Abstract
Previous studies have shown that a variety of animals including humans are sensitive to social cues from others and shift their attention to the same objects attended to by others. However, little is known about how animals process conspecifics' and another species' actions, although primates recognize conspecific faces better than those of another species. In this study, using unrestrained eye-tracking techniques, we first demonstrated that conspecific social cues modulated looking behaviours of chimpanzees more than human cues, whereas human observers were equally sensitive to both species. Additionally, first pass gaze duration at the face indicates that chimpanzees looked at the chimpanzees' face longer than the human face, suggesting that chimpanzees might extract more referential information from a conspecific face. These results also imply that a unique ability for extracting referential information from a variety of social objects has emerged during human evolution.
Keywords: social cognition, non-human primates, eye-tracking, comparative cognitive science
1. Introduction
When we see others looking in a particular direction, we orient our attention in the same direction (Langton & Bruce 1999). This gaze-following reflex, which begins to appear in six-month-old humans (Butterworth & Jarrett 1991) is considered to be an important precursor to more complex social abilities, such as theory of mind (Baron-Cohen 1995).
Although previous studies demonstrated that such gaze-following reflex occur in non-human species (Emery et al. 1997; Tomasello et al. 1998; Anderson & Mitchell 1999; Ferrari et al. 2000; Deaner & Platt 2003; but see Tomonaga 2007; Tomonaga & Imura 2009), little is known about how species identity of the models affects the sensitivity to others' actions. Given that most primates including humans, live in highly social communities (Aureli & de Waal 2000) where various developed social cognitive abilities (Matsuzawa et al. 2006) are necessary, sensitivity to conspecific social cues is probably much more important than sensitivity to allospecific cues.
The present study directly investigated whether static images of conspecific or allospecific social cues affect differently on scanning patterns of chimpanzees and humans. This question is crucially important for understanding an evolutionary origin of our ability to process social information from others, which underlies higher social cognitive abilities, such as theory of mind.
2. Material and methods
Eight chimpanzees (two males and six females, 9–33 years old) and eight humans (two males and six females 21–25 years old) participated in this study. The chimpanzees live as a social group of 14 individuals in an environmentally enriched outdoor compound (770 m2) connected to the experimental room by a tunnel (Matsuzawa et al. 2006). Informed consent was obtained from all human participants. We used the same apparatus for both participant species in order to compare them directly. Participants sat freely in the experimental booth (2.5 m × 2.5 m × 2.1 m, W × D × H) and viewed a 17-inch LCD display (1280 × 1024 pixels) at 60-cm distance, while their eye movements were recorded by a table-mounted eye-tracker (60 Hz; Tobii X120, Tobii Technology AB).
In total, 10 digital colour pictures of a familiar female chimpanzee and a familiar woman were used in this experiment. Whole bodies were cut out from the original background and placed on a 1280 × 1024 pixels mid-grey background and a black object was placed on each side of the model. We made three kinds of postures for each model, which are defined as ‘Neutral’ (looking at straight ahead), ‘Look’ (looking at one of the two objects) and ‘Reach’ (reaching for one of the two objects) (figure 1).
Figure 1.
Example of stimuli.
We showed two randomly selected stimuli per day to the chimpanzees (one of a chimpanzee model and one of a human model) and tested for 5 days. For humans, we showed all 10 stimuli for 1 day. Each stimulus was shown for 3 s. Two-point and five-point calibrations were conducted, respectively, for the chimpanzee and human participants and repeated until we obtained sufficient accuracy (Kano & Tomonaga 2009, 2010). The test trial was initiated by the subjects looking at a fixation point that appeared at the centre of the screen. If the chimpanzees held the fixation position for 250 ms, a picture was presented for 3 s. Once a picture appeared it could be looked at freely (Hirata et al. in press; Kano & Tomonaga 2009, 2010). The chimpanzees were rewarded after the presentation, regardless of their viewing behaviours in order to maintain their motivation for participating. Human participants were given a book token as a form of payment after the test session.
We measured total looking durations to objects, face and hands. The target object was defined as the one attended to by the model; the other one was the distractor object. We also measured first pass gaze durations to face. First path gaze duration is the measure of sum of all fixation durations in a region from first entry to exit, which expresses the same thing as the first looking duration. Some researchers studying scene perception have analysed looking durations at a more fine-grained level, suggesting that visual information available in a fixation affects the duration of that fixation (Henderson & Hollingworth 1999) and pointed out that first pass gaze duration was longer for semantically informative objects (Loftus & Mackworth 1978; Henderson et al. 1999). Thus, when the chimpanzees viewed the social cues, if a conspecific face was more informative for the chimpanzees, first pass gaze duration to the face of the chimpanzee model should be longer than to the human model.
3. Results
(a). Looking duration analysis: target versus distractor
Separate three-way analysis of variances (ANOVAs; model (chimpanzee versus human) × condition (Look versus Reach) × object (target versus distractor)) with looking durations to objects were conducted for each species to examine the effects of social cues. The Neutral condition was not included in these analyses.
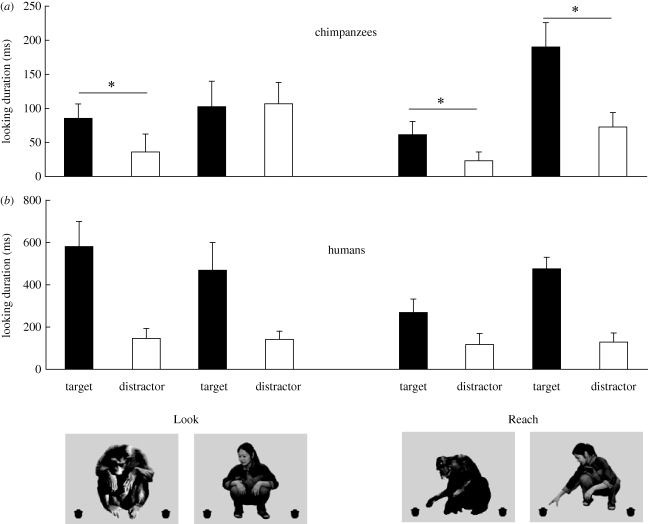
For chimpanzees (n = 8), we found a main effect of model (F1,7 = 5.648, p = 0.049) and an interaction among model, condition and object (F1,7 = 7.491, p = 0.029), but no other significant effects. Post hoc paired t-tests (two-tailed) with Bonferroni correction revealed that the chimpanzees looked longer at the target than the distractor in all conditions except Look condition-human model (Look condition-chimpanzee model t7 = 2.527, p = 0.039; Reach condition-chimpanzee model t7 = 2.690, p = 0.031; Reach condition-human model t7 = 2.384, p = 0.049; Look condition-human model t7 = −0.102, p = 0.922) (figure 2a). These results indicate that chimpanzees' scanning patterns were engaged to look at the target when they observed another chimpanzee looking at or reaching for it. However, such engagement occurred only when they observed the human model reaching for the target, although the significance of the main effect of model indicates that overall, they looked at objects with the human model for longer than those with the chimpanzee model.
Figure 2.
Effect of social cues on looking duration in Look and Reach conditions. (a) Mean (+s.e.m.) looking duration of eight chimpanzees. (b) Mean (+s.e.m.) looking duration of eight humans. Post hoc t-test, *p < 0.05.
For humans (n = 8), only a main effect of the object was found (F1,7 = 12.907, p = 0.009), indicating that humans looked at the target longer than the distractor regardless of the species of the model (figure 2b). The absence of any other effects shows that humans did not look at objects differentially as a function of the species of the model. These results suggest that humans were equally sensitive to social cues from both chimpanzee and human models.
For the Neutral condition, two-tailed paired t-tests confirmed that neither species looked at the objects differentially when the stimuli contained no attention-directing actions (chimpanzees: t7 = 1.369, p = 0.213; humans: t7 = −0.156, p = 0.88).
(b). Looking duration analysis: other AOIs (face and hands)
We further analysed whether the duration of looking at other features (i.e. face and hands) changed when participants viewed either the chimpanzee or human models. Two-way ANOVAs were conducted for each species with models (chimpanzee and human) and conditions (Neutral, Look and Reach) as within-subject factors.
Concerning looking time at the face, we found no significant difference for chimpanzee participants (n = 8), whereas in humans (n = 8) the main effect of condition was significant (F2,14 = 7.452, p = 0.006), but not the effect of model and interaction. Post hoc paired t-tests indicate that humans looked at the face significantly longer in the Neutral condition than in the other two conditions (Neutral–Look: t7 = 3.467, p = 0.0038; Neutral–Reach: t7 = 3.203, p = 0.0064). This increased duration of looking at the face in the Neutral condition indicates that directed gaze attracts attention in humans, but not in chimpanzees.
For looking time to hands, there were no significant differences in either species.
(c). First pass gaze duration analysis
Two-way ANOVAs with model (chimpanzees versus model) and condition (Look versus Reach) run for each species separately revealed that chimpanzees (n = 8) looked at the face of the chimpanzee model significantly longer than that of the human model (main effect of model F1,7 = 6.404, p = 0.0392). Additionally, they also looked at the face significantly longer in the Reach condition than in the Look condition (F1,7 = 9.360, p = 0.0183). We found no interaction. In contrast, there were no significant effects for humans. We also analysed the data from the neutral condition with paired t-tests to assess any effect of model, but found no significance in either species. Overall, these results suggest that for chimpanzees, at least when viewing others attending to something, a conspecific face conveys more information.
4. Discussion
In this study, we have demonstrated that conspecific social cues modulate chimpanzees' scanning patterns more effectively than human cues. Specifically, chimpanzees looked at the target more than the distractor by the chimpanzee model looking but not the human model. Although previous studies report that non-human primates follow human gaze (e.g. Tomasello et al. 1999), dynamic movement might have contributed their visual co-orientation with humans. On the other hand, humans were equally sensitive to social cues for both human and chimpanzee models, which may reflect a superior ability to read social information beyond species barriers.
Species-superiority of chimpanzees in conveying social information found in this study is also indicated by other recent studies. For example Ruiz et al. (2009) showed that lemurs co-orient with conspecific photographs to find hidden food but failed with humans (Anderson & Mitchell 1999). In addition, species-superiority in face perception is already known as an expertise effect for both humans and non-human primates (Tomonaga et al. 1993; Parr et al. 1998; Dahl et al. 2009). We also found that chimpanzees looked at a conspecific face longer with respect to their first pass gaze duration. This may suggest that conspecific faces contain more information to be processed for chimpanzees and might explain modulated looking of chimpanzees at the objects. Thus, how species-superiority in scanning pattern affect other actions such as those in object choice tasks is worthy of future study.
In conclusion, we first demonstrated that conspecific social cues triggered attentional shifts in chimpanzees more readily than allospecific cues, whereas humans were equally sensitive to both chimpanzee and human models. Increased first path gaze duration may explain the conspecific cue-modulated looking behaviour in chimpanzees.
Acknowledgements
The care and use of the chimpanzees adhered to the 2002 version of the Guide for the Care and Use of Laboratory Primates by the KUPRI. The experimental protocol for chimpanzees was approved by the Animal Welfare and Animal Care Committee of the KUPRI and Animal Research Committee of Kyoto University. All procedures adhered to the Japanese ‘Act on Welfare and Management of Animals’.
This study and preparation of this manuscript were financially supported by Grants-in-Aid for Scientific Research from the Japanese Ministry of Education, Cultures, Sports, Science and Technology (MEXT); the Japan Society for the Promotion of Science (JSPS) (20-1240, 21-2299, 16002001, 19300091, 20002001); and by MEXT Grants-in-Aid for the global COE programme (A06 and D07).
We thank the staff of the Language and Intelligence Section for their help and invaluable comments. We also thank the Centre for Human Evolution Modelling Research at the Primate Research Institute for daily care of the chimpanzees.
References
- Anderson J. R., Mitchell R. W.1999Macaques but not lemurs co-orient visually with humans. Folia Primatol. 70, 17–22 (doi:10.1159/000021670) [DOI] [PubMed] [Google Scholar]
- Aureli F., de Waal F. B. M.2000Natural conflict resolution. Berkeley, CA: University of California Press [Google Scholar]
- Baron-Cohen S.1995Mindblindness: an essay on autism and theory of mind. Cambridge, UK: MIT Press [Google Scholar]
- Butterworth G., Jarrett N.1991What minds have in common is space: spatial mechanisms serving joint visual attention in infancy. Br. J. Dev. Psychol. 9, 55–72 [Google Scholar]
- Dahl C. D., Wallraven C., Bulthoff H. H., Logothetis N. K.2009Humans and macaques employ similar face-processing strategies. Curr. Biol. 19, 509–513 (doi:10.1016/j.cub.2009.01.061) [DOI] [PubMed] [Google Scholar]
- Deaner R. O., Platt M. L.2003Reflexive social attention in monkeys and humans. Curr. Biol. 13, 1609–1613 (doi:10.1016/j.cub.2003.08.025) [DOI] [PubMed] [Google Scholar]
- Emery N. J., Lorincz E. N., Perrett D. I., Oram M. W., Baker C. I.1997Gaze following and joint attention in rhesus monkeys (Macaca mulatta). J. Comp. Psychol. 111, 286–293 (doi:10.1037/0735-7036.111.3.286) [DOI] [PubMed] [Google Scholar]
- Ferrari P. F., Kohler E., Fogassi L., Gallese V.2000The ability to follow eye gaze and its emergence during development in macaque monkeys. Proc. Natl Acad. Sci. USA 97, 13 997–14 002 (doi:10.1073/pnas.250241197) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson J. M., Hollingworth A.1999High-level scene perception. Annu. Rev. Psychol. 50, 243–271 (doi:10.1146/annurev.psych.50.1.243) [DOI] [PubMed] [Google Scholar]
- Henderson J. M., Weeks P. A., Jr, Hollingworth A.1999The effects of semantic consistency on eye movements during scene viewing. J. Exp. Psychol. Hum. Percept. Perform. 25, 210–228 (doi:10.1037/0096-1523.25.1.210) [Google Scholar]
- Hirata S., Fuwa K., Sugama K., Kusunoki K., Fujita S.In press Facial perception of conspecifics: chimpanzees (Pan troglodytes) preferentially attend to proper orientation and open eyes. Anim. Cogn. [DOI] [PubMed] [Google Scholar]
- Kano F., Tomonaga M.2009How chimpanzees look at pictures: a comparative eye-tracking study. Proc. R. Soc. B 276, 1949–1955 (doi:10.1098/rspb.2008.1811) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano F., Tomonaga M.2010Face scanning in chimpanzees and humans: continuity and discontinuity. Anim. Behav. 79, 227–235 (doi:10.1016/j.anbehav.2009.11.003) [Google Scholar]
- Langton S. R. H., Bruce V.1999Reflexive visual orienting in response to the social attention of others. Vis. Cogn. 6, 541–567 (doi:10.1080/135062899394939) [Google Scholar]
- Loftus G. R., Mackworth N. H.1978Cognitive determinants of fixation location during picture viewing. J. Exp. Psychol. Hum. Percept. Perform. 4, 565–572 (doi:10.1037/0096-1523.4.4.565) [DOI] [PubMed] [Google Scholar]
- Matsuzawa T., Tomonaga M., Tanaka M.2006Cognitive development in chimpanzees. Tokyo, Japan: Springer [Google Scholar]
- Parr L. A., Dove T., Hopkins W. D.1998Why faces may be special: evidence for the inversion effect in chimpanzees (Pan troglodytes). J. Cogn. Neurosci. 10, 615–622 (doi:10.1162/089892998563013) [DOI] [PubMed] [Google Scholar]
- Ruiz A., Gómez J. C., Roeder J. J., Byrne R. W.2009Gaze following and gaze priming in lemurs. Anim. Cogn. 12, 427–434 (doi:10.1007/s10071-008-0202-z) [DOI] [PubMed] [Google Scholar]
- Tomasello M., Call J., Hare B.1998Five primate species follow the visual gaze of conspecifics. Anim. Behav. 55, 1063–1069 (doi:10.1006/anbe.1997.0636) [DOI] [PubMed] [Google Scholar]
- Tomasello M., Hare B., Agnetta B.1999Chimpanzees, Pan troglodytes, follow gaze direction geometrically. Anim. Behav. 58, 769–777 (doi:10.1006/anbe.1999.1192) [DOI] [PubMed] [Google Scholar]
- Tomonaga M.2007Is chimpanzee (Pan troglodytes) spatial attention reflexively triggered by the gaze cue? J. Comp. Psychol. 121, 156–170 [DOI] [PubMed] [Google Scholar]
- Tomonaga M., Imura T.2009Human gestures trigger different attentional shifts in chimpanzees (Pan troglodytes) and humans (Homo sapiens). Anim. Cogn. 12, S11–S18 [DOI] [PubMed] [Google Scholar]
- Tomonaga M., Itakura S., Matsuzawa T.1993Superiority of conspecific faces and reduced inversion effect in face perception by a chimpanzee (Pan troglodytes). Folia Primatol. 61, 110–114 (doi:10.1159/000156737) [DOI] [PubMed] [Google Scholar]