Abstract
Computer reconstructions of Archimylacris eggintoni, a Carboniferous stem-group dictyopteran (‘roachoid’), are presented. A siderite-hosted specimen was scanned using high-resolution X-ray microtomography (µCT), and a ‘virtual fossil’ was created with a resolution of 17.7 µm. This has revealed the morphology in great detail, including adhesive limb structures indicative of climbing and specializations for rapid movement. The antennae are filiform, and the mandibles are comparable to those of certain extant cockroaches, suggesting a similar generalist, saprophagous diet. The reconstruction reveals a high degree of specialization, and provides insights into the mode of life of these common Palaeozoic insects. Further µCT study of insect fossils has the potential to supplement wing venation with new characters, and hence improve fossil insect phylogenies.
Keywords: carboniferous, computer tomography, roachoid, Dictyoptera, siderite, Blattoptera
1. Introduction
Prior to the Upper Carboniferous, the insect fossil record is limited to a small number of incomplete specimens. Insects are relatively common in Coal Measures Lagerstätten, by which time the crown groups of many clades (e.g. Odonatoptera, Palaeodictyoptera) are present, together with the ‘roachoid’ Blattoptera, a diverse paraphyletic assemblage of stem-group Dictyoptera (cockroaches, termites and mantises). Such fossils are commonly voids within siderite (FeCO3) nodules, preserving three-dimensional representations of the organisms. Nodules typically split along the animal's dorsal surface; structures enclosed within part or counterpart (e.g. limbs) are normally visually inaccessible. Accordingly, taxonomic and phylogenetic work on Carboniferous insects relies heavily upon wing venation. Many fossils comprise only wings, and thus venation remains vital, but is unfortunately not an ideal character. It can prove problematic taxanomically at species level owing to intraspecific variation in both fossil (Brauckmann 1991) and living (Jarzembowski 2008) insects. Further, in some groups (e.g. cockroaches), asymmetry—which results in variation on a single individual—is common (Bell et al. 2007). Venation is less problematic for family level taxonomy, but proves phylogenetically less useful at this level, being frequently convergent (Kristensen 1998), with vein translocation (rapid fusion) common (Béthoux & Wieland 2009). Additionally, different paradigms provide significantly different phylogenetic implications (Béthoux 2008). Limited venation data for extant insects compounds these problems (Béthoux & Wieland 2009). Perhaps unsurprisingly, the phylogeny of these taxa is poorly resolved. Appendage details could provide new characters for phylogeny and greatly improve our knowledge of the palaeoecology of Carboniferous insects, but relatively few have hitherto been described (e.g. Béthoux 2009). Garwood et al. (2009) demonstrated that a microtomography (µCT)-based ‘virtual palaeontology’ approach can extract appendage data from siderite nodule fossils; we apply this method here to a blattopteran fossil.
2. Material and methods
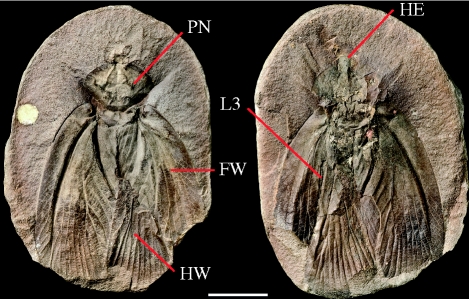
The studied specimen is the holotype of Archimylacris eggintoni (Bolton 1921; =Aphthoroblattina eggintoni, see Schneider 1983), from the Natural History Museum (NHM), London (In 31273, figure 1); Middle Coal Measures (between the Brooch and Thick coals, Bolton 1921), of the Clay croft open-cast works, Coseley Lagerstätte, Staffordshire, UK; Duckmantian in age (ca 311 Ma; Garwood et al. 2009). The fossil is void within a siderite nodule; it was scanned twice at the NHM on a Metris X-Tek HMX-ST. An unfiltered tungsten reflection target, and 3142 projections were used for both scans, one of the entire fossil (200 µA/225 kV), the 2000 × 2000 detector providing a resolution of 25.5 µm, and one of just the anterior to increase limb resolution (180 µA/225 kV, 17.7 µm). Computer models were created using the custom software (SPIERS) written by one of us (M.D.S.; e.g. Garwood et al. 2009). Each slice was manually cleaned digitally; distinct structures were assigned to masks and rendered as individual isosurfaces. Images in figure 2 were ray-traced with Blender (blender.org).
Figure 1.
Scanned specimen—siderite-hosted holotype of A. eggintoni (Bolton 1921) from the Coseley Lagerstätte. (NHM In 31273). Scale bar, 1 cm. Copyright NHM. Photographer Phil Crabb. FW, forewing; HE, head; HW, hindwing; L3, hindleg; PN, pronotum.
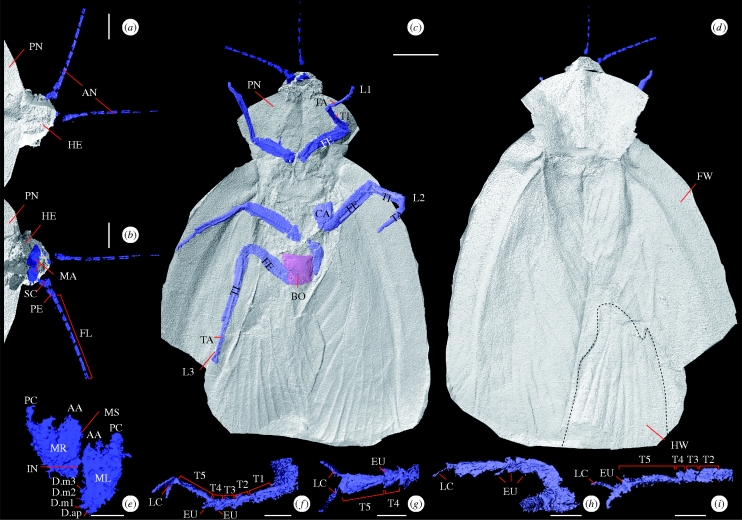
Figure 2.
Computer reconstructions of A. eggintoni. (a) Dorsal view of anterior. (b) Ventral view of anterior. (c) Ventral view, showing all limbs and body. (d) Dorsal view showing wings, pronotum and head. (e) Mandibles. Looking posteriorly, labelling after Zhuzhikov (2007). (f) Right foreleg in the lateral view. (g) Right ventral foreleg. (h) Right midleg in the lateral view. (i) Right ventral midleg. Scale bars, (a,b) 1 mm; (c,d) 5 mm, (e–i) 0.5 mm. All images from the higher resolution model except (c,d). AA, anterior acetabulum; AN, antennae; CA, coxa; D.ap, apical tooth; D.m1–D.m3, marginal teeth; EU, euplantulae; FE, femur; FL, flagellum; FW, forewing; HE, head; HW, hindwing; IN, incisor; L1, foreleg; L2, midleg; L3, hindleg; LC, lateral claw; MA, mandibles; ML, left mandible; MR, right mandible; MS, molar surface; PC, posterior condyle; PE, pedicel; PN, pronotum; SC, scape; T1–5, tarsomeres 1–5; TA, tarsus; TI, tibia.
3. Results
The wings of A. eggintoni are fully resolved (figure 2b,c, see also animation, electronic supplementary material); broad tegminous forewings are absent in one area (figure 2d) where the underlying hindwing is revealed. Venation is resolved, and is as described by Bolton (1921).
The models show antennae (figure 2a,b) with two basal segments—the scape and pedicel—and a minimum of 10 anuli in the flagellum. Ventral and posterior to their attachment are the mandibles (figure 2e), which are dicondylic (i.e. possessing two pivotal points with motion roughly confined to a single plane) with a ventral serrated ridge bearing four denticles. Dorsal to this is a more robust grinding (molar) surface. The head is poorly preserved, with only lateral margins resolved. The antennae and mandibles attach anterior to the pronotum (head shield), suggesting that the head protruded, an interpretation supported by localized discoloration (figure 1). The models' cephalic boundaries (figure 2) were approximated on the basis of the preserved lateral margins.
Limbs are fully resolved. The coxae appear flattened (as in extant cockroaches)—best shown in the left midleg (figure 2c), which also displays the trochanter. The femur—typically the second longest article—is well resolved in all limbs. Distal to this is the tibia. Both tibia and femur are dorso-ventrally flattened and bear longitudinal ridges. The tarsus is split into five tarsomeres (figure 2f–i), the distal and basal being longer than the middle three pseudosegments. Tarsomeres distally bear ventral euplantulae (=pulvilli; adhesive pads), which increase in size distally. Terminally, a small pretarsus is present, with lateral claws (ungues). The limbs are gracile and elongate (figure 2c,d); all are held at a low angle to the body, creating a flattened appearance. The forelegs—the shortest limbs (femur, 4.1 mm; tibia, 4.6 mm; tarsus, 2.7 mm)—are missing the base of the coxae. The midlegs are entirely resolved, with the exception of the distal tarsus on the left limb, which is truncated by the nodule edge. The other is complete (coxa, 3.0 mm; femur, 6.7 mm; tibia, 5.3 mm; tarsus, 3.7 mm). Only the coxa of the left hindleg is preserved (3.2 mm), but the other elements of the right are preserved. It is significantly longer than the anterior limbs as a result of an elongate tibia (femur, 5.5 mm; tibia, 8.2 mm; tarsus, 4.3 mm) and is posteriorly directed.
A small portion of the posterior body has also been preserved. It is flattened in form and preserves no ovipositor or cerci. No other elements of the abdomen are resolved.
The specimen is well preserved, with little preservational distortion or flattening. The midleg tibia demonstrate this; one lies almost within the plane of the wings (probably aligned with bedding), while the other is near perpendicular to this. If significant compression had occurred, a disparity in length between the two would be expected, yet both measure 4.6 mm.
4. Discussion
This study resolves novel characters that may inform blattopteran phylogeny, but a revision of this populous assemblage and the position of A. eggintoni will require analysis of multiple taxa, and is beyond the scope of this paper; we focus instead on mode of life.
The antennae preserve a scape, pedicel and flagellum. They are filiform, and while probably incomplete, long annuli suggest the flagellum could comprise significantly fewer pseudosegments than found in crown-group cockroaches, which can possess in excess of 150. One is preserved parallel to the long axis of the organism, with the other at 70° to this. This flexibility suggests that A. eggintoni had well-developed sensory abilities, using its antennae to sweep a broad sensory arc as it moved, in a manner analogous to extant cockroaches.
The mandibles of A. eggintoni are closely comparable to the mouthparts of crown-group cockroaches, which are generalists, with great dietary versatility. The asymmetry preserved (left mandible larger) is often seen in extant cockroaches (Zhuzhikov 2007), the left overlapping the right, allowing the teeth to interdigitate (figure 2e). Archimylacris eggintoni has four sizeable denticles in the incisor region, an arrangement also seen in Blaberus atropos (Zhuzhikov 2007, fig. 4d), but simpler to that in pest species such as Periplaneta americana, which has several additional denticles (Zhuzhikov 2007, fig. 4c). Blaberus atropos is often found in decaying litter, epiphytes, hollows of trees and inside rotting logs (Bell et al. 2007). This—coupled with the Coal Measures environments in which the Blattoptera are typically found—suggests that A. eggintoni and other Carboniferous forest-dwelling stem-group Dictyoptera were saprophagous/detritivorous.
The limbs display adaptations that are indicative of rapid movement in extant cockroaches (i.e. a cursorial habit), including flattened, thin podomeres (Full & Tu 1990), a long femora and tibiae in all limbs (Gullan & Cranston 2005) and a low angle between the limbs and the body (Kram et al. 1997). Motion studies (Frazier et al. 1999) have shown that the five tarsomere arrangement is highly advantageous for rapid traversing of irregular terrain. The proportionally longer hind limbs of A. eggintoni are also present in pest species such as P. americana. This species takes a bipedal stance when running, overcoming limitations imposed upon stride length by shorter anterior limbs (Full & Tu 1990); P. americana is one of the fastest invertebrates known, relative to mass (Bell et al. 2007). The specializations towards cursorial habit seen in A. eggintoni suggest that it too was capable of very rapid movement.
Pretarsal claws are used in crown-group cockroaches only for climbing rough surfaces (Bell et al. 2007), suggesting that A. eggintoni was capable of venturing beyond the relative safety of the leaf litter. This is supported by the presence of euplantulae (figure 2f–i), used in modern forms for climbing smooth vertical surfaces such as those of plants (Bell et al. 2007). These structures tend to be lost by pure leaf-litter dwelling cockroaches, but retained by those which perch, forage or oviposit in leaves and on plants (e.g. Deans & Roth 2003).
The posterior of a flattened body is preserved (figure 2c). This, in conjunction with the low-angle limbs, creates a flat insect that could fit easily into crevices, suggesting a cryptic habit similar to that of crown roaches. Cerci (paired tail appendages) are known in all crown-group cockroaches, and their presence in primitive winged insects, e.g. the Palaeoptera, and Polyneoptera, suggests they are plesiomorphic to the Dictyoptera. Their absence here could result from decay. Duncan et al. (2003) present a series of cockroach decay experiments in which the disarticulation of cerci precedes that of the limbs, which themselves precede the loss of the ovipositor. The left hindleg in this fossil is missing, suggesting that partial limb decay is recorded, and hence that decay has progressed beyond the loss of the cerci. The remaining limbs appear well preserved, suggesting that the absence of an ovipositor is not taphonomic. A more plausible explanation for its absence is that the specimen is a male.
This study demonstrates the use of µCT-based approaches in the study of siderite-hosted fossils; otherwise unobtainable characters are resolved that—in insects—provide additional characters to wing venation for building phylogenies. The resolution of appendage morphology can provide new insights into the mode of life of Carboniferous organisms. Archimylacris eggintoni shows a high degree of specialization early in the evolution of insects. It was likely to have been a fast runner in life, but also had the ability to climb plant surfaces. Its diet was probably comparable to that of modern forest cockroaches. Its anatomical—and by inference functional and ecological— similarity to crown-group cockroaches (e.g. Bell et al. 2007, figs 1.4, 2.7A, 2.8 and 3.7) is apparent; this emphasizes the cockroach-like nature of the dictyopteran stem group, highlighting in contrast the derived and specialized nature of the mantids.
Acknowledgements
We thank Claire Mellish (NHM) and Andy Ross (NMS) for the loan of material. Thanks also to Andy Ross, and two anonymous referees for valuable comments on the manuscript. R.G. thanks Richard Abel for CT training and Olivier Béthoux for valuable discussion and advice. R.G.'s work is funded by an NERC PhD studentship.
References
- Bell W., Roth L., Nalepa C., Edward O.2007Cockroaches: ecology, behavior, and natural history. Baltimore, MD: Johns Hopkins University Press [Google Scholar]
- Béthoux O.2008Groundplan, nomenclature, homology, phylogeny, and the question of the insect wing venation pattern. Alavesia 2, 219–232 [Google Scholar]
- Béthoux O.2009Head and leg morphology of Elongata Brongniart, 1893: 433 (Late carboniferous, Archaeorthoptera): phylogenetic and palaeoecological implications. Ann. Zool. 59, 141–147 (doi:10.3161/000345409X463949) [Google Scholar]
- Béthoux O., Wieland F.2009Evidence for Carboniferous origin of the order Mantodea (Insecta: Dictyoptera) gained from forewing morphology. Zool. J. Linn. Soc. 156, 79–113 (doi:10.1111/j.1096-3642.2008.00485.x) [Google Scholar]
- Bolton H.1921A monograph of the fossil insects of the British coal measures. London, UK: Palaeontographical Society [Google Scholar]
- Brauckmann C.1991Morphologie und Variabilität von Homoioptera vorhallensis (Insecta: Palaeodictyoptera, Ober-Karbon). Geolog. Palaeontol. 25, 193–213 [Google Scholar]
- Deans A., Roth L.2003Nyctibora acaciana (Blattellidae: Nyctiborinae), a new species of cockroach from Central America that oviposits on Ant-Acacias. Trans. Am. Entomol. Soc. 129, 267–283 [Google Scholar]
- Duncan I. J., Titchener F., Briggs D.2003Decay and disarticulation of the cockroach: implications for preservation of the Blattoids of Writhlington (Upper Carboniferous), UK. Palaios 18, 256–265 (doi:10.1669/0883-1351(2003)018<0256:DADOTC>2.0.CO;2) [Google Scholar]
- Frazier S., Larsen G., Neff D., Quimby L., Carney M., DiCaprio R., Zill S. N.1999Elasticity and movements of the cockroach tarsus in walking. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 185, 157–172 (doi:10.1007/s003590050374) [Google Scholar]
- Full R. J., Tu M.1990The mechanics of six-legged runners. J. Exp. Biol. 148, 129–146 [DOI] [PubMed] [Google Scholar]
- Garwood R. J., Dunlop J., Sutton M. D.2009High-fidelity X-ray micro-tomography reconstruction of siderite-hosted Carboniferous arachnids. Biol. Lett. 5, 841–844 (doi:10.1098/rsbl.2009.0464) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gullan P. J., Cranston P. S.2005The insects: an outline of entomology, 3rd edn Oxford, UK: Blackwell Publishing Ltd [Google Scholar]
- Jarzembowski E.2008The oldest insect from Romania: a new Carboniferous blattodean. Stud. Geolog. Polonica 129, 43–50 [Google Scholar]
- Kram R., Wong B., Full R. J.1997Three-dimensional kinematics and limb kinetic energy of running cockroaches. J. Exp. Biol. 200, 1919–1929 [DOI] [PubMed] [Google Scholar]
- Kristensen N. P.1998The groundplan and basal diversification of the hexapods. In Arthropod relationships, Systematics Association Special Volume Series 55 (eds Fortey R. A., Thomas R. H.), pp. 281–293 London, UK: Chapman & Hall [Google Scholar]
- Schneider J.1983Die Blattodea (Insecta) des Palaeozoikums. Teil 1: Systematik, Oekologie und Biostratigraphie. Freiberger Forschungshefte C382, 106–146 [Google Scholar]
- Zhuzhikov D. P.2007The mouthparts of cockroaches (Blattodea). Entomol. Rev. 87, 25–37 (doi:10.1134/S0013873807010034) [Google Scholar]