Abstract
Processed and red meat consumption is associated with the risk of colorectal cancer. Meta-analyses have suggested that the risk associated with processed meat is higher. Most processed meats are cured and cooked, which leads to formation of free nitrosyl heme. We speculated that free nitrosyl heme is more toxic than native myoglobin. The promoting effect of a freeze-dried, cooked, cured ham diet was looked for in a 100-day study. Colon carcinogenesis endpoints were aberrant crypt foci and mucin depleted foci (MDF). A second study (14 days) was designed 1) to compare the effect of ham, hemoglobin, and hemin; and 2) to test the effect of sodium chloride, nitrite, and phosphate in diet on early biomarkers associated with heme-induced promotion. In the 100-day study, control and ham-fed rats had 3.5 and 8.5 MDF/colon, respectively (P < 0.0001). Promotion was associated with cytotoxicity and lipid peroxidation. In the short-term study, cytotoxicity and lipid peroxidation of fecal water, and the urinary marker of lipid peroxidation, increased dramatically in ham- and hemin-fed rat. In contrast, the hemoglobin diet, sodium chloride, nitrite, phosphate diet had no effect. Freeze-dried cooked ham can promote colon carcinogenesis in a rodent model. Hemin, but not hemoglobin, mimicked ham effect on early biochemical markers associated with carcinogenesis.
Keywords: ham, jambon, charcuterie, cured eat, processed meat, cancer, colon, colorectal, prevention, diet, freeze-drying, lyophylisation, peroxydation, peroxides, rats, azoxymethane, ACF, MDF
Introduction
Colorectal cancer is one of the main causes of death in affluent countries. Environmental factors are involved in this cancer, particularly diet. Modifications in dietary habits could reduce this cancer burden up to 70% (1). In its 2007 report, the World Cancer Research Fund panel judges that “the evidence that red meat and processed meat are a cause of colorectal cancer is convincing”. The panel thus recommends: “Limit intake of red meat and avoid processed meat”. Three recent meta-analyzes show that consumption of red or processed meat is associated with a modest but significant risk of colorectal cancer (2–4). We have estimated, from these meta-analyses, that one gram of processed meat increases the risk of colorectal cancer respectively eleven times, six times or twice more than one gram of fresh red meat (5). Thus processed meat seems more closely associated with the risk of colorectal cancer than fresh red meat. This causes a challenge for meat processing industry to (i) understand the mechanisms involved in the relationship between colorectal cancer and processed meat and (ii) develop research to solve the problem (6).
Several mechanisms have been proposed to explain the relationship between the risk of colorectal cancer and red meat intake. Red meat enhances the formation of putative carcinogenic N-nitroso compounds (NOC) in human feces (7–9). But NOC in rat feces from a bacon-based diet do not initiate or promote preneoplastic lesions in rat colon (10). Meat cooked at a high temperature contains mutagenic heterocyclic amines (HCA) that induce colon, mammary and prostate tumors in rodents and monkeys (11). But HCA might not play an important role in colorectal cancer incidence, since (i) chicken intake is a major contributor of HCA intake, but it is not associated with the risk (12), and (ii) doses of HCA that induce cancer in animals are 1000 to 100,000 times higher than doses ingested by humans (13). Red meat also contains heme, the iron-bearing prosthetic group of myoglobin. Dietary hemin (free heme stabilized by freely exchangeable axial chloride group) in rat diet increases colonic epithelial proliferation and induces cytotoxicity of fecal water in rats (14). Dietary hemin, hemoglobin, and heme in meat promote dose-dependently the formation of preneoplastic lesions in the colon, aberrant crypt foci (ACF) and mucin depleted foci (MDF). We have observed in these previous studies that dietary free heme in the form of hemin was more detrimental than hemoprotein in the form hemoglobin (15–17). Usual processes to make processed meat include salting, adding nitrites, smoking and cooking (5). The addition of nitrite can nitrosylate heme iron and cooking can release nitrosyl heme from myoglobin. We speculated that free nitrosyl heme in processed meat is more toxic than native heme in fresh meat, which would explain why processed meat intake is more closely associated with the risk of colorectal cancer than fresh red meat intake. This hypothesis is corroborated by differences of effect between hemin and hemoglobin in our previous studies, in which hemin is more toxic (16). An alternative hypothesis would be that processed meat nitrite enhances the endogenous formation of carcinogenic NOC, but we did not test this hypothesis in the present study.
Few experimental studies had been conducted on the impact of processed meat on colorectal carcinogenesis (10, 15, 18). Here, the promoting effect of cooked ham was looked for in a 100-day study. Carcinogenesis endpoints were dimethylhydrazine (DMH)-induced preneoplastic lesions (ACF and MDF) in rats. To test the hypothesis that free heme might be more toxic than globin-bound heme, a 14-day study was designed to compare the effect of cooked ham, hemoglobin and hemin on early biomarkers previously associated with heme-induced promotion (17). This study showed for the first time that a cured meat, cooked ham, can promote colon carcinogenesis in a rodent model. Hemin, but not hemoglobin, mimicked ham effect on early biochemical markers associated with carcinogenesis.
Materials and Methods
Animals and Diets
Fifty five Fischer 344 female rats were purchased at four weeks of age from Iffa Credo (St.Germain l’Arbresle, France). Animal care was in accordance with the guidelines of the European Council on animals used in experimental studies. The animal colony and staff got official agreement #31-121 for animal studies by French government.
Short-term study
Thirty five rats were housed individually in metabolic cages. The room was kept at a temperature of 22°C on a 12-h light-dark cycle. After 2 days of acclimatization to the AIN76 powdered, rats were randomly allocated to 5 dietary groups (5 rats per group) and fed experimental diets for two weeks. Body weights and food intake were monitored at the beginning, at the middle and at the end of the experiment. Feces were collected for the last five days and were frozen at −20°C. Urines were collected 1 day before the end of the experiment. Animals were killed 14 days after the start of the experimental diets.
Long-term study
Twenty rats were housed by pairs in stainless steel wire bottomed cages. The room was kept at a temperature of 22°C on a 12-h light-dark cycle. Rats were allowed 7 d of acclimatization to the room and to the control diet, before being injected i.p. with the carcinogen 1,2 dimethylhydrazine (Sigma chemical, St.Quentin, France; 190 mg/kg body wt) in NaCl (9 g/L). Usually, several injections are given to rats. We reasoned that the first shot initiates carcinogenesis, and the following shots promote it, blurring diet-induced promotion. We thus chose a single-shot protocol, following Glauert (19). Seven days later, rats were randomly allocated to two groups of ten, and allowed free access to a control diet or a ham-based diet for 100 days. We chose to initiate all rats with the carcinogen, since the study was designed to show dietary promotion, and because a 2.5% hemoglobin diet does not initiate ACF in rats (Pierre and Corpet, unpublished results).
Diets
Seven experimental diets shown in Table 1 were based on a modified standard AIN-76 diet (20), prepared and formulated in a powdered form by UPAE (INRA, Jouy-en-Josas, France). Diets were made every 14 days and maintained at −20°C. Calcium level is critical for heme promotion (17), calcium was thus excluded from mineral mix but dibasic calcium phosphate was included in all diets at a low concentration of 2.7 g/kg. Wet cured cooked ham (Jambon de Paris) was obtained from Récapé S.A. (Lanta, France). Ham diet of short term study contained 55% cured cooked ham (freeze-dried) w/w, providing 0.25 μmol/g heme. Hemin and hemoglobin (Sigma chemical, St.Quentin, France) diets were formulated to provide the same amount of heme (0.25 μmol/g), and of sodium phosphate, nitrite and chloride as the ham-based diet. All diet of short term study were designed to match ham levels of sodium chloride, nitrite and phosphate, but a low salt control diet contained no added sodium nitrite and phosphate, and little sodium chloride (Table 1). Ham diet of long term study provided 0.036 μmol/g heme.
Table 1.
Composition of diets (g/kg)
| Long-Term Study | Short-Term Study | ||||||
|---|---|---|---|---|---|---|---|
| Control | Ham | Control Low Salt (LoSaCo) | Hemin High Salt (Hemin) | Hemoglobin High Salt (Hemog) | Control High Salt (HiSaCo) | Ham High Salt (Ham2) | |
| Ham | 0 | 550 | 0 | 0 | 0 | 0 | 550 |
| Beef | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hemin | 0 | 0 | 0 | 0.2 | 0 | 0 | 0 |
| Hemoglobin | 0 | 0 | 0 | 0 | 6.6 | 0 | 0 |
| Lard | 160 | 97 | 180 | 180 | 180 | 180 | 117 |
| Safflower Oil | 50 | 50 | 50 | 50 | 50 | 50 | 50 |
| Caseina | 500 | 127 | 450 | 450 | 443 | 450 | 77 |
| Corn Starch | 60 | 60 | 60 | 60 | 60 | 60 | 60 |
| Sucrose | 127 | 14 | 157 | 111 | 111 | 111 | 44 |
| Cellulose | 50 | 50 | 50 | 50 | 50 | 50 | 50 |
| Methionine | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| Mineral mixb | 35 | 35 | 35 | 35 | 35 | 35 | 35 |
| Vitamin mixb | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| Choline bitartrate | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| CaHPO4.2H2O | 2.7 | 2.35 | 2.7 | 2.7 | 2.7 | 2.7 | 2.35 |
| Ferric Citrate | 0.13 | 0 | 0.13 | 0.02 | 0.02 | 0.08 | 0 |
| Sodium Phosphate | 0 | 0 | 0 | 13.7 | 13.7 | 13.7 | 0 |
| Sodium Nitrite | 0 | 0 | 0 | 0.19 | 0.19 | 0.19 | 0 |
| Sodium Chloride | 0 | 0 | 0.54 | 33 | 33 | 33 | 0 |
Low-calcium casein
AIN76 mix, but 500g/kg of dibasic calcium phosphate replaced by sucrose in mineral mix.
Independent study
ACF Assay
Rats were killed by CO2 asphyxiation in a random order at day 100 of the long-term study. Colons were excised from rats immediately post mortem, flushed with cold Krebs solution (Sigma chemical, St.Quentin, France), opened longitudinally and fixed flat between two sheets of filter paper in 10% formalin (Sigma chemical, St.Quentin, France), marked with a two-digit blinding code. Colons picked up in random order were stained for 6 min in a 0.05% filtered solution of methylene blue (21). Number of ACF per colon, and number of crypts in each ACF, were counted under light microscope at x40 magnification in duplicate by two readers, blinded for the origin of the colon.
MDF Assay
MDF may predict colon carcinogenesis better than ACF, since Apc mutations are present in MDF with a frequency similar to that of tumors (22). Colons, after being scored for ACF, were stained with high iron diamine-Alcian blue procedure (HID-AB) to evaluate mucin production (23). Briefly, colons were rinsed in distilled water and left overnight in freshly prepared HID solution (50 mL of distilled water with 120 mg N-N′-dimethyl-m-phenylene diamine, 20 mg N-N′-dimethyl-p-phenylene diamine, and 1.4 mL of 60% ferric chloride). After rinsing, colons were counterstained in 1% alcian blue solution for 30 min. MDF number, and number of crypts per MDF, were scored blindly under light microscope at ×40 magnification by a single reader.
Preparation of Fecal Water
Fecal pellets were collected under each cage of two rats for 24 h, thus leading to five samples per group. Freeze-dried feces were used to calculate dry fecal mass and to prepare fecal water by adding 1 mL of sterilized water to 0.3 g of feces. Samples were then incubated at 37°C for one hour, stirring thoroughly every 20 min, followed by centrifugation at 20 000g for 10 min. The aqueous phase was re-centrifuged at the same speed and duration and the subsequent supernatant (fecal water) collected and conserved at −20°C until use.
TBARS and Heme Assay
Thiobarbituric acid reactive substances (TBARS) were measured in fecal water according to Ohkawa et al. (24), exactly as previously described (15). Heme contents of freeze-dried feces and of fecal water were measured by fluorescence according to Van den Berg et al. (25) and Sesink et al. (14), respectively, as already described (15).
Cytotoxicity Assay of Fecal Water
Cytotoxicity of fecal water was quantified on a cell line according to Bonneson et al. (26), and as previously described (15). Briefly, cancerous mouse colonic epithelial cell line, CMT93 (ECAC), was seeded in 96-well microtiter plates (1.6 × 104 cells per well in 200 μL of medium) and treated for 24 h with fecal water sample diluted at 10% (v/v) in the culture medium. Cytotoxicity of each fecal water was quantified by the 3-(4,5-dimethyldiazol-2-yl)-2,5 diphenyl tetrazolium bromide (MTT) test.
Urinary DHN-MA assay
Each rat was placed alone in a metabolic cage, for two days during the fifth week of experimental diet of long-term study, and at the end of the first week of short-term study. The 24-hour urine was collected under each cage of one rat, thus leading to ten and five samples per group for respectively long and short-term study. DHN-MA assay was done by competitive enzyme immunoassay (EIA) as previously described (27), using DHN-MA-linked acetylcholinesterase enzyme. Each urine sample was assayed in duplicate.
Statistical Analysis
Results were analyzed using Systat 10 software for Windows, and reported as mean ± SD. Long-term study data were analyzed by Student’s t test or Welsh’s test when variances were not similar. ACF data were analyzed by two-way analysis of variance (ANOVA, dietary group and reader). Short-term study data were considered firstly using one-way ANOVA. If a significant difference was found between groups (p<0.05) then pairwise comparisons were made with Tukey multiple comparison test (all other data). The Pearson correlation coefficient was used to determine the relations between ACF, MDF and fecal or urinary values, and P values were calculated with Bonferroni correction for multiple comparisons.
Results
Weight and food intake
Final body weight of rats was 200 ± 2 g after 100 d on experimental diets in the long term study, without significant differences between both groups. Food intake was the same (data not shown), but ham-fed rats drank more water than control rats (23.8 ± 0.5 and 16.4 ± 0.3 mL/d, respectively, p<0.0001).
Final body weight of rats was 132 ± 4 g in the short term study, without significant differences between groups. Food intake was the same in all groups of rats, but rats given a low salt diet drank less water than the other rats (data not shown).
ACF and MDF data
Ham-based diet strikingly increased the number of MDF per colon (Student’s t test p<0.0001, Table 2 and Figure 1). Ham also increased the number of ACF per colon (ANOVA p=0.048, no difference between readers). No difference was observed between groups in the ACF and MDF size (Table 2).
Table 2.
Effect of meat-based diets on aberrant crypt foci and mucin-depleted foci in the colon of rats 107 d after the injection of dimethylhydrazinea
| Dietsb | Heme intake | Aberrant Crypt Foci |
Mucin Depleted Foci |
||||||
|---|---|---|---|---|---|---|---|---|---|
| ACF/colon | Crypts/ACF | MDF/colon | Crypts/MDF | ||||||
| μmol/day | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Control | 0 | 105 | 24 | 2.3 | 0.2 | 3.5 | 2.0 | 4.6 | 1.7 |
| Ham | 0.45 | 119c | 16 | 2.1 | 0.1 | 8.5c | 2.2 | 4.3 | 1.2 |
Values are means and SD, N = 10 rats/dietary group
Diets were based on a low calcium formula, as shown in Table 1.
Significantly different from control group by 2-way ANOVA (ACF, P<0.05) and by Welsh’s test (MDF, P<0.0001)
Figure 1.
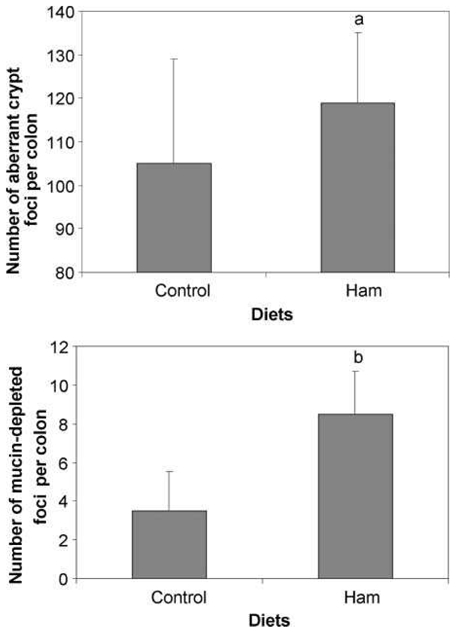
Effect of a ham-based diet on putative precancerous lesions per rat colon 100 days after the injection of dimethylhydrazine. Values are means ± SD; N = 10. Top panel: number of aberrant crypt foci; a, significantly different from control group by 2-way ANOVA (P < 0.05).Bottom panel: number of mucin-depleted foci; b, significantly different from control group by Welsh's test (P < 0.0001).
TBARS and Cytotoxicity of Fecal Water
Heme intake values matched study design: Ham, hemin and hemoglobin groups had similar heme intake (Table 3). Heme can induce the formation of peroxyl radicals in fats, which may be cytotoxic and cleave DNA in vivo. Lipid peroxidation and cytotoxicity were thus measured in fecal water respectively by TBARS assay and MTT assay. Lipid peroxidation and cytotoxicity of fecal water of long-term ham-fed rats were 3-times higher than control values (Table 3, top panel, p<0.0001, Welsh’s test for unequal variances). Ham, hemin and hemoglobin diets increased lipid peroxidation in fecal water in the short-term study (p<0.0001), but the increase was smaller in hemoglobin-fed rats than in ham- and hemin-fed rats (p<0.0001), although heme level was the same in the three experimental diets. Furthermore, as previously published, fecal water from hemin-fed rats was highly cytotoxic to CMT93 cells. Fecal water from ham-fed rats was also cytotoxic, but fecal water from hemoglobin-fed rats was not (Table 3).
Table 3.
Effect of meat-based diets on fecal and urinary values in rats, notably, lipoperoxides and cytotoxicitya
| Study length | Diets b | No. of Rats | Heme intake | TBARS MDA equiv. | Cytotoxicity on CMT93 cells | Urinary DHN-MA | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| days | μmol/d | SD | μM | SD | % lysed | SD | ng/d | SD | ||
| 100 | Control | 10 | 0 | 0 | 33 | 9 | 35 | 8 | 58 | 7 |
| 100 | Ham | 10 | 0.45c | 0.1 | 113c | 24 | 93c | 2 | 11448c | 963 |
| 14 | LoSaCo | 5 | 0 | 0 | 40 | 30 | 0 | 0 | 139 | 7 |
| 14 | HiSaCo | 5 | 0 | 0 | 29 | 14 | 0 | 0 | 197 | 12 |
| 14 | Ham2 | 5 | 2.75d | 0.1 | 278d | 23 | 68 d | 2 | 5107d | 19 |
| 14 | Hemin | 5 | 2.87d | 0.3 | 284d | 30 | 98 d | 2 | 9707d | 37 |
| 14 | Hemog | 5 | 3d | 0 | 110d, e | 22 | 0 | 0 | 71 e | 4 |
Values are means and SD.
Diets were based on a low calcium formula. See Table 1 for precise composition.
Significantly different from control group by Student’s t test (P<0.05)
Significantly different from control group by Tukey multiple comparison test (P<0.05)
Significantly different from ham and hemin fed groups by Tukey multiple comparison test (P<0.05)
Urinary DHN-MA Excretion
Table 3 shows that ham-based diet increased urinary DHN-MA excretion by approximately 200, compared with control diet in the long-term study (P<0.0001). This striking change in DHN-MA excretion is an early event, since a 36-fold increase in DHN-MA was seen after two weeks on ham-based diet in the short-term study. A striking increase in urinary DHN-MA level was also observed in hemin-fed rats, but not in hemoglobin-fed rats (Table 3).
Discussion
The present data establish for the first time that processed meat in a low calcium diet promotes colon carcinogenesis in rats. Processed meat promoted number of ACF and MDF in the colon of carcinogen-induced rats and number of large ACF and MDF (lesions > 4 crypts per foci, from 5.8 to 7.5 for ACF and 1.5 to 3.5 for MDF for respectively control and ham diets) and ham promotion of MDF was correlated with fecal water cytotoxicity (r=0.78, n=20, p<0.001) and TBARS (r=0.68, n=20, p<0.01), and with urinary excretion of DHN-MA (r=0.51, n=20, p<0.05) in line with our previous studies on red meat (15, 17). Correlations in this study between ACF and biomarkers were not significant, which can be explained by the weak number of individuals and groups, but tendencies were strong and close of significant for TBARS and DHN-MA. As, MDF predicts tumor outcome better than ACF, as shown in studies of synbiotics, cholic acid, and piroxicam (23, 28), this result supported our conclusion on correlation between biomarkers and promotion of carcinogenesis.
Red meat promotion can be fully explained by its heme content, since the number of preneoplastic lesions is the same in the colon of beef meat and hemoglobin-fed rats (15). We speculated that ham promotion was related to heme intake, and may be linked to the stimulation of peroxidation and cytotoxicity (15–17). However, in cured meat, the heme molecule can be modified by processing: the addition of nitrite can nitrosylate heme iron, and cooking can release nitrosyl heme from myoglobin to form mononitrosylheme (29). Heme in cooked cured meat is thus different from heme in fresh red meat. In addition, ham contains much more salts than beef meat.
In the short term study, we first looked for the involvement of nitrite and phosphate salts that are usually added to ham. We previously suggested that fecal cytotoxicity and TBARS, and urinary DHN-MA, can be used as short-term biomarkers to screen meat induced promotion of colon cancer (17). Furthermore, in this study we have observed a strong correlation between MDF promotion by Ham and short-term biomarkers modulation. Here, no difference in these biomarkers was seen between high-salt diet and low-salt diet-fed rats (table 3). We thus suggest that nitrite and phosphate salts alone were not responsible for ham-induced modulation of the short-term biomarkers, and we can therefore extrapolate that they were not responsible for ham-induced promotion.
We then looked for the effect of heme in the form of free nitrosyl heme brought by cooked ham or hemin or globin-bound heme. Three diets were formulated with similar heme content in the form of 55% freeze-dried cooked cured ham, 0.2% hemin (free heme with freely exchangeable axial chloride group), and 6.6% hemoglobin (hemoprotein). Sodium chloride, nitrite and phosphate were added to hemin and hemoglobin diets to match ham levels. The magnitude of effect of the ham-diet on the three short-term biomarkers was closely similar to the effect of the hemin diet. By contrast, hemoglobin-diet induced fewer TBARs in fecal water, no cytotoxicity on CMT93 cells, and no urinary excretion of DHN-MA (table 3). In two independent experimentations, we have previously observed that beef-diet (60% MS) induced fewer TBARs in fecal water and cytotoxicity on CMT93 cells, than Hemin2 or Ham groups of this study, after 100 days (17) or after 7 days (data not shown). This is similar to the differences observed between hemin and hemoglobin for a same heme intake of 1.5μmol/g of diet (16). Hemin and ham nitrosyl heme were thus clearly more toxic than hemoglobin and beef. We also made an attempt to synthesize pure mononitrosylheme by Sahidi and Pegg’s protocol (29, 30), and to give it by daily gavages to rats. However, no effect was seen on toxicity biomarkers in rat feces and urine, and we suppose that pure nitrosyl heme was not stable enough to resist stomach gavages. We speculated that processes leading to release of heme from denatured protein could explain difference of toxicity between red and processed meat (5). Indeed, if we compare the effect of Ham on the promotion of MDF to the effect of Beef diets in two independents studies, we can observe the same tendency on the promotion but with a heme intake 10 times higher for the beef diets (0.4μmol/g of beef-diet vs 0.036 μmol/g of ham diet). Furthermore, at equivalent molar dietary concentrations, hemin is more potent than hemoglobin to promote colon carcinogenesis (16), and hemoglobin and beef meat have the same potency in rat (15). Here, hemin and ham-diet induced the same effects on early biomarkers. As MDF promotion and modulation of early biomarkers were correlated in the long term study, we can propose that (i) processes leading to release of heme from globin could participate to the promotive effect of ham and that (ii) hemin may be used as a model agent to study the effect of processed meat on colon carcinogenesis, and hemoglobin a model agent for fresh red meat studies.
The mechanism of ham promotion is not known but, as stated above, it can be linked to peroxidation and cytotoxicity. The high urinary excretion of DHN-MA indicated a high endogenous and/or exogenous formation of 4-hydroxynonenal (HNE) and increased with heme intake in rats and Human (31). We have recently explored the effect of fecal water rich in HNE on normal (Apc +/+) and premalignant colonic cells (Apc Min/+) (32). Apc mutated cells survive heme-induced fecal lipoperoxides, notably 4-hydroxynonenal (HNE) that is toxic to normal cells. Selection of mutated cells by cytotoxic lipoperoxides may explain promotion of colon carcinogenesis by diet inducing large amount of HNE. However in this study, we can suspect an over-production of HNE in ham diets due to freeze-drying. Indeed, formation of HNE, facilitated by concomitant presence of heme iron and omega 6 fatty acids, is enhanced by freeze-drying (33). It would be therefore preferable in future studies to work with a meat that is not freese-dried.
An alternative hypothesis for processed meat promotion tells that nitrite in cured meat enhances the formation of carcinogenic NOC, in food and endogenously (7, 9, 34, 35). This hypothesis was not tested in the present study. Parnaud et al. (10, 18) showed that fried bacon-fed rats excrete 10 to 20 times more NOCs in feces than controls. Mirvish et al. showed that hot-dog contains 10 times more NOCs than fresh red meat (36). This high level of NOCs could explain why processed meat is associated more strongly than no nitrite-fresh red meat with colorectal cancer risk (5). However, fecal NOCs do not initiate or promote preneoplastic lesions in the colon of rats (10, 18), and we thus think this hypothesis is not supported by experimental studies in rodents, although it receives much support from human volunteer’s studies (7, 9, 34, 35).
In conclusion, the present study shows for the first time that a cured meat, cooked ham, can promote colon carcinogenesis in a rodent model. It also suggests that hemin, but not hemoglobin, may be used as a model agent for processed meat studies.
Acknowledgments
We thank Xavier Blanc (UPAE) for the preparation of experimental diets, Raymond Gazel and Florence Blas Y Estrada for the care of the animals.
The studies were supported in part by the INRA, by the DGER, and by a grant of the French region Midi-Pyrénées
Abbreviations
- ACF
aberrant crypt foci
- MDF
mucin-depleted foci
- TBARS
thiobarbituric acid reactive substances
- DHN-MA
1,4-dihydroxynonane mercapturic acid
References
- 1.Cummings JH, Bingham SA. Fortnightly review - diet and the prevention of cancer. British Medical Journal. 1998;317:1636–1640. doi: 10.1136/bmj.317.7173.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sandhu MS, White IR, Mcpherson K. Systematic review of the prospective cohort studies on meat consumption and colorectal cancer risk: a meta-analytical approach. Cancer Epidemiology Biomarkers & Prevention. 2001;10:439–446. [PubMed] [Google Scholar]
- 3.Norat T, Lukanova A, Ferrari P, Riboli E. Meat consumption and colorectal cancer risk: dose-response meta-analysis of epidemiological studies. International Journal of Cancer. 2002;98:241–256. doi: 10.1002/ijc.10126. [DOI] [PubMed] [Google Scholar]
- 4.Larsson SC, Wolk A. Meat consumption and risk of colorectal cancer: a meta-analysis of prospective studies. Int J Cancer. 2006;119:2657–2664. doi: 10.1002/ijc.22170. [DOI] [PubMed] [Google Scholar]
- 5.Santarelli RL, Pierre F, Corpet DE. Processed meat and colorectal cancer: a review of epidemiologic and experimental evidence. Nutrition and Cancer. 2008;60:131–44. doi: 10.1080/01635580701684872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Demeyer D, Honikel K, De Smet S. The World Cancer Research Fund report 2007: a challenge for the meat processing industry. Meat Science. 2008;80:953–959. doi: 10.1016/j.meatsci.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 7.Bingham SA, Pignatelli B, Pollock JRA, Ellul A, Malaveille C, Gross G, Runswick S, Cummings JH, Oneill IK. Does increased endogenous formation of N-nitroso compounds in the human colon explain the association between red meat and colon cancer? Carcinogenesis. 1996;17:515–523. doi: 10.1093/carcin/17.3.515. [DOI] [PubMed] [Google Scholar]
- 8.Cross AJ, Pollock JRA, Bingham SA. Haem, not protein or inorganic iron, is responsible for endogenous intestinal n-nitrosation arising from red meat. Cancer Research. 2003;63:2358–2360. [PubMed] [Google Scholar]
- 9.Lunn JC, Kuhnle G, Mai V, Frankenfeld C, Shuker DE, Glen RC, Goodman JM, Pollock JR, Bingham SA. The effect of haem in red and processed meat on the endogenous formation of N-nitroso compounds in the upper gastrointestinal tract. Carcinogenesis. 2007;28:685–690. doi: 10.1093/carcin/bgl192. [DOI] [PubMed] [Google Scholar]
- 10.Parnaud G, Pignatelli B, Peiffer G, Tache S, Corpet DE. Endogenous N-nitroso compounds, and their precursors, present in bacon, do not initiate or promote aberrant crypt foci in the colon of rats. Nutrition and Cancer. 2000;38:74–80. doi: 10.1207/S15327914NC381_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sugimura T, Wakabayashi K, Nakagama H, Nagao M. Heterocyclic amines: Mutagens/carcinogens produced during cooking of meat and fish. Cancer Sci. 2004;95:290–299. doi: 10.1111/j.1349-7006.2004.tb03205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sinha R, Rothman N, Brown ED, Mark SD, Hoover RN, Caporaso NE, Levander OA, Knize MG, Lang NP, Kadlubar FF. Pan-fried meat containing high levels of heterocyclic aromatic amines but low levels of polycyclic aromatic hydrocarbons induces cytochrome p4501a2 activity in humans. Cancer Research. 1994;54:6154–6159. [PubMed] [Google Scholar]
- 13.Stavric B. Biological significance of trace levels of mutagenic heterocyclic aromatic amines in human diet: a critical review. Food and Chemical Toxicology. 1994;32:977–994. doi: 10.1016/0278-6915(94)90093-0. [DOI] [PubMed] [Google Scholar]
- 14.Sesink ALA, Termont DSML, Kleibeuker JH, Vandermeer R. Red meat and colon cancer: the cytotoxic and hyperproliferative effects of dietary heme. Cancer Research. 1999;59:5704–5709. [PubMed] [Google Scholar]
- 15.Pierre F, Freeman A, Tache S, Van der Meer R, Corpet DE. Beef meat and blood sausage promote the formation of azoxymethane-induced mucin-depleted foci and aberrant crypt foci in rat colons. Journal of Nutrition. 2004;134:2711–2716. doi: 10.1093/jn/134.10.2711. [DOI] [PubMed] [Google Scholar]
- 16.Pierre F, Tache S, Petit CR, Van der Meer R, Corpet DE. Meat and cancer: haemoglobin and haemin in a low-calcium diet promote colorectal carcinogenesis at the aberrant crypt stage in rats. Carcinogenesis. 2003;24:1683–1690. doi: 10.1093/carcin/bgg130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pierre F, Santarelli R, Tache S, Gueraud F, Corpet DE. Beef meat promotion of dimethylhydrazine-induced colorectal carcinogenesis biomarkers is suppressed by dietary calcium. Br J Nutr. 2008;99:1000–1006. doi: 10.1017/S0007114507843558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parnaud G, Peiffer G, Tache S, Corpet DE. Effect of meat (beef, chicken, and bacon) on rat colon carcinogenesis. Nutrition and Cancer. 1998;32:165–173. doi: 10.1080/01635589809514736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karkare MR, Clark TD, Glauert HP. Effect of Dietary Calcium on Colon Carcinogenesis Induced by a Single Injection of 1,2-Dimethylhydrazine in Rats. Journal of Nutrition. 1991;121:568–577. doi: 10.1093/jn/121.4.568. [DOI] [PubMed] [Google Scholar]
- 20.American Institute of Nutrition. Report of the American Institute of Nutrition Ad Hoc Committee on standards for nutritional studies. J Nutr. 1977;107:1340–1348. doi: 10.1093/jn/107.7.1340. [DOI] [PubMed] [Google Scholar]
- 21.Bird RP. Observation and quantification of aberrant crypts in murine colon treated with a colon carcinogen: preliminary findings. Cancer Lett. 1987;37:147–151. doi: 10.1016/0304-3835(87)90157-1. [DOI] [PubMed] [Google Scholar]
- 22.Femia AP, Dolara P, Giannini A, Salvadori M, Biggeri A, Caderni G. Frequent mutation of Apc gene in rat colon tumors and mucin-depleted foci, preneoplastic lesions in experimental colon carcinogenesis. Cancer Research. 2007;67:445–449. doi: 10.1158/0008-5472.CAN-06-3861. [DOI] [PubMed] [Google Scholar]
- 23.Caderni G, Femia AP, Giannini A, Favuzza A, Luceri C, Salvadori M, Dolara P. Identification of mucin-depleted foci in the unsectioned colon of azoxymethane-treated rats: correlation with carcinogenesis. Cancer Research. 2003;63:2388–2392. [PubMed] [Google Scholar]
- 24.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Analytical Biochemistry. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 25.Van den Berg JW, Koole-Lesuis R, Edixhoven-Bosdijk A, Brouwers N. Automating the quantification of heme in feces. Clinical Chemistry. 1988;34:2125–2126. [PubMed] [Google Scholar]
- 26.Bonneson C, Eggleston IM, Hayes JD. Dietary indoles and isothiocyanates that are generated from cruciferous vegetables can both stimulate apoptosis and confer protection against DNA damage in human colon cell lines. Cancer Research. 2001;61:6120–6130. [PubMed] [Google Scholar]
- 27.Gueraud F, Peiro G, Bernard H, Alary J, Creminon C, Debrauwer L, Rathahao E, Drumare MF, Canlet C, et al. Enzyme immunoassay for a urinary metabolite of 4-hydroxynonenal as a marker of lipid peroxidation. Free Radic Biol Med. 2006;40:54–62. doi: 10.1016/j.freeradbiomed.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 28.Femia AP, Dolara P, Caderni G. Mucin-depleted foci (MDF) in the colon of rats treated with azoxymethane (AOM) are useful biomarkers for colon carcinogenesis. Carcinogenesis. 2004;25:277–281. doi: 10.1093/carcin/bgh005. [DOI] [PubMed] [Google Scholar]
- 29.Pegg RB, Shahidi F. The color of meat. Food & Nutrition Press Inc; Trumbull, Connecticus 06611 USA: 2000. Nitrite curing of meat: the N-nitrosamine problem and nitrite alternatives; pp. 23–66. [Google Scholar]
- 30.Shahidi F, Pegg RB. Novel synthesis of cooked cured-meat pigment. J Food Science. 1991;5:1205–1208. [Google Scholar]
- 31.Pierre F, Peiro G, Tache S, Cross AJ, Bingham SA, Gasc N, Gottardi G, Corpet DE, Gueraud F. New marker of colon cancer risk associated with heme intake: 1,4-dihydroxynonane mercapturic Acid. Cancer Epidemiol Biomarkers Prev. 2006;15:2274–2279. doi: 10.1158/1055-9965.EPI-06-0085. [DOI] [PubMed] [Google Scholar]
- 32.Pierre F, Tache S, Gueraud F, Rerole AL, Jourdan ML, Petit C. Apc mutation induces resistance of colonic cells to lipoperoxide-triggered apoptosis induced by faecal water from haem-fed rats. Carcinogenesis. 2007;28:321–327. doi: 10.1093/carcin/bgl127. [DOI] [PubMed] [Google Scholar]
- 33.Gasc N, Tache S, Rathahao E, Bertrand-Michel J, Roques V, Gueraud F. 4-hydroxynonenal in foodstuffs: heme concentration, fatty acid composition and freeze-drying are determining factors. Redox Rep. 2007;12:40–44. doi: 10.1179/135100007X162257. [DOI] [PubMed] [Google Scholar]
- 34.Kuhnle GG, Bingham SA. Dietary meat, endogenous nitrosation and colorectal cancer. Biochem Soc Trans. 2007;35:1355–1357. doi: 10.1042/BST0351355. [DOI] [PubMed] [Google Scholar]
- 35.Zhou L, Haorah J, Perini F, Carmella SG, Shibamoto T, Mirvish SS. Partial purification from hot dogs of N-nitroso compound precursors and their mutagenicity after nitrosation. J Agric Food Chem. 2006;54:5679–5687. doi: 10.1021/jf0604788. [DOI] [PubMed] [Google Scholar]
- 36.Mirvish SS, Haorah J, Zhou L, Hartman M, Morris CR, Clapper ML. N-nitroso compounds in the gastrointestinal tract of rats and in the feces of mice with induced colitis or fed hot dogs or beef. Carcinogenesis. 2003;24:595–603. doi: 10.1093/carcin/24.3.595. [DOI] [PubMed] [Google Scholar]


