Abstract
Bioluminescence is a common feature in the permanent darkness of the deep-sea. In fishes, light is emitted by organs containing either photogenic cells (intrinsic photophores), which are under direct nervous control, or symbiotic luminous bacteria (symbiotic photophores), whose light is controlled by secondary means such as mechanical occlusion or physiological suppression. The intrinsic photophores of the lantern shark Etmopterus spinax were recently shown as an exception to this rule since they appear to be under hormonal control. Here, we show that hormones operate what amounts to a unique light switch, by acting on a chromatophore iris, which regulates light emission by pigment translocation. This result strongly suggests that this shark's luminescence control originates from the mechanism for physiological colour change found in shallow water sharks that also involves hormonally controlled chromatophores: the lantern shark would have turned the initial shallow water crypsis mechanism into a midwater luminous camouflage, more efficient in the deep-sea environment.
Keywords: bioluminescence, chromatophore, Etmopterus spinax, lantern shark, midwater fish, photophore
1. Introduction
In the permanent darkness of the deep ocean a surprising diversity of organisms, from bacteria to fishes, generate their own light to find food, communicate or avoid predation (Buck 1978; Haddock et al. 2010). In fishes, which are the only luminous vertebrates, this bioluminescence is either self-produced by intrinsic photophores or harnessed from glowing symbiotic bacteria (extrinsic photophores), light sources that have different ‘on/off switches’. Whereas photophores are typically under nervous control, fishes regulate their bacterial light by secondary means, either physiologically by controlling the physico-chemical parameters of the bacterial chamber (Haygood 1993), or simply by hiding it with chromatophores, dark shutters or by rotation of the entire bacterial organ into a dark pocket (Herring & Morin 1978; Herring 1985).
Lantern sharks (Etmopteridae), one of the few luminous shark families, were recently demonstrated as an exception to this rule (Claes & Mallefet 2009a). Although their simple light organs are intrinsic, made of tiny clusters of photogenic cells (photocytes) sheathed in a pigmented layer and topped by one or more lens cells (Hubbs et al. 1967), work on the velvet belly lantern shark (Etmopterus spinax) demonstrated that, unlike any other known system, their luminescence is under hormonal control (Claes & Mallefet 2009a): prolactin and melatonin trigger the light emission using specific extrinsic and intrinsic pathways while α-MSH inhibited these light emissions.
Since these hormones are also involved in the physiological control of colour changes in elasmobranchs (sharks, skates and rays) whose functional unit is a pigmented cell, the melanophore (Visconti et al. 1999; Gelsleichter 2004), it has been postulated that the hormones could act on an iris-like structure present between the photocytes and the lens cells, and therefore that the shark E. spinax would control its intrinsic luminescence mechanically (Claes & Mallefet 2009a).
2. Material and methods
Adult lantern shark (E. spinax) specimens were collected by longlines lowered in a deep area (180–250 m) of the Raunefjord (Norway) and brought to Espeland Marine Station where they were maintained in water tanks placed in a dark cold (6°C) room until they were killed by a blow to the head before experimentation took place, following the local rules for experimental fish care.
Using the method of Claes & Mallefet (2009a), we excised photophore-containing skin patches from the sharks and placed them in Perspex chambers filled with a shark saline (292 mM NaCl, 3.2 mM KCl, 5 mM CaCl2, 0.6 mM MgSO4, 1.6 mM Na2SO4, 300 mM urea, 150 mM trimethylamine N-oxide (TMAO), 10 mM glucose, 6 mM NaHCO3; total osmolarity: 1.080 mosM; pH 7.7; from Bernal et al. 2005).
The hormones prolactin (PRL; Sigma Chemical Co., St Louis, MO, USA) and melatonin (MT; Across Organics, Pittsburgh, PA, USA) were diluted in the saline and applied at a final concentration of 10−3 mM (which is known to be the concentration to which the tissue is the most sensitive; Claes & Mallefet 2009a) on the skin patches in separate trials (n = 12 for each hormone) to stimulate photophores to glow and a luminometer (Berthold FB12, Berthold Technologies, Pforzheim, Germany) was used to record the time course of the light emission during 45 min. Concomitantly, similar hormone-stimulated photophore preparations were digitally photographed (Canon D07, Canon, Tokyo, Japan) under a binocular microscope (Leitz Diaplan, Oberkochen, Germany) to investigate possible morphological changes of the chromatophores in the superficial layer of the skin; the apparent photogenic surface area i.e. the ‘transparency index’ of six different photophores was measured using the image-analysing software Image J (National Institutes of Health, Bethesda, MD, USA) over the course of each post-stimulation trial.
3. Results
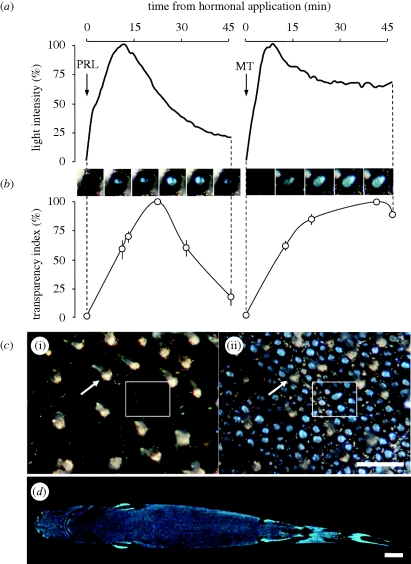
Immediately after application, both hormones always induced light emission from the photophores in the investigated lantern shark specimens. The two hormones, however, showed two different luminescence time courses (figure 1a), similar to those described by Claes & Mallefet (2009a).
Figure 1.
Mechanism for luminescence control in Etmopterus spinax. (a) Photophore luminescence recordings after melatonin (MT) and prolactin (PRL) application (arrows). (b) Evolution of the ‘transparency index’ of photophores (dots, mean data points calculated on six different photophores) after hormonal stimulation. Serial pictures (top) illustrate individual photophore evolution after MT and PRL stimulation. (c) Photophore-containing skin patch before hormonal stimulation (i) and at luminescence peak (ii). The same spiny dermal denticle (white arrow) and group of photophores (white box) are represented in both panels. Scale bar, 1 mm. (d) Ventral luminous pattern of an adult E. spinax specimen. Scale bar, 2 cm.
Concomitantly, both hormones also provoked an irising of the pigmented layer that revealed the glowing underlying photocytes. The ‘transparency index’ of the photophores varied according to the hormone, showing general patterns of evolution similar to the time course patterns of hormonal-induced luminescence curves, although luminescence intensity peaked before transparency index (figure 1b). Whereas pre-stimulation photophores appeared as dark blotches, they were black rings surrounding luminescent bluish photocytes at maximum opening, due to the pigment translocation occurring in chromatophores (figure 1c). The photophores are small (about 150 μm wide) and yet because of their high density, formed homogeneous luminous areas at greater distance (figure 1d).
4. Discussion
The ‘irising’ feature of lantern shark photophores is to our knowledge a unique mechanism of light regulation among luminescent animals and the only one known to be under hormonal control (Claes & Mallefet 2009a). The use of occlusive structures to control luminescence was believed to be restricted to fishes with continuously glowing bacterial organs (Herring 1985; Haygood 1993; Claes & Mallefet 2009a). In the case of E. spinax, however, the discrepancy between the highest values of luminescence intensity and ‘transparency index’ suggests that hormones also stimulate photocytes in addition to provoking pigment retraction in melanophores. The lantern shark mechanism illustrates the range of physiological and morphological means by which diverse taxa have separately arrived at bioluminescent capability.
Bioluminescence is a soft-tissue phenomenon that leaves no fossil track; we therefore rely on extant taxa for clues to evolutionary pathways (Johnsen et al. 1999). Our data provide the valuable suggestion that this lantern shark's luminescence is the result of a co-opting of existing skin structures. The hormonally controlled retraction/expansion capabilities of elasmobranch melanophores (Gelsleichter 2004) provided the basis for the control mechanism of lantern shark photophores.
Skin physiological colour change has been found in shallow water elasmobranch species, which use this adaptation to remain cryptic against variable backgrounds and defeat visual detection by prey and predators (Gelsleichter 2004). On the other hand, E. spinax's extended ventral pattern of photophores radiating a long lasting blue light suggests that this shark uses its luminescence to cloak its silhouette from below (Claes & Mallefet 2008, 2009b), a camouflage mechanism known as counter-illumination that is found in numerous other midwater organisms including crustaceans, molluscs (squids) and bony fishes (Clarke 1963; Warner et al. 1979; Young et al. 1980). We therefore suggest that this species turned the initial shallow water crypsis mechanism into a midwater bioluminescent camouflage, more efficient in the darkness of the deep-sea. This functional transition might have occurred during colonization of the deep-sea by lantern sharks during the late Cretaceous (Adnet & Capetta 2001).
Acknowledgements
Research was carried out under approval from the Norwegian Animal Research Authority (NARA) and under University of Bergen (UiB)/High Technology Centre in Bergen (HiB)/Espeland Marine Station ethics guidelines.
J.M.C. has a scholarship from the National Fund for Scientific Research (FNRS, Belgium). J.M. is a research associate at FNRS. Financial support was provided by a FNRS grant 1.5.278.08. We acknowledge A. Aadnesen, for logistical support, T. Sørlie for his skilful help during field collections, Prof. R. N. Finn for support and helpful advices during this research, Dr M. Dean for valuable help in the writing of the paper, L. de Lichtervelde for valuable comments on an earlier version of the manuscript, as well as the three anonymous reviewers and the editor for their helpful comments on a recent version of the manuscript. Contribution to the Biodiversity Research Center (BDIV) and to the Centre Interuniversitaire de Biologie Marine (CIBIM).
References
- Adnet S., Capetta H.2001A palaeontological and phylogenetical analysis of squaliform sharks (Chondrichthyes: Squaliformes) based on dental characters. Lethaia 34, 234–248 (doi:10.1111/j.1502-3931.2001.tb00052.x) [Google Scholar]
- Bernal D., Donley J. M., Shadwick R. E., Syme D. A.2005Mammal-like muscle power swimming in a cold-water shark. Nature 437, 1349–1352 (doi:10.1038/nature04007) [DOI] [PubMed] [Google Scholar]
- Buck J. B.1978Functions and evolutions of bioluminescence. In Bioluminescence in action (ed. Herring P. J.), pp. 419–460 London, UK: Academic Press [Google Scholar]
- Claes J. M., Mallefet J.2008Early development of bioluminescence suggests camouflage by counter-illumination in the velvet belly lantern shark, Etmopterus spinax. J. Fish. Biol. 73, 1337–1350 (doi:10.1111/j.1095-8649.2008.02006.x) [Google Scholar]
- Claes J. M., Mallefet J.2009aHormonal control of luminescence from lantern shark (Etmopterus spinax) photophores. J. Exp. Biol. 212, 3684–3692 (doi:10.1242/jeb.034363) [DOI] [PubMed] [Google Scholar]
- Claes J. M., Mallefet J.2009bOntogeny of photophore pattern in the velvet belly lantern shark, Etmopterus spinax. Zoology 112, 433–441 (doi:10.1016/j.zool.2009.02.003) [DOI] [PubMed] [Google Scholar]
- Clarke W. D.1963Function of bioluminescence in mesopelagic organisms. Nature 198, 1244–1246 (doi:10.1038/1981244a0) [Google Scholar]
- Gelsleichter J.2004Hormonal regulation of elasmobranch physiology. In Biology of sharks and their relatives (eds Carrier J., Musick J., Heithaus M.), pp. 287–323 Boca Raton, FL: CRC Press [Google Scholar]
- Haddock S. H. D., Moline M. A., Case J. F.2010Bioluminescence in the sea. Ann. Rev. Mar. Sci. 2, 443–493 (doi:10.1146/annurev-marine-120308-081028) [DOI] [PubMed] [Google Scholar]
- Haygood M. G.1993Light organ symbioses in fishes. Crit. Rev. Microbiol. 19, 191–216 (doi:10.3109/10408419309113529) [DOI] [PubMed] [Google Scholar]
- Herring P. J.1985How to survive in the dark: bioluminescence in the deep sea. Symp. Soc. Exp. Biol. 39, 323–350 [PubMed] [Google Scholar]
- Herring P. J., Morin J. G.1978Bioluminescence in fishes. In Bioluminescence in action (ed. Herring P. J.), pp. 273–329 London, UK: Academic Press [Google Scholar]
- Hubbs C. L., Iwai T., Matsubara K.1967External and internal characters, horizontal and vertical distribution, luminescence, and food of the dwarf pelagic shark Euprotomicrus bispinatus. Bull. Scripps Inst. Oceanogr. 10, 1–64 [Google Scholar]
- Johnsen S., Balser E. J., Widder E. A.1999Light-emitting suckers in an octopus. Nature 398, 113–114 (doi:10.1038/18131) [Google Scholar]
- Visconti M. A., Ramanzini G. C., Camargo C. R., Castrucci A. M. L.1999Elasmobranch color change: a short review and novel data on hormone regulation. J. Exp. Zool. 284, 485–491 (doi:10.1002/(SICI)1097-010X(19991001)284:5<485::AID-JEZ3>3.0.CO;2-5) [DOI] [PubMed] [Google Scholar]
- Warner J. A., Latz M. I., Case J. F.1979Cryptic bioluminescence in a midwater shrimp. Science 203, 1109–1110 (doi:10.1126/science.203.4385.1109) [DOI] [PubMed] [Google Scholar]
- Young R. E., Kampa E. M., Maynard S. D., Mencher F. M., Roper C. F. E.1980Counterillumination and the upper depth of midwater animals. Deep-Sea Res. 27, 671–691 (doi:10.1016/0198-0149(80)90022-9) [Google Scholar]