Philippines, biology. In Encyclopedia of islands (eds Gillespie R. M., Clague D.), pp. 723–732 Berkeley, CA: University of California Press [Google Scholar]
- Brown R. M., McGuire J. A., Ferner J. W., Icarangal N., Jr, Kennedy R. S.2000aAmphibians and reptiles of Luzon Island, II: preliminary report on the herpetofauna of Aurora Memorial National Park, Philippines. Hamadryad 25, 175–195 [Google Scholar]
- Brown R. M., McGuire J. A., Diesmos A. C.2000bStatus of some Philippine frogs related to Rana everetti (Anura: Ranidae), description of a new species, and resurrection of Rana igorota Taylor 1922. Herpetologica 56, 81–104 [Google Scholar]
- Brown R. M., Diesmos A. C., Duya M. V.2007A new species of Luperosaurus (Squamata: Gekkonidae) from the Sierra Madre mountain range of northern Luzon Island, Philippines. Raffles Bull. Zool. 55, 153–160 [Google Scholar]
- Davenport T. R. B., Stanley W. T., Sargis E. J., De Luca D. W., Mpunga N. E., Machaga S. J., Olson L. E.2006A new genus of African monkey Rungwecebus. Science 312, 1378 (doi:10.1126/science.1125631) [DOI] [PubMed] [Google Scholar]
- Dung U. V., Mong-Giao P., Ngoc-Chinh N., Tuoc D., Arctander P., Mackinnon J.1993A new species of living bovid from Vietnam. Nature 363, 443–445 (doi:10.1038/363443a0) [Google Scholar]
- Gaulke M.1992Taxonomy and biology of Philippine water monitors (Varanus salvator). Philippine J. Sci. 121, 345–381 [Google Scholar]
- Gaulke M., Curio E.2001A new monitor lizard from Panay Island, Philippines. Spixiana 24, 275–286 [Google Scholar]
- Gaulke M., Altenbach A. V., Demegillo A., Struck U.2005On the distribution and biology of Varanus mabitang. Silliman J. 46, 89–117 [Google Scholar]
- Hall R.2002Cenozoic geological and plate tectonic evolution of SE Asia and the SW Pacific: computer-based reconstructions and animations. J. Asian Earth Sci. 20, 353–434 (doi:10.1016/S1367-9120(01)00069-4) [Google Scholar]
- Harvey M. B., Barker D. G.1998A new species of blue-tailed monitor lizard (genus Varanus) from Halmahera Island, Indonesia. Herpetologica 54, 34–44 [Google Scholar]
- Mallari N. A. D., Jensen A.1993Biological diversity in Northern Sierra Madre, Philippines: its implications for conservation and management. Asia Life Sci. 2, 1–12 [Google Scholar]
- Pianka E. R., King D. R., King R. A.2004Varanoid lizards of the world. Bloomington, IN: Indiana University Press [Google Scholar]
- Taylor E. H.1922The lizards of the Philippine Islands. Manila, Philippines: The Philippine Bureau of Science [Google Scholar]
- van Weerd M., Strijk J., Snelder D.2004The importance of forest fragments for birds and local communities in Northeast Luzon, Philippines. Sylvatrop 13, 1–30 [Google Scholar]
- Yumul G., Dimalanta C., Queaño K., Marquez E.2009Philippines, geology. In Encyclopedia of islands (eds Gillespie R., Calgue D.), pp. 732–738 Berkeley, CA: University of California Press [Google Scholar]
- Ziegler T., Böhme W., Schmitz A.2007A new species of the Varanus indicus group (Squamata, Varanidae) from Halmahera Island, Moluccas: morphological and molecular evidence. Mitt. Mus. Nat. kd Berlin Zool. Reihe 83(Suppl.), 109–119 (doi:10.1002/mmnz.200600034) [Google Scholar]