Abstract
Invasive species can have profound impacts on communities and it is increasingly recognized that such effects may be mediated by parasitism. The ‘enemy release’ hypothesis posits that invaders may be successful and have high impacts owing to escape from parasitism. Alternatively, we hypothesize that parasites may increase host feeding rates and hence parasitized invaders may have increased community impacts. Here, we investigate the influence of parasitism on the predatory impact of the invasive freshwater amphipod Gammarus pulex. Up to 70 per cent of individuals are infected with the acanthocephalan parasite Echinorhynchus truttae, but parasitized individuals were no different in body condition to those unparasitized. Parasitized individuals consumed significantly more prey (Asellus aquaticus; Isopoda) than did unparasitized individuals. Both parasitized and unparasitized individuals displayed Type-II functional responses (FRs), with the FR for parasitized individuals rising more steeply, with a higher asymptote, compared with unparasitized individuals. While the parasite reduced the fitness of individual females, we predict a minor effect on population recruitment because of low parasite prevalence in the peak reproductive period. The parasite thus has a large per capita effect on predatory rate but a low population fitness effect, and thus may enhance rather than reduce the impact of this invader.
Keywords: enemy release, functional-response, invasive-species, parasites, predation, trait-mediated-indirect-effects
1. Introduction
Parasitism is recognized as a powerful force in shaping biological communities (Hatcher et al. 2006; Hudson et al. 2006) and parasites may play critical roles in the success and impacts of invasive species (Dunn 2009). Invaders often show lower parasite diversity and load in their new ranges and it has been proposed that such ‘enemy release’, and consequent increase in competitive ability, can aid the invasion process and impacts (Keane & Crawley 2002; Torchin et al. 2003). A reduction in food intake by parasitized animals is well documented, but parasites may also increase host feeding and growth (e.g. Arnott et al. 2000; Wright et al. 2006). Thus, counter to perceived wisdom, parasites might increase the competitive and/or predatory impacts of invading individuals. Further, if the negative fitness consequences of such a parasite are low, the net population effect of the parasite might be to enhance the impact of the invader.
The amphipod crustacean Gammarus pulex is native to Europe but invasive in Ireland and elsewhere (Dick 2008). Gammarus pulex often actively replaces native amphipods and significantly alters community structure, for example, decreasing macro-invertebrate species diversity (Kelly et al. 2006). In Ireland, the fish acanthocephalan parasite Echinorhynchus truttae uses either the native G. duebeni celticus or the invasive G. pulex as its intermediate host. Prevalence is low in the native (0–1%), but high in the invader (up to 70% in stream patches; MacNeil et al. 2003) and thus any impact of the parasite on invader host predatory strength is likely to have substantial community ramifications.
The ‘functional response’ (FR) of predators is the relationship between prey density and prey consumption. Derivation of FRs illuminates predator behaviour and their impacts on prey populations (Holling 1959). Furthermore, comparative FRs can explain and predict higher invader versus native species community impacts (Bollache et al. 2008). Here, in the invasive G. pulex, we take the novel approach of examining the FRs of parasitized and unparasitized individuals to illuminate their relative population and community impacts. First, we measured parasite prevalence in the field and examined parasite : host mass ratio and the body condition of G. pulex with and without E. truttae. Second, we examined if there are major negative fitness consequences of the parasite by measuring its potential effect on host population recruitment, that is female reproductive output. Third, we investigate the impact of parasitism on predatory strength by deriving FRs for individuals with and without the parasite.
2. Material and methods
From January–September 2008, we collected adult male and female (more than 8 mm) G. pulex from the River Lagan, N. Ireland (J308646) and juvenile (3–5 mm) Asellus aquaticus from Kiltonga Lake (J334716). Experimental animals were maintained as described in Bollache et al. (2008).
To estimate parasite prevalence, at least 150 adult G. pulex were screened each month for infection (presence of an orange/red cystacanth confirmed on dissection as E. truttae). For a subset of unparasitized and E. truttae parasitized males (n = 63 each group) and all females in the peak reproductive period (more than 85% females with bristled oostegites) of June–September (n = 570 unparasitized and 21 parasitized), we measured the body length, body mass (blotted wet weight with the mass of the parasite subtracted) and parasite mass. We used these data to calculate: (i) parasite : host mass ratio; and (ii) mass/length as an index of body condition of individuals with and without the parasite. Numbers of embryos carried by females capable of reproduction (setae present on oostegites) in June–September were compared between those unparasitized and parasitized (ANCOVA, length as covariate).
For the FR experiment, we selected similar sized male G. pulex (mean body length 14.2 mm) both unparasitized and parasitized (status confirmed by later dissection). We presented single males (starved for 24 h) with A. aquaticus at seven prey densities (4, 6, 8, 10, 16, 20, 30; n = 3 per density) in glass dishes (7.5 cm dia.) with 250 ml of continuously aerated water (mixed 50 : 50 amphipod/isopod source). Controls were three replicates of each prey density without predators. Replicates were initiated at 18.00 h and examined after 40 h. Mean prey eaten was examined with respect to ‘parasite status’ and ‘prey density’ (2-factor ANOVA). FRs were modelled (SigmaPlot 8) using a Monod function (y = ax/(1 + bx)), providing estimates of a (the scale parameter) and b (saturation parameter), maximum feeding rate (the asymptote a/(bh), where h is experimental time) and adjusted R2 values for the fitted curves (see Bollache et al. 2008).
3. Results
Hosts always harboured a single parasite and prevalence ranged from 1.2–30.4% (mean 10.3%) for males and 2.7–22.4% (mean 10.0%) for females (paired t8 = 0.2, n.s.). However, during the peak reproductive period, only 2.7–3.7% of females were parasitized. Echinorhynchus truttae weighed up to 17 per cent (for males) and 24 per cent (for females) of the (corrected) body mass of hosts. There was no significant difference in ‘body condition’ (corrected mass/length) of unparasitized and parasitized G. pulex males (t124 = 1.5, n.s.) or females (t589 = 1.8, n.s.). Unparasitized males and females were significantly smaller than those parasitized (mean ± s.e. males: 10.7 mm ± 0.05 versus 12.0 mm ± 0.17, t1445 = 7.1, p < 0.001; females 9.11 ± 0.03 versus 9.99 ± 0.16, t589 = 5.1, p < 0.001). Mean numbers of embryos carried by females was significantly higher in unparasitized compared with parasitized females (12.5 ± 0.3 versus 8.4 ± 1.6, ANCOVA F1,588 = 33.9, p < 0.001).
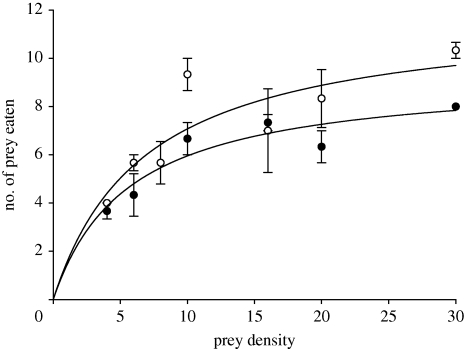
Control A. aquaticus had high survival, with 98.2 per cent alive at 40 h. Thus, experimental deaths were owing to amphipod predation, which we also directly observed. Parasitized G. pulex consumed significantly more prey than did those unparasitized (F1,28 = 8.12, p < 0.01; figure 1), with more prey eaten at higher prey densities (F6,28 = 11.2, p < 0.0001), but the interaction was not significant (F6,28 = 1.2, n.s.). Monod models of FRs achieved high goodness of fit (table 1), reflecting Type-II FRs (see Bollache et al. 2008). The FR for parasitized animals rose more steeply and with a higher asymptote compared with unparasitized individuals (figure 1 and table 1).
Figure 1.
Relationship between the number of prey eaten and prey density, the ‘functional response’ (FR), at 40 h, for unparasitized Gammarus pulex (black circles) and those parasitized with Echinorhynchus truttae (white circles).
Table 1.
Functional response parameters (a and b) with goodness of fit (adjusted R2) for unparasitized and parasitized G. pulex (see figure 1) and predicted maximum intake rate a/(bh).
| parasite | a | b | adj. R2 | a/(bh) |
|---|---|---|---|---|
| absent | 1.65 | 0.18 | 0.87 | 0.23 |
| present | 1.74 | 0.15 | 0.73 | 0.30 |
4. Discussion
Parasites can mediate predator–prey interactions through long-term density effects (Anderson & May 1981; Wilmers et al. 2006). However, there is also growing interest in short-term effects (‘trait-mediated indirect effects’) of parasites on host–host interactions (Werner & Peacor 2003; Hatcher et al. 2006, 2008). There is also considerable evidence that behavioural manipulation by parasites can lead to increased vulnerability of the host to predation (Thomas et al. 2005). Here, we further demonstrate that parasitism of a predator can also modify predator/prey interactions by increasing the predatory impact of the host.
Parasitism with E. truttae significantly enhanced the predation rate of G. pulex on A. aquaticus, with a Type-II FR that rises more steeply and with a higher asymptote compared with unparasitized individuals. Considering that parasitized G. pulex preyed at a 30 per cent higher rate than those unparasitized, parasitism may effectively increase the pressure on prey populations by 3 per cent (at 10.3% mean prevalence) or up to nearly 10 per cent (at 30.4% prevalence). Further, in our study population, E. truttae can infect up to 70 per cent of individuals (MacNeil et al. 2003), increasing the impact of G. pulex on prey by over 20 per cent. Since we size matched males in the two experimental groups to remove any confounding effect of body size, and since parasitized males were significantly larger than unparasitized males in the field, our observed differences in FRs may be conservative and hence actual population level impacts of parasitism may be greater.
On the other hand, if the parasite has major fitness consequences for the invader, then any population level impacts owing to increased per capita effects on feeding rate might be negated. However, as parasite prevalence was low (maximum 3.7%) in the peak reproductive period, even with the parasite's effect of reducing fecundity by 32 per cent, we estimate a reduction of only 1.2 per cent in juvenile recruitment to the population. Hence, direct density effects of the parasite may be more than counterbalanced by behavioural changes, i.e. the per capita predatory impact. Another possible fitness effect, reduced longevity of hosts, seems unlikely as parasitized G. pulex were on average larger than those unparasitized.
Although a previous study reported decreased predation by G. pulex parasitized with E. truttae (Fielding et al. 2003), only a single, low prey density was offered in that study, with intakes in accord with the low densities in the present study. The divergence in FRs we find here occurred at much higher densities, highlighting the utility of the FR approach over other experimental designs. The increase in FR of parasitized G. pulex probably results from direct metabolic demands of the parasite as well as from parasitic manipulation of the host. Echinorhynchus truttae cystacanths were large (up to 24% of corrected host weight), with parasitized G. pulex in a similar body condition as those unparasitized, suggesting that hosts compensate for the nutritional demands of the parasite. Furthermore, E. truttae induces increased activity and hence presumably the vulnerability of G. pulex to predation by the definitive host (MacNeil et al. 2003), which is likely to increase the nutritional demands of the host. For G. pulex with another acanthocephalan, Pomphorynchus laevis, elevated levels of glycogen (Plaistow et al. 2001) and respiratory pigment suggest higher oxygen consumption (Bentley & Hurd 1993).
Parasite-driven changes in host trophic interaction strengths have the potential to alter the wider community structure (Hatcher et al. 2006; Wood et al. 2007). Here, we find that the predatory impact of the invasive G. pulex is enhanced by parasitism. Gammarus pulex decreases the diversity and richness of communities it invades, particularly through predation on other macro-invertebrates (Kelly et al. 2002, 2006). The increase in predatory FR of parasitized hosts may enhance the impact of this invader. Clearly, there is a need to consider the effects of parasitism on trophic interactions between invasive and native species when investigating and predicting the impact of an invasion. Further, the ‘enemy release’ hypothesis must be critically evaluated in other invasion scenarios, since parasites may in fact increase the success and impacts of invasive species.
Acknowledgements
We acknowledge NERC funding, grant NE/G01 521X/1.
References
- Anderson R. M., May R. M.1981The population dynamics of microparasites and their invertebrate hosts. Phil. Trans. R. Soc. Lond. B 291, 451–524 (doi:10.1098/rstb.1981.0005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnott S. A., Barber I., Huntingford F. A.2000Parasite-associated growth enhancement in a fish–cestode system. Proc. R. Soc. Lond. B 267, 657–663 (doi:10.1098/rspb.2000.1052) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley C. R., Hurd H.1993Pomphorhynchus laevis (Acanthocephala) elevation of hemolymph protein concentrations in the intermediate host, Gammarus pulex (Crustacea, Amphipoda). Parasitology 107, 193–198 (doi:10.1017/S0031182000067305) [Google Scholar]
- Bollache L., Dick J. T. A., Farnsworth K. D., Montgomery W. I.2008Comparison of the functional responses of invasive and native amphipods. Biol. Lett. 4, 166–169 (doi:10.1098/rsbl.2007.0554) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick J. T. A.2008Role of behaviour in biological invasions and species distributions; lessons from interactions between the invasive Gammarus pulex and the native G. duebeni (Crustacea: Amphipoda). Contrib. Zool. 77, 91–98 [Google Scholar]
- Dunn A. M.2009Parasites and biological invasions. Adv. Parasitol. 68, 161–184 (doi:10.1016/S0065-308X(08)00607-6) [DOI] [PubMed] [Google Scholar]
- Fielding N. J., MacNeil C., Dick J. T. A., Elwood R. W., Riddell G. E., Dunn A. M.2003Effects of the acanthocephalan parasite Echinorhynchus truttae on the feeding ecology of Gammarus pulex (Crustacrea: Amphipoda). J. Zool. 261, 321–325 (doi:10.1017/S0952836903004230) [Google Scholar]
- Hatcher M. J., Dick J. T. A., Dunn A. M.2006How parasites affect interactions between competitors and predators. Ecol. Lett. 9, 1253–1271 (doi:10.1111/j.1461-0248.2006.00964.x) [DOI] [PubMed] [Google Scholar]
- Hatcher M. J., Dick J. T. A., Dunn A. M.2008A keystone effect for parasites in intraguild predation? Biol. Lett. 4, 534–537 (doi:10.1098/rsbl.2008.0178) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holling C. S.1959Some characteristics of simple types of predation and parasitism. Can. Entomol. 91, 385–398 (doi:10.4039/Ent91385-7) [Google Scholar]
- Hudson P. J., Dobson A. P., Lafferty K. D.2006Is a healthy ecosystem one that is rich in parasites? Trends Ecol. Evol. 7, 381–385 [DOI] [PubMed] [Google Scholar]
- Keane R. M., Crawley M. J.2002Exotic plant invasions and the enemy release hypothesis. Trends Ecol. Evol. 17, 164–170 [Google Scholar]
- Kelly D. W., Dick J. T. A., Montgomery W. I.2002Predation on mayfly nymph, Baetis rhodani, by native and introduced Gammarus: direct effects and the facilitation of salmonid predation. Freshwat. Biol. 47, 1257–1268 (doi:10.1046/j.1365-2427.2002.00864.x) [Google Scholar]
- Kelly D. W., Bailey R. J. E., MacNeil C., Dick J. T. A., McDonald R. A.2006Invasion by the amphipod Gammarus pulex alters community composition of native freshwater macroinvertebrates. Divers. Distrib. 12, 525–534 (doi:10.1111/j.1366-9516.2006.00275.x) [Google Scholar]
- MacNeil C., Fielding N. J., Hume K. D., Dick J. T. A., Elwood R. W., Hatcher M. J., Dunn A. M.2003Parasite altered microdistribution of Gammarus pulex (Crustacea: Amphipoda). Int. J. Parasitol. 33, 57–64 (doi:10.1016/S0020-7519(02)00229-1) [DOI] [PubMed] [Google Scholar]
- Plaistow S. J., Troussard J.-P., Cezilly F.2001The effect of the acanthocephalan parasite Pomphorynchus laevis on the lipid and glycogen content of its intermediate host Gammarus pulex. Int. J. Parasitol. 31, 346–351 (doi:10.1016/S0020-7519(01)00115-1) [DOI] [PubMed] [Google Scholar]
- Thomas F., Adamo S., Moore J.2005Parasitic manipulation: where are we and where should we go? Behav. Process. 68, 185–199 (doi:10.1016/j.beproc.2004.06.010) [DOI] [PubMed] [Google Scholar]
- Torchin M. E., Lafferty K. D., Dobson A. P., McKenzie V. J., Kuris A. M.2003Introduced species and their missing parasites. Nature 421, 628–630 (doi:10.1038/nature01346) [DOI] [PubMed] [Google Scholar]
- Werner E. E., Peacor S. D.2003A review of trait-mediated indirect interactions in ecological communities. Ecology 84, 1083–1100 (doi:10.1890/0012-9658(2003)084[1083:AROTII]2.0.CO;2) [Google Scholar]
- Wilmers C. C., Post E., Peterson R. O., Vucetich J. A.2006Predator disease out-break modulates top-down bottom-up and climatic effects on herbivore population dynamics. Ecol. Lett. 9, 383–389 (doi:10.1111/j.1461-0248.2006.00890.x) [DOI] [PubMed] [Google Scholar]
- Wood C. L., Byers J. E., Cottingham K. L., Altman I., Donahue M. J.2007Parasites alter community structure. Proc. Natl Acad. Sci. USA 104, 9335–9339 (doi:10.1073/pnas.0700062104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright H. A., Wootton R. J., Barber I.2006The effect of Schistocephalus solidus infection on meal size of three-spined stickleback. J. Fish. Biol. 68, 801–809 (doi:10.1111/j.0022-1112.2006.00966.x) [Google Scholar]