Abstract
Biological rhythms that oscillate with periods close to 24 h (circadian cycles) are pervasive features of mammalian physiology, facilitating entrainment to the 24 h cycle generated by the rotation of the Earth. In the absence of environmental time cues, circadian rhythms default to their endogenous period called tau, or the free-running period. This sustained circadian rhythmicity in constant conditions has been reported across the animal kingdom, a ubiquity that could imply that innate rhythmicity confers an adaptive advantage. In this study, we found that the deviation of tau from 24 h was inversely related to the lifespan in laboratory mouse strains, and in other rodent and primate species. These findings support the hypothesis that misalignment of endogenous rhythms and 24 h environmental cycles may be associated with a physiological cost that has an effect on longevity.
Keywords: circadian, tau, lifespan, free-running, rodent, primate
1. Introduction
The free-running endogenous circadian rhythm, or ‘tau’ is generated by a network of transcriptional and translational feedback loops controlling the expression of a panel of ‘clock’ genes (Reppert & Weaver 2002), and lies close to 24 h in all animals (Pittendrigh & Daan 1976; electronic supplementary material, tables S1 and S2). Free-running periods that are longer or shorter than 24 h necessitate daily re-entrainment to external time cues that have a 24 h period, such as the light–dark cycle, and this chronic re-entrainment may be associated with a physiological cost that affects survival. There are clear examples of this cost in invertebrates, for example, the lifespan of fruit flies (Drosophila melanogaster) and blowflies (Phormia terraenovae) were significantly longer when raised on 24 h light dark cycles compared with cycles that were shorter than tau (Pittendrigh & Minis 1972; von Saint Paul & Aschoff 1978). Furthermore, in Arabidopsis strains grown under photoperiods not equal to tau produced less chlorophyll and had reduced growth and survival (Dodd et al. 2005).
The circadian resonance hypothesis was proposed several decades ago by Pittendrigh (e.g. Pittendrigh & Bruce 1959), and states that fitness is enhanced when endogenous periods are synchronized with the period of environmental cycles. Such an adaptive advantage of circadian resonance seems intuitive, but remarkably, this concept is disproved in mammals. Supportive evidence is derived from rather ambiguous epidemiological investigations of prevalence of the disease among human shift workers (Knutsson 2003), and from studies of increased morbidity in rodent models with disruption to the molecular mechanisms of the circadian clock (Martino et al. 2008), neither of which conclusively demonstrates specific beneficial effects of circadian resonance in the healthy, wild-type phenotype.
Ageing is associated with disruption of the circadian timing system, including drifting of tau, reduced entrainment to light, reduced amplitude of clock gene expression and desynchronization of physiological rhythms (Valentinuzzi et al. 1997; Duffy et al. 2002; Hofman & Swaab 2006). Transplantation of the master circadian clock located in the suprachiasmatic nuclei (SCN) of the hypothalamus, from young mice into aged mice restored youthful rhythmicity, and increased lifespan (Hurd & Ralph 1998), suggesting an adaptive advantage of robust circadian timing. This finding, along with the beneficial effects of circadian resonance in plants (Dodd et al. 2005) and invertebrates (Pittendrigh & Minis 1972), led us to investigate whether longer mammalian lifespans are associated with values of tau that are close to 24 h.
The specific hypothesis tested in this study was that values of tau that deviate away from 24 h are associated with reduced lifespan, possibly because they are less easily synchronized with 24 h environmental cycles.
2. Material and methods
Values for tau, body mass and for lifespan were taken from the literature for strains of laboratory mice (n = 9), primate (n = 13) and rodent species (n = 25) (electronic supplementary material, tables S1–S3). Data for laboratory mice were taken from a single study where tau was measured under identical conditions for each strain (Schwartz & Zimmerman 1990). Linear regression analysis was used to investigate associations between tau, lifespan and body mass and phylogenetic independent contrasts were computed to account for evolutionary relationships between the species (see electronic supplementary material for further details of methods and sources). Data for tau and lifespan for the rodent and primate species were subjected to a log-transformation in order to comply with the parameters of the normal distribution and to facilitate analysis using parametric statistical tests. Data were analysed using Minitab software with p < 0.05 taken to indicate statistical significance.
3. Results
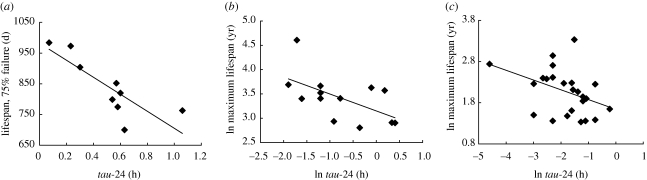
Values for the proximity of tau to 24 (tau-24) were significantly related to lifespan (75% failure) in individual strains of laboratory mice (figure 1a; r = 0.83; p = 0.003), with strains with greater values for tau-24 having shorter lifespans. Multiple linear regression analysis confirmed a similar negative relationship between maximum lifespan and tau-24 in 13 species of primate (figure 1b) and in 25 species of rodent (figure 1c), where body mass and clade (rodent or primate) were included as factors in the model (r2adj = 72.2%; F37,3 = 33.04; p < 0.001). The standardized partial regression coefficient (β) was used to assess the relative importance of each variable in the final model indicating that both tau-24 (β = −0.24; p < 0.05) and body mass (β = 0.37; p < 0.05) exerted significant and independent effects on lifespan. Correction for phylogenetic non-independence confirmed a statistically significant relationship between tau-24 and lifespan in the primate group (r2 = 0.43; p = 0.05), but not in the rodent group (electronic supplementary material, figure S2).
Figure 1.
(a) Relationship between tau-24 and lifespan (time to 75% failure, i.e. when 75% of the animals have died) in nine strains of laboratory mouse (values are mean ± s.e.m.; r2 = 0.7; p < 0.01). (b) relationship between tau-24 and maximum lifespan of 13 primates (Otolemur garnettii, Aotus trivirgatus, Aotus lemurinus griseimembra, Microcebus murinus, Homo sapiens, Saimiri sciureus, Macaca mulatta, Macaca fascicularis, Callithrix jacchus, Macaca nemestrina, Aotus azarai boliviensis, Galago senegalensis, Eulemur fulvus albifrons) (r2 = 0.33; p < 0.05). (c) relationship between tau-24 and maximum lifespan of 25 rodents (Mesocricetus auratus, Phodopus sungorus, Mus musculus, Rattus norvegicus, Ammospermophilus leucurus, Arvicanthis niloticus, Cryptomys damarensis, Georychus capensis, Acomys russatus, Cryptomys hottentotus, Heterocephalus glaber, Rhabdomys pumilio, Spalax ehrenbergi, Octodon degus, Peromyscus maniculatus, Dipodomys merriami, Tamiasciurus hudsonicus, Tamias striatus, Sigmodon hispidus, Glaucomys volans, Meriones unguiculatus, Peromyscus leucopus, Tamias sibiricus, Lemniscomys barbarus and Spalacopus cyanus) (r2 = 0.16; p < 0.05).
4. Discussion
The consistent inter- and intra-species relationship between tau and lifespan reported here suggests that this association reflects a biological constraint that limits lifespan when endogenous rhythms do not match the 24 h environmental cycle. The reasons for such a wide inter-species diversity in the values of tau are unclear, but it has been suggested that having a tau that deviates from 24 h confers some additional functional capacity, possibly related to environmental or seasonal adaptation (Pittendrigh & Daan 1976).
Maximum lifespan was the only available index of lifespan in rodents and primates, and because this parameter is often based on a small sample of animals maintained in captivity, it may not represent the true maximum lifespan for any species (Speakman et al. 2002). It is also probable that values for tau in some of the rodents and primates may differ from the true values; many of these measurements were taken using different methods for detection of locomotor activity, and few studies accounted for previous light exposure of the animals, which is known to affect the length of tau (Pittendrigh & Daan 1976). Furthermore, correction for evolutionary relationships among species was based on an estimated phylogenetic tree, as full mitochondrial gene sequences were not available for all animals, also necessitating the assumption of equal branch lengths (Garland et al. 2005). These limitations might account for the lack of association between the phylogenetic independent contrasts in rodents (electronic supplementary material, figure S2). Nevertheless, interpretation of the results in the rodent and primate groups is aided by the concurrent relationship between tau and lifespan among strains of laboratory mice (figure 1), where highly accurate data for tau and lifespan are available (Schwartz & Zimmerman 1990). However, the contribution of strain-related pathology to lifespan cannot be excluded. In this study, tau was measured under identical experimental conditions in all strains, with previous light exposure, age and sex all controlled (Schwartz & Zimmerman 1990), and tau was compared with well-established indices of lifespan (Paigen, Mouse Phenome Database).
The endogenous nature of circadian rhythms was confirmed through SCN lesion studies less than 50 years ago, and evidence to support the enormous significance of circadian control for human health and physiology is now rapidly accumulating. For example, it is evident that circadian clock genes oscillate in virtually all mammalian cells (Peirson et al. 2006), and these genes control the transcription of at least 10 per cent of the human genome (Storch et al. 2002). Most physiological parameters are subject to endogenously generated circadian rhythms, as are demographic factors such as the time of death (Mitler et al. 1987) and birth (Honnebier et al. 1991). Clock genes regulate the cell cycle, cell proliferation and tumour suppression (Fu et al. 2002; Miller et al. 2007; Moriya et al. 2007), and play a central role in metabolic physiology (Rudic et al. 2004; Alenghat et al. 2008). The results of this investigation support the hypothesis that values of tau that deviate from 24 h impose an increased requirement for daily re-entrainment, and this may result in a cumulative physiological cost that negatively affects lifespan.
Circadian rhythms can no longer be viewed as artefacts of mammalian evolution under a 24 h light–dark cycle, but rather as fundamental molecular synchronizing mechanisms that permeate every aspect of physiology. The relatively recent desynchronization of human life schedules with environmental cycles by increased shift work, trans-meridian travel and electric lighting may have significant implications on human health, and the circadian resonance hypothesis requires timely re-examination.
Acknowledgements
C. Wyse was supported by Research into Ageing and the BBSRC.
References
- Alenghat T., et al. 2008Nuclear receptor corepressor and histone deacetylase 3 govern circadian metabolic physiology. Nature 456, 997–1000 (doi:10.1038/nature07541) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd A. N., Salathia N., Hall A., Kévei E., Tóth R., Nagy F., Hibberd J. M., Millar A. J., Webb A. A.2005Plant circadian clocks increase photosynthesis, growth, survival, and competitive advantage. Science 309, 630–633 (doi:10.1126/science.1115581) [DOI] [PubMed] [Google Scholar]
- Duffy J. F., Zeitzer J. M., Rimmer D. W., Klerman E. B., Dijk D. J., Czeisler C. A.2002Peak of circadian melatonin rhythm occurs later within the sleep of older subjects. Am. J. Physiol. 282, E297–E303 [DOI] [PubMed] [Google Scholar]
- Fu L., Pelicano H., Liu J., Huang P., Lee C.2002The circadian gene Period2 plays an important role in tumor suppression and DNA damage response in vivo. Cell 111, 41–50 (doi:10.1016/S0092-8674(02)00961-3) [DOI] [PubMed] [Google Scholar]
- Garland T., Bennett A. F., Rezende E. L.2005Phylogenetic approaches in comparative physiology. J. Exp. Biol. 208, 3015–3035 (doi:10.1242/jeb.01745) [DOI] [PubMed] [Google Scholar]
- Hofman M. A., Swaab D. F.2006Living by the clock: the circadian pacemaker in older people. Ageing Res. Rev. 5, 33–51 (doi:10.1016/j.arr.2005.07.001) [DOI] [PubMed] [Google Scholar]
- Honnebier M. B., Jenkins S. L., Wentworth R. A., Figueroa J. P., Nathanielsz P. W.1991Temporal structuring of delivery in the absence of a photoperiod: preparturient myometrial activity of the rhesus monkey is related to maternal body temperature and depends on the maternal circadian system. Biol. Reprod. 45, 617–625 (doi:10.1095/biolreprod45.4.617) [DOI] [PubMed] [Google Scholar]
- Hurd M. W., Ralph M. R.1998The significance of circadian organisation for longevity in the golden hamster. J. Biol. Rhythms 13, 430–436 (doi:10.1177/074873098129000255) [DOI] [PubMed] [Google Scholar]
- Knutsson A.2003Health disorders of shift workers. Occup. Med. 53, 103–108 (doi:10.1093/occmed/kqg048) [DOI] [PubMed] [Google Scholar]
- Martino T. A., et al. 2008Circadian rhythm disorganization produces profound cardiovascular and renal disease in hamsters. Am. J. Physiol. 294, R1675–R1683 [DOI] [PubMed] [Google Scholar]
- Miller B. H., et al. 2007Circadian and CLOCK-controlled regulation of the mouse transcriptome and cell proliferation. Proc. Natl Acad. Sci. USA 104, 3342–3347 (doi:10.1073/pnas.0611724104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitler M. M., Hajdukovic R. M., Shafor R., Hahn P. M., Kripke D. F.1987When people die. Cause of death versus time of death. Am. J. Med. 82, 266–274 (doi:10.1016/0002-9343(87)90067-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriya T., Hiraishi K., Horie N., Mitome M., Shinohara K.2007Correlative association between circadian expression of mousePer2 gene and the proliferation of the neural stem cells. Neuroscience 146, 494–498 (doi:10.1016/j.neuroscience.2007.02.018) [DOI] [PubMed] [Google Scholar]
- Peirson S. N., Butler J. N., Duffield G. E., Takher S., Sharma P., Foster R. G.2006Comparison of clock gene expression in SCN, retina, heart, and liver of mice. Biochem. Biophys. Res. Commun. 351, 800–807 (doi:10.1016/j.bbrc.2006.10.118) [DOI] [PubMed] [Google Scholar]
- Pittendrigh C. S., Bruce V. G.1959. In Photoperiodism and related phenomena in plants and animals (ed. Withrow R. B.), pp. 475–505 Washington, DC: American Association for the Advancement of Science [Google Scholar]
- Pittendrigh C. S., Daan S.1976A functional analysis of circadian pacemakers in nocturnal rodents. The stability and lability of spontaneous frequency. J. Comp. Physiol. 106, 223–252 (doi:10.1007/BF01417856) [Google Scholar]
- Pittendrigh C. S., Minis D. H.1972Circadian systems: longevity as a function of circadian resonance in Drosophila melanogaster. Proc. Natl Acad. Sci. USA 69, 1537–1539 (doi:10.1073/pnas.69.6.1537) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reppert S. M., Weaver D. R.2002Coordination of circadian timing in mammals. Nature 418, 935–941 (doi:10.1038/nature00965) [DOI] [PubMed] [Google Scholar]
- Rudic R. D., McNamara P., Curtis A. M., Boston R. C., Panda S., Hogenesch J. B., Fitzgerald G. A.2004BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. PLoS Biol. 2, e377 (doi:10.1371/journal.pbio.0020377) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz W. J., Zimmerman P.1990Circadian timekeeping in BALB/c and C57BL/6 inbred mouse strains. J. Neurosci. 10, 3685–3694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speakman J. R., Selman C., McLaren J. S., Harper E. J.2002Living fast, dying when? The link between aging and energetics. J. Nutr. 132, 1583S–1597S [DOI] [PubMed] [Google Scholar]
- Storch K. F., Lipan O., Leykin I., Viswanathan N., Davis F. C., Wong W. H., Weitz C. J.2002Extensive and divergent circadian gene expression in liver and heart. Nature 417, 78–83 (doi:10.1038/nature744) [DOI] [PubMed] [Google Scholar]
- Valentinuzzi V. S., Scarbrough K., Takahashi J. S., Turek F. W.1997Effects of aging on the circadian rhythm of wheel-running activity in C57BL/6 mice. Am. J. Physiol. 273, R1957–R1964 [DOI] [PubMed] [Google Scholar]
- von Saint Paul U., Aschoff J.1978Longevity among blowflies, Phormia terraenovae kept in non-24-hour light–dark cycles. J. Comp. Physiol. 127, 191–195 [Google Scholar]