Abstract
Non-human primates are marked by well-developed prosocial and cooperative tendencies as reflected in the way they support each other in fights, hunt together, share food and console victims of aggression. The proximate motivation behind such behaviour is not to be confused with the ultimate reasons for its evolution. Even if a behaviour is ultimately self-serving, the motivation behind it may be genuinely unselfish. A sharp distinction needs to be drawn, therefore, between (i) altruistic and cooperative behaviour with knowable benefits to the actor, which may lead actors aware of these benefits to seek them by acting cooperatively or altruistically and (ii) altruistic behaviour that offers the actor no knowable rewards. The latter is the case if return benefits occur too unpredictably, too distantly in time or are of an indirect nature, such as increased inclusive fitness. The second category of behaviour can be explained only by assuming an altruistic impulse, which—as in humans—may be born from empathy with the recipient's need, pain or distress. Empathy, a proximate mechanism for prosocial behaviour that makes one individual share another's emotional state, is biased the way one would predict from evolutionary theories of cooperation (i.e. by kinship, social closeness and reciprocation). There is increasing evidence in non-human primates (and other mammals) for this proximate mechanism as well as for the unselfish, spontaneous nature of the resulting prosocial tendencies. This paper further reviews observational and experimental evidence for the reciprocity mechanisms that underlie cooperation among non-relatives, for inequity aversion as a constraint on cooperation and on the way defection is dealt with.
Keywords: cooperation, prosocial behaviour, non-human primates, reciprocity
1. Introduction
The common claim that humans are the only truly altruistic species, since all non-human animals are self-interested and only care about return benefits (e.g. Dawkins 1976; Kagan 2000; Fehr & Fischbacher 2003; Silk et al. 2005), conflates individual motivation with the possible reason for a behaviour's evolution, i.e. it confuses proximate and ultimate causes. In order to be literally selfishly motivated, an animal needs to be aware how its behaviour will ultimately benefit itself or its immediate kin. For most altruistic behaviour (e.g. behaviour that increases the fitness of the recipient while decreasing the actor's direct fitness), evidence for such awareness is lacking. Therefore, the more parsimonious assumption about the proximate motivation behind altruistic behaviour is that it is either unconcerned with outcomes or simply altruistic.
It may be useful to divide cooperative and altruistic behaviour into two categories: (i) behaviour that benefits others, but also benefits the actor in such a way that the actor can potentially learn about these benefits and (ii) behaviour that benefits others, whereas its potential return benefits remain obscure either because they are not open to direct experience (e.g. increased inclusive fitness) or so unpredictable and/or distant in time that it is unlikely that the actor will associate them with the original behaviour. Whereas the first category may lead to selfishly motivated altruism and cooperation, this cannot hold for the second category. Even though the second category may very well be evolutionarily self-serving (e.g. ultimately increases the actor's fitness through reciprocal altruism or inclusive fitness), such behaviour is best considered motivationally autonomous in the same way that sexual motivation is autonomous, i.e. independent of the ultimate goal of reproduction. Thus, from a proximate perspective, aiding behaviour may be genuinely altruistic in that the actor performs it without selfish ends in mind (de Waal 2008).
In humans, the most widely assumed autonomous motivation for altruism is empathy (Batson 1991), which has also been proposed for other mammals (de Waal 1996, 2008). Empathy is the capacity to (i) be affected by and share the emotional state of another (e.g. emotional contagion), (ii) assess the reasons for the other's state and/or (iii) identify with the other, adopting his or her perspective (de Waal 2008). Not all altruistic behaviour requires empathy, though. When animals alert others to an outside threat, sacrifice themselves by stinging an intruder or vocally attract others to discovered food, biologists may speak of altruism or cooperation, yet such behaviour is unlikely to be based on empathy with the beneficiary. Indeed, these behaviours are probably inborn responses to certain stimuli performed with little consideration for the exact situation of the recipients. The role of empathy is limited to so-called directed altruism, defined as helping or comforting behaviour directed at an individual in need, pain or distress. A detailed discussion of the neural basis of this mechanism is beyond the scope of this paper, but it has been proposed that empathy relies on automatically activated state-matching that produces shared representations and similar emotions (Preston & de Waal 2002; Decety & Jackson 2006). Probably, this mechanism is a mammalian universal, given that part of its assumed neural underpinnings were first discovered in macaques (e.g. mirror neurons; Rizzolatti et al. 1996) and that emotional contagion (often considered the starting point of empathy) is increasingly studied in rodents (Langford et al. 2006; Chen et al. 2009; Grenier & Lüthi 2010). Mirroring mechanisms permit one individual to resonate with the emotional state of another, thus priming this individual for actions appropriate to the other's state, such as when a mother is distressed at hearing the distress calls of her young resulting in comforting behaviour (Panksepp 1996). Oxytocin acts as a hormonal mechanism to facilitate empathy, which in game theory experiments causes humans to become more generous (Zak et al. 2007; Barraza & Zak 2009).
This paper introduces terminology related to the proximate side of cooperation and prosocial behaviour and starts out with behaviour of which the pay-offs are knowable to the actors, thus permitting them to strive for these pay-offs. Next, it will address cooperation and altruism that serve others without any direct, knowable benefits to the actor, ranging from so-called other-regarding preferences to spontaneous consolation of distressed parties. Finally, we will consider circumstances under which these behavioural mechanisms are inhibited or thwarted, and how animals handle cheaters. By the nature of our own research, this review will be biased towards non-human primates, even though the discussed mechanisms probably apply outside the primate order.
2. Potentially selfish cooperation and contingent reciprocity
(a). Learning the need for a partner
Perhaps the easiest way to learn the benefits of cooperation is when all parties receive benefits immediately following the cooperative act. Such cooperation has been observed in a great variety of taxa, including the mutual grooming of impala, mobbing of predators by European blackbirds and cooperative predation on the embryos of large fish by schools of wrasse in order to overcome paternal defences (Dugatkin 1997).
A typical example is cooperative hunting in which the pay-off quickly follows the effort. Among primates, group hunting and meat-sharing are known of capuchin monkeys (Perry & Rose 1994; Rose 1997) and chimpanzees (Boesch 1994). Among chimpanzees at Taï Forest, in Ivory Coast, meat is almost always shared, and active participants in the hunt have easier access to it than individuals that did not help capture the prey (Boesch 1994). It is important to note, however, that meat-sharing following hunting seems to be dependent on whether or not multiple individuals are required to catch the prey. Thus, in Gombe National Park chimpanzees do not reliably hunt cooperatively, since there is high success for single hunters, and as a result there may be less emphasis on sharing (Stanford 1996). Differences in meat-sharing may therefore be explained by differing levels of cooperation required to obtain the food, so that sharing functions more as reciprocal benefits for participating in the hunt than as altruistic provisioning.
Cooperative hunting can be mimicked in the laboratory by letting individuals work together to gain access to food. The first such experiment was conducted by Crawford (1937), who let two juvenile chimpanzees pull ropes simultaneously to bring in a box with food too heavy for a single individual to bring in. After training, the apes worked together and demonstrated their understanding of the task by recruiting reluctant partners whose motivation had been reduced by food intake prior to the test. They activated these partners by gently slapping their backs.
Crawford's classical mutualism experiment inspired many others, including a test by Melis et al. (2006a) on the chimpanzees' understanding of their partner's role. Chimpanzees were allowed to choose whether or not to recruit a helper. In the solo condition, the apparatus was set up such that the individual could pull in a drawer with food alone. In the mutualism condition, the individual needed a partner to help them obtain food for both. The chimpanzees were able to open a door to give partners access to the testing apparatus and did so significantly more often when they needed help than when they were able to pull by themselves. Furthermore, after learning that certain individuals were more reliable collaborators than others, when given the option of recruiting different collaborators, chimpanzees preferentially gave access to the best ones. A follow-up study gave subjects the option of recruiting a ‘nice’ partner (who had collaborated with them in the past) and a ‘mean’ partner (who had chosen to collaborate with another partner than the subject in the past). After a brief learning period to establish the reputation of the partner as ‘nice’ or ‘mean’, the chimpanzees more often recruited the ‘nice’ partner than they had done before (Melis et al. 2008).
Whereas these experiments confirm and expand upon Crawford's (1937) initial chimpanzee study, the same level of understanding was thought to be lacking in non-apes. But this may have been due to the fact that the cooperative skills of monkeys were initially tested with a different, less intuitive paradigm. Instead of pulling a box towards themselves, two capuchin monkeys had to press levers or buttons at exactly the same time to receive food. They did succeed at this task, yet without any indication that they actually understood their partner's contribution (Chalmeau et al. 1997; Visalberghi et al. 2000; Brosnan & de Waal 2002). A related experiment with blue jays rewarded two birds for simultaneously pecking at a ‘cooperation’ key (Clements & Stephens 1995). But since the jays were equally successful regardless of whether or not they could see their partner, and since success seemed to reflect accidental co-occurrence of pecking, it has been argued that their behaviour had little to do with cooperation (Roberts 1997).
In sum, when monkeys (or birds) cannot observe the incremental results of collective action, and need to act in perfect synchrony, they seem to have trouble learning about each other's contributions. They never achieve true cooperation which requires an understanding that their partner is necessary to achieve a goal. But does this mean that such cooperation is beyond their capacity? Cotton-top tamarins were more likely to act in a handle-pulling task when their partner was present than when their partner was absent (Cronin et al. 2005). Although partner presence may have served as a conditioned stimulus for action, without the monkeys realizing that their partner was actually helping, there were indications that the monkeys understood they needed their partner's help. For example, individuals would sustain pulling on the apparatus and wait for their partner to pull their handle also before releasing.
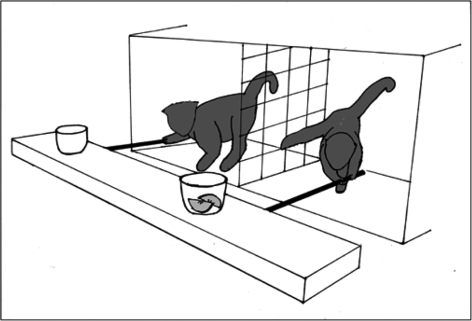
When capuchin monkeys were tested with an apparatus similar to Crawford's (1937; figure 1), they showed immediate success. The pulling task has the advantage of both visual and kinesthetic feedback in the course of collective action towards a shared goal, which may be essential to learn about the partner's contribution. When an opaque barrier was placed between both monkeys, their success rate dropped dramatically even though the partner's presence was clear: both monkeys could see each other through a hole at the back of the partition. Knowing that another monkey was there, they nevertheless failed to coordinate. In other tests, the same monkeys would wait for the return of partners who had wandered away before initiating pulling actions. These monkeys gave every indication, therefore, of understanding the need for coordination (Mendres & de Waal 2000).
Figure 1.
In the cooperative pulling task two capuchin monkeys are situated in adjacent sections of a test chamber, separated by mesh. The apparatus consists of a counter-weighted tray with two pull bars, with each monkey having access to one bar. If both cups are filled, success requires mutualistic cooperation, whereas if only one cup is filled (as shown here) cooperation is sustained by sharing through the mesh by the advantaged individual, who pays for the other's labour (de Waal & Berger 2000). Drawing by Sarah Brosnan.
Outside of the primate order, social carnivores such as hyenas exhibit cooperation and coordination with other individuals in the group. In one experiment, hyenas coordinated their behaviour with a partner to obtain food from a hidden platform (Drea & Carter 2009). They modified their behaviour in response to social stimuli, showing sensitivity to the need for a partner and coordination with other individuals in their group. In fact, experienced hyenas facilitated cooperation with a naive partner by increasing visual monitoring and coordination.
An even higher level of understanding was demonstrated in an experiment in which capuchin monkeys had to perform a closely coordinated sequence to obtain food (Hattori et al. 2005). First one partner had to pull a tab, which then allowed the other partner to slide a block. If both actions were done in sequence, both individuals would obtain food. The second individual spent more time looking at its partner when they needed help than when they were able to solve the task by themselves, which suggests visual coordination. As opposed to the lever or button pressing paradigms, where individuals can act independently and succeed by coincidence, in ‘communicative cooperation’ individuals need to coordinate closely to succeed (reviewed by Noë 2006).
What these mutualism experiments demonstrate is that both monkeys and apes are able to learn the benefits and pay-offs of cooperation and develop a fairly good grasp of the need for and role of a partner, thus achieving true cooperation. The same learning process probably underlies collective action in the field, such as hunting together followed by sharing. Since cooperation produces benefits that are hard or impossible to attain by any individual alone, the resulting behaviour is essentially self-serving even if it benefits others at the same time.
(b). Contingent reciprocity
Not all forms of cooperation produce immediate benefits, however. Whenever benefits are exchanged after a time interval, we speak of reciprocal altruism, or reciprocity (Trivers 1971). In the way reciprocity is modelled, it requires certain cognitive abilities (Brosnan et al. 2010b), which are sometimes assumed too complex for most or all animals (Hammerstein 2003; Stevens & Hauser 2004), whereas in fact reciprocity can be produced by a range of proximate mechanisms, not all of which are cognitively demanding. There is evidence for a variety of these mechanisms in non-human primates (de Waal & Brosnan 2006).
One such mechanism is attitudinal reciprocity, according to which individuals mirror the attitudes of their partners over short time intervals (table 1). This type of reciprocity was first experimentally demonstrated in capuchin monkeys using a delayed exchange task. First, one partner was given pieces of apple for 20 min while her partner sat at the other side of a mesh partition. In the following 20 min, the other was given pieces of carrot. It was found that the amount of food shared through the mesh by the second individual correlated with the amount of food he/she had received from the first. It is important to note that these results do not necessarily indicate that the monkeys were keeping track of food amounts, repaying food with food, even though this was the end result. They may simply have been responding to their partner's tolerant or intolerant attitude by being, respectively, tolerant or intolerant in return (de Waal 2000). The same monkeys exchanged food preferentially with partners who had just helped them in a cooperative pulling task (figure 1; de Waal & Berger 2000).
Table 1.
Various proximate mechanisms that ensure a reciprocal distribution of helping behaviour. These mechanisms are arranged from top to bottom from the least to the most cognitively demanding. Modified from de Waal and Brosnan (2006).
| mechanism | catch phrase | definition |
|---|---|---|
| generalized reciprocity | ‘Thank goodness!’ | increased tendency to assist any others after having received assistance: no partner-specific contingency |
| symmetry-based reciprocity | ‘We're buddies’ | symmetrical relationship characteristics (e.g. association) prompt similar behaviour in both directions within a dyad without a high degree of contingency |
| attitudinal reciprocity | ‘If you're nice, I'll be nice’ | Parties mirror each other's social attitudes with a high degree of short-term contingency |
| calculated reciprocity | ‘What have you done for me lately?’ | scorekeeping of given and received favours resulting in partner-specific delayed contingency |
Benefits exchanged in close temporal succession allow actors to learn about behavioural contingencies. These contingencies may be used to develop successful cooperation. In other words, they learn that their partner's behaviour at trial t is contingent upon their own behaviour at trial t − 1. If they additionally learn to change their own behaviour so as to manipulate their partner's future behaviour, we speak of contingent reciprocity. Whereas capuchin monkeys seem capable of establishing these contingencies over short time intervals, longer delays may interfere with the learning process. Contingent reciprocity is constrained, therefore, by the time delay between exchanges and the memory capacity of the species under study (see Brosnan et al. 2010b).
Experiments with apes have yielded conflicting results. Chimpanzees failed to change their behaviour in response to their partner's previous behaviour: they were equally likely to donate food to a partner regardless of whether or not they had previously received food from this partner (Brosnan et al. 2009). This study, however, used a set-up that has thus far never produced altruistic giving in chimpanzees (Silk et al. 2005; Jensen et al. 2006), thus removing any basis to learn the advantages of reciprocity. Another ape study provides a contrast in that two orangutans learned to reciprocally exchange tokens when each had access to tokens that were of value only to the other (Dufour et al. 2009). The orangutans' behaviour was particularly interesting in that one individual seemed to initiate token transfers, but that over time reciprocity emerged and both partners began to alternate transfers to each other. Similarly, when chimpanzees were given the opportunity to exchange other rewarding tokens, they learned to alternate donating rewards to each other (Yamamoto & Tanaka 2009). However, this type of exchange might be limited to a very specific situation facilitated by human experimenters. For example, a comparative study of many ape species found exchanges to be limited to orangutans (Pelé et al. 2009), whereas chimpanzees have thus far failed to show reciprocal exchange without human facilitation (Brosnan & Beran 2009; Yamamoto & Tanaka 2009).
Despite these negative experimental results, we should not forget that observations of reciprocity in chimpanzee's natural behaviour strongly suggest learning over longer time intervals, i.e. the development not just of attitudinal reciprocity, but also calculated reciprocity based on scorekeeping of given and received favours (§3; table 1). For example, male chimpanzees in Bossou, Guinea, sometimes raid papaya plantations (a risky endeavour) and share the highly prized fruits specifically with females, which they hypothesized was a strategy for obtaining future copulations (Hockings et al. 2007). This is similar to observations of Stanford et al. (1994) of male chimpanzees at Gombe National Park hunting especially at times when there are oestrus females around and sharing meat with these females. It should be added, though, that this ‘meat-for-sex’ hypothesis has come under debate. Other researchers have reported no effect of a female's reproductive state on the frequency of hunting or food-sharing (Mitani & Watts 2001; Gilby 2006; Gilby et al. 2006). Rather, it has been suggested that the primary function of meat-sharing is to foster reciprocal relationships among males. Thus, one population of wild chimpanzees showed a strong association between meat-sharing and agonistic support, thus suggesting that meat plays a ‘political’ role (Mitani & Watts 2001).
Throughout the literature one finds similar suggestions of chimpanzees currying favours with others for strategic reasons in situations which may involve planning. Some of these reports are anecdotal, such as a male at a zoo who secured hard to obtain food and shared it generously with potential supporters at around the time that he began to challenge the established alpha male (de Waal 1982) or the wild male chimpanzee who retained alpha status for an extraordinarily long time while selectively rewarding allies through a ‘bribery’ system (Nishida et al. 1992). There is at least one systematic study confirming these reports for a large zoo colony. Chimpanzees selectively groomed supporters the day before they needed their help in an agonistic confrontation that they themselves initiated. The investigators suggest that chimpanzees groom others in anticipation of future recruitment of their assistance (Koyama et al. 2006). If so, reciprocity in chimpanzees may involve more than learning the benefits of exchange, but include planning for these benefits and undertaking actions to secure them. Other studies have confirmed future planning in other contexts in a variety of ape species, for example, in collecting and storing tools or weapons that were needed many hours later (Mulcahy & Call 2006; Osvath 2009).
But even if primates learn the benefits of exchange after considerable time intervals, we should keep in mind that spontaneous prosocial tendencies are a pre-condition for such learning (§3). Reciprocity is never purely a product of learning, but rather of a prosocial tendency fortified by learning. In addition, learned reciprocity is not the only kind in existence. The majority of exchanges may not depend on cognitively monitored contingencies, but rather grow out of long-term social bonds. If members of a species preferentially direct favours to their closest associates, the distribution of favours will automatically be reciprocal owing to the symmetrical nature of association (i.e. if individual A associates with B, B also associates with A). Such symmetry-based reciprocity obviates the need for scorekeeping, hence should be the default assumption whenever animals show reciprocity in long-term relations—such as between ‘mates’, ‘friends’ or ‘buddies’—whether it is among vampire bats (Wilkinson 1984) or primates (e.g. Barrett et al. 1999; Gomes & Boesch 2009). Matrix correlations between favours given and received across all dyads in a population can be fully explained by this cognitively less demanding mechanism (de Waal & Luttrell 1988). Affiliative ties act as an overarching emotional and neurohormonal mechanism (such as oxytocin; see Soares et al. 2010) to produce mutual benefits, as also suggested for humans (Brown & Brown 2006).
3. Unselfish cooperation and the altruistic impulse
(a). Observational data
Qualitative descriptions of spontaneous assistance among primates are abundant, ranging from bringing a mouthful of water to an incapacitated individual to slowing down travel for injured companions (Boesch 1992; de Waal, 1996, 1997a). Similar descriptions exist for both elephants (e.g. Hamilton-Douglas et al. 2006; Bates et al. 2008) and cetaceans (e.g. Caldwell & Caldwell 1966; Connor & Norris 1982). The help provided can be quite costly. For example, when a female chimpanzee reacts to the screams of her closest associate by defending her against an aggressive male, she takes enormous risks on behalf of the other. Alliances are among the best documented forms of cooperation in primatology, involving many studies and thousands of observations (de Waal 1982, 1992).
Another well-known form of assistance is food-sharing. Outside the mother–offspring relation or immediate kin-group, sharing is rare in the primate order (Feistner & McGrew 1989), yet common in callitrichid monkeys, capuchin monkeys and chimpanzees. The two main hypotheses to explain this kind of food-sharing are (i) the sharing-under-pressure hypothesis and (ii) the reciprocity hypothesis. According to the sharing-under-pressure hypothesis, individuals share in order to be left alone by potentially aggressive beggars (Blurton-Jones 1987; Stevens & Stephens 2002; Gilby 2006). This hypothesis is contradicted, however, by the fact that the most generously sharing individuals are often fully dominant (de Waal 1989; Nishida et al. 1992), aggression is more often shown by food possessors than non-possessors (figure 2; de Waal 1989), food transfers occur even if negative behaviour is prevented by physical separation (Nissen & Crawford 1932; de Waal 1997a) and many primates—including wild chimpanzees (Wrangham 1977)—vocally announce the presence of sharable food, thus attracting beggars. In fact, chimpanzee begging behaviour is rarely of a threatening nature as it derives from infant and juvenile expressions of need aimed at the mother (e.g. pouting, whimpering and holding out a hand; van Lawick-Goodall 1968). None of the above observations fits the sharing-under-pressure hypothesis.
Figure 2.
Interactions over sharable food are generally tolerant and peaceful, such as here in a cluster of chimpanzees at the Yerkes Field Station. The female in the top-right corner is the possessor of branches with leaves. The female in the lower left corner is tentatively reaching out for the first time. Whether or not she will be able to feed will depend on the possessor's reaction. Photograph by Frans de Waal.
The reciprocity hypothesis, on the other hand, predicts that food is part of a service economy, hence exchanged for other favours. It has indeed been shown that adult chimpanzees are more likely to share with individuals who have groomed them earlier in the day. In other words, if A groomed B in the morning, B was more likely than usual to share food with A in the afternoon. Rather than representing generalized reciprocity (i.e. increased altruism to any partner upon receipt of a favour, cf. Rutte & Taborsky 2007, for rats), food-for-grooming exchanges among chimpanzees have been shown to be partner-specific (de Waal 1997b). Of all examples of reciprocal altruism in non-human animals, these exchanges come closest to fulfilling the requirements of calculated reciprocity, i.e. exchange with the same partner after a significant time delay reflecting memory of previous events and a psychological mechanism described, which Trivers (1971) described as ‘gratitude’ (Bonnie & de Waal 2004).
The extent to which non-human primates engage in reciprocity is not well recognized in the human literature, however, which often attributes non-human primate altruism and cooperation to kin selection, thus calling human cooperation with non-relatives a ‘huge anomaly’ in the animal kingdom (Fehr & Fischbacher 2003; Gintis et al. 2003; Boyd 2006; see Melis & Semmann 2010, for further discussion of this topic). Even though there is ample evidence that this claim does not hold for captive chimpanzees (de Waal 1982, 1992, 1997b; Koyama et al. 2006), it has only recently been effectively countered for wild chimpanzees. DNA data from the field demonstrates that most of the cooperative relationships among male chimpanzees are of a reciprocal nature and concern individuals without family ties (Mitani 2006; Langergraber et al. 2007). Bonobos may show the same pattern, since females maintain a close cooperative network that allows them to collectively dominate the males (Furuichi 1997; de Waal 1997c) despite the fact that females are also the migratory sex, hence largely unrelated within each community (Kano 1992). It seems, then, that both of our closest relatives are marked by frequent cooperation among non-relatives.
A final common form of spontaneous assistance is so-called consolation, defined as friendly, reassuring contact directed by an uninvolved bystander at the loser of a previous aggressive incident (figure 3). For example, a third party goes over to the loser and puts an arm around his or her shoulders or provides calming grooming. de Waal & van Roosmalen (1979) based their conclusions on hundreds of post-conflict observations, and a replication by de Waal & Aureli (1996) included an even larger sample in which they sought to test two simple predictions. If third-party contacts indeed serve to alleviate the distress of conflict participants, these contacts should be directed more at recipients of aggression than at aggressors, and more at recipients of intense than mild aggression. Comparing third-party contact rates with baseline levels, the authors found support for both predictions.
Figure 3.
Consolation behaviour is common in humans and apes, but largely absent in monkeys. A juvenile chimpanzee puts an arm around a screaming adult male, who has been defeated in a fight. Photograph by Frans de Waal.
Whether consolation produces any direct benefits for the actor remains unclear. In one study, this behaviour was disproportionately directed at conflict participants likely to aggress the actor, hence may have served to forestall aggression (Koski & Sterck 2009). Yet, given the extreme rarity of redirected aggression in chimpanzees (i.e. <0.5% of agonistic incidents) and that other studies have found consolation to be predominantly provided by friends and relatives, the chief function of this behaviour is probably reassurance of distressed parties (Fraser et al. 2008; Romero & de Waal in press). In support of this hypothesis, Fraser et al. (2008) found that consolation reduced stress in the victims of aggression.
(b). Experimental approaches
The above observational studies show how common helping is, especially among chimpanzees. This behaviour may be partly based on learned contingencies between help given and received (§2), yet since these contingencies are highly probabilistic and occur over intervals lasting days, weeks or longer, it is hard to see how they might explain high-risk helping, such as when Washoe, the world's first language-trained chimpanzee, heard another female scream and hit the water. Fouts & Mills (1997, p. 180) describe how Washoe raced across two electric wires, which normally contained the apes, to reach the victim and waded into the slippery mud to reach the wildly thrashing female and grab one of her flailing arms to pull her to safety. Washoe barely knew this female, having met her only a few hours before.
Even if contingent reciprocity were to play a role, it is good to realize that it is impossible to learn behavioural contingencies without spontaneously engaging in the behaviour in the first place. We must therefore assume an impulse that propels individuals to defend, share with or rescue others. In the case of Washoe, this impulse needed to be strong enough to overcome her species' hydrophobia (chimpanzees cannot swim). Empathy has the potential to provide such an impulse as it produces a stake in the recipient's well-being through shared representations. In the words of Hoffman (1981, p. 133), empathy has the unique property of ‘transforming another person's misfortune into one's own feeling of distress’. Inasmuch as both humans and other animals are most empathic towards past cooperators and socially close individuals, empathy biases altruistic behaviour precisely as predicted by theories of kin selection and reciprocal altruism (Preston & de Waal 2002; de Waal 2008).
For both practical and ethical reasons, however, there is a scarcity of experiments on emotionally charged situations that could trigger costly altruism. This is not only true for animal altruism, but equally so for human altruism. Instead, experiments concern low-cost altruism, sometimes called ‘other-regarding preferences’. A typical paradigm is to offer one member of a pair the option to either secure food for itself by manipulating part A of an apparatus or secure food for both itself and the other by manipulating part B of the same apparatus. In the first such experiment, Colman et al. (1969) found 1 of 4 tested macaques to be consistently other-regarding. When replications failed to find the same tendency in chimpanzees, however, this led to the suggestion that other-regarding preferences may be uniquely human (Silk et al. 2005; Jensen et al. 2006). It is impossible to prove the null hypothesis, however, and recent studies with different methodologies have yielded results more in line with expectations based on naturalistic primate behaviour.
In one study, investigators tried to rule out reciprocity by having apes interact with humans they barely knew, and on whom they did not depend for food or other favours (Warneken et al. 2007). The investigators also ruled out the role of immediate return benefits by manipulating the availability of rewards. In this experiment, chimpanzees spontaneously assisted persons regardless of whether or not this yielded rewards and were also willing to open a door for conspecifics so that these could reach a room with food. One would think that rewards for the actor, even if not strictly necessary, at least stimulated helping actions, but in fact rewards proved irrelevant. The decision to help did not seem based on a cost/benefit calculation, therefore, consistent with predictions from empathy-induced altruism.
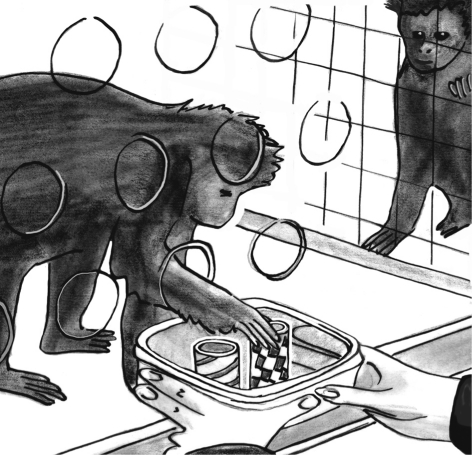
Spontaneous helping has also been experimentally demonstrated in both capuchin monkeys (de Waal et al. 2008; Lakshminarayanan & Santos 2008) and marmosets (Burkart et al. 2007; although not in closely related cotton-top tamarins, Cronin et al. 2009; see also Jaeggi et al. 2010). In our study, two capuchin monkeys were placed side by side separated by mesh. One of them needed to barter with us with small plastic tokens, which we would first give to a monkey, after which we would hold out an open hand to let them return the token for a tidbit (figure 4). The critical test came when we offered a choice between two differently coloured tokens with different meaning: one token was ‘selfish’, the other ‘prosocial’. If the bartering monkey picked the selfish token, it received a small piece of apple for returning it, but its partner remained unrewarded. The prosocial token, on the other hand, rewarded both monkeys with apple at the same time. Since the monkey who did the bartering was rewarded either way, the only difference was in what the partner received.
Figure 4.
One capuchin monkey reaches through an armhole to choose between differently marked pieces of pipe while her partner looks on. The pipe pieces can be exchanged for food. One token feeds both monkeys; the other feeds only the chooser. Capuchins typically prefer the ‘prosocial’ token (de Waal et al. 2008). Drawing from a video still by Frans de Waal.
Monkeys preferentially bartered with the prosocial token. This preference could not be explained by fear of future punishment because dominant partners proved to be more prosocial than subordinate ones. Familiarity biased the choices in the predicted direction: the stronger the social tie between two monkeys, as measured by how much time they associated in the group, the more they favoured the prosocial token. Moreover, choices were reflected in accompanying behaviour, with greater orientation towards the partner during prosocial choices (de Waal et al. 2008).
In short, there is mounting evidence from both naturalistic observations and experiments that primates care about each other's welfare and follow altruistic impulses in some contexts, probably based on empathy, which in both humans and other animals increases with familiarity. The empathy mechanism automatically produces a stake in the other's welfare, i.e. the behaviour comes with an intrinsic reward, known in the human literature as the ‘warm-glow’ effect. Actions that improve another's condition come with pleasant feelings (Andreoni 1989), so that humans report feeling good when they do good and show activation of reward-related brain areas (Harbaugh et al. 2007). It will be important to determine if the same self-reward system extends to other primates.
4. Constraints on cooperation and altruism
(a). Inequity aversion and tolerance
How skewed can a pay-off distribution get before it begins to undermine cooperation? Fehr & Schmidt (1999) have argued that the well-known human aversion to disadvantageous inequity relates to the need to maintain cooperation. Similarly, cooperative animals may be guided by a set of expectations about pay-off distribution. Thus, de Waal (1996, p. 95) proposed a sense of social regularity, defined as ‘a set of expectations about the way in which oneself (or others) should be treated and how resources should be divided’. Note that the expectations are not specified: they are species-typical. Some primates are so hierarchical that subordinate individuals cannot expect anything, whereas in other species dominant individuals are prepared to share and, correspondingly, the species has evolved a repertoire of begging signals to extract food from them. These animals negotiate their share and may protest if it is too small.
In one experiment, capuchin monkeys were paired to perform a simple task 25 times in a row, alternating between both of them. Food rewards varied from low value (a cucumber piece) to high value (a grape). In equity tests, both the subject and its partner did the same work for the same low-value food. In inequity tests, the subject received low-value rewards, whereas its partner received high-value rewards for the same effort. It turned out that the capuchins were far less willing to complete the task or accept the reward if their partner received a better deal. Subjects receiving the low-value reward in inequity tests showed both passive negative reactions (i.e. refusal to perform or refusing the reward) and active negative reactions (i.e. throwing out the token or the reward; Brosnan & de Waal 2003).
It could be argued that the mere presence of high-value food is what triggers these reactions (e.g. a contrast effect; Roma et al. 2006; Silberberg et al. 2009). In other words, subjects are holding out for something better. The first argument against this alternative is that if food is merely made available, without any task, there is no sign of inequity aversion even in the same monkeys as those of the original study (e.g. Dubreuil et al. 2006; Dindo & de Waal, 2007; Fontenot et al. 2007). The second counter-argument is that showing grapes before every equity trial, in which both monkeys receive cucumber, has no effect: the monkeys do not work any less for cucumber after having seen grapes. The grapes need to serve as rewards for the partner to affect a monkey working for cucumber, which implies that the social aspect of the task plays a critical role (van Wolkenten et al. 2007). Other task-oriented studies have found signs of inequity aversion in chimpanzees (Brosnan et al. 2005, 2010a), capuchin monkeys (Fletcher 2008; Takimoto et al. 2010) and domestic dogs (Range et al. 2008), whereas one study yielded mixed results, with an apparent inequity response in bonobos but not in other apes (Bräuer et al. 2009). A study on cotton-top tamarins, finally, found behavioural changes over time that might reflect inequity aversion (Neiworth et al. 2009).
Given the above, it is not surprising that unequal outcomes reduce cooperative tendencies. For example, when capuchin monkeys pull cooperatively to obtain unequally distributed food, the most successful pairs are those that alternate positions so that both parties share in the best rewards. In contrast, pairs tend to fail if one individual tries to monopolize the best food (Brosnan et al. 2006). Similarly, when inequity was introduced in the aforementioned prosocial versus selfish choice paradigm with capuchin monkeys, their prosociality disappeared. In other words, when prosocial choices produced better food for the partner than the chooser herself, prosocial tendencies fell to chance levels (de Waal et al. 2008). However, in a similar study also with capuchin monkeys, unequal rewards led to higher prosocial behaviour (although this could have resulted from the training procedures, rather than an understanding of the task; Lakshminarayanan & Santos 2008).
Outcome calculations are very much part of the decision to cooperate: tolerance promotes cooperation and competition undermines it. A real-life example is group hunting (i.e. several individuals cooperate, but only one of them obtains the prize), which is common in both wild chimpanzees and capuchin monkeys (Boesch 1994; Perry & Rose 1994). Since group hunting is sustainable only if the prey is shared at the end, Rose (1997) has proposed a convergent evolution of food-sharing in these two distant primates. The way tolerance affects cooperation has been tested in the laboratory by comparing the effect of clumped versus dispersed food rewards. The more competitive the dominant party in a pair of cooperating capuchin monkeys, the less cooperation will take place when food is monopolizable, whereas cooperation is unaffected under the dispersed condition (de Waal & Davis 2003). When chimpanzees and bonobos face a similar task, both species cooperate equally for a dispersed food source, but with a clumped source the bonobos are more successful because of their more effective conflict resolution techniques (de Waal 1987) resulting in increased tolerance around a clumped reward (Hare et al. 2007). Another illustration of the same principle is that both capuchins and chimpanzees cooperate most readily with partners with whom they are socially close, hence enjoy the greatest food tolerance (figure 5; de Waal & Davis 2003; Melis et al. 2006b).
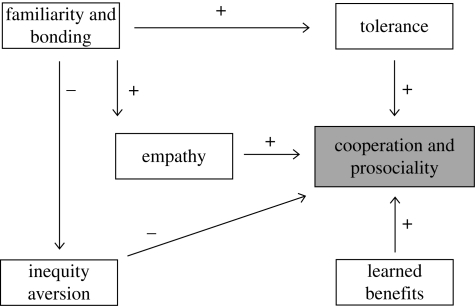
Figure 5.
Cooperative and prosocial behaviour is enhanced by familiarity and bonding between parties both via the empathy mechanism, thought to regulate the altruistic impulse and by increased social tolerance, which ensures rewards for the subordinate party. Familiarity and bonding also reduce sensitivity to inequity, while sensitivity undermines cooperative and prosocial behaviour if certain individuals gain conspicuously more than others. Finally, whenever cooperation produces knowable return benefits for the actor, there is the potential of learned reciprocity in which individuals cooperate in order to secure future return favours.
(b). Free loaders
Individuals who give less than they receive need to be discouraged if cooperation is to survive (Trivers 1971). Active punishment may be rare in non-human primates, yet in the food-for-grooming study of de Waal (1997b), food possessors showed increased aggressive resistance to approaching beggars who had failed to groom them. They were more than three times as likely to threaten such beggars than those with whom they had previously groomed. This is not punishment per se, but an aggressive reaction to those who try to get without giving, which psychologically speaking may not be far removed.
Chimpanzees also reciprocate in the negative sense: retribution is the flip side of reciprocity. Data on several thousand aggressive interventions show a healthy correlation between how often individual A intervenes against B and how often B intervenes against A. As a result, every choice has multiple consequences, both positive and negative. The supported party in a conflict may repay the favour, whereas the slighted party may try to get even in what has been called a revenge system (de Waal & Luttrell 1988; see also Jensen 2010).
By far the most common tool to enforce cooperation, however, is partner choice. Unsatisfactory relationships can be abandoned and replaced by ones with greater benefits. With each individual shopping for the best partners and selling its own services, the framework becomes one of supply and demand, as formalized in Noë & Hammerstein's (1994) Biological market theory. This theory applies whenever trading partners can choose with whom to deal. Market mechanisms are an effective way of sidelining profiteers.
It takes much energy, though, to keep a watchful eye on cheaters and the flow of favours. This is why both humans and other animals rely on simpler forms of reciprocity much of the time. Instead of keeping track of each and every instant of positive or negative behaviour, events get pooled into the larger framework of a social bond with mutually positive attitudes, resulting in symmetry-based reciprocity (§2). When it comes to distant relations, on the other hand, we tend to keep mental records and react more strongly to inequities. In humans, the reciprocity dynamics of close relationships are radically different from those between distant partners (Clark & Grote 2003). The better two individuals know each other, the longer the time frame over which they seem to evaluate their relationships so that momentary imbalances matter less. This may also apply to chimpanzees, in which familiarity appears to reduce sensitivity to inequity (Brosnan et al. 2005) and in which one-on-one exchanges of favours are less pronounced the closer the social relationship between both parties (de Waal 1997b).
5. Conclusion
Prosocial behaviour can be categorized based on whether or not the actor knows or can potentially learn about its long-term consequences. There is considerable support from both field observations and laboratory experiments that non-human primates exhibit prosocial behaviour both when they know its benefits, such as when they help each other reach a common goal, and when there is little chance for them to have this knowledge. In the latter case, they must be motivated by an altruistic impulse perhaps similar to the main mechanism thought to underlie human altruism: empathy with the distress, pain or need of another. While considerable attention has been given to the ultimate explanations for altruistic behaviour, this paper reviewed proximate mechanisms and concluded that since ultimate reasons rarely figure at the proximate level, primate altruism must occur in many cases without any selfish motivations. On the other hand, inequity aversion, intolerance and negative reactions to cheating constitute constraints on prosocial behaviour. Together, this array of mechanisms provides an understanding of the proximate decision-making regarding prosocial behaviour.
Acknowledgements
We thank the editors of this volume as well as two anonymous reviewers for constructive comments on this review. Writing was supported by Emory's College of Arts and Sciences, the Living Links Center, as well as the Base Grant by the National Institutes of Health to the Yerkes National Primate Research Center (YNPRC) (RR-00165). The YNPRC is fully accredited by the American Association for Accreditation for Laboratory Animal Care.
Footnotes
One contribution of 14 to a Theme Issue ‘Cooperation and deception: from evolution to mechanisms’.
References
- Andreoni J.1989Giving with impure altruism: applications to charity and ricardian equivalence. J. Pol. Econ. 97, 1447–1458 (doi:10.1086/261662) [Google Scholar]
- Barraza J., Zak P. J.2009Empathy towards strangers triggers oxytocin release and subsequent generosity. Ann. NY Acad. Sci. 1167, 182–189 (doi:10.1111/j.1749-6632.2009.04504.x) [DOI] [PubMed] [Google Scholar]
- Barrett L., Henzi S. P., Weingrill T., Hill R. A.1999Market forces predict grooming reciprocity in female baboons. Proc. R. Soc. Lond. B 266, 665–670 (doi:10.1098/rspb.1999.0687) [Google Scholar]
- Bates L. A., Lee P. C., Njiraini N., Poole J. H., Sayialel K., Sayialel S., Moss C. J., Byrne R. W.2008Do elephants show empathy? J. Conscious. Stud. 15, 204–225 [Google Scholar]
- Batson C. D.1991The altruism question: toward a social-psychological answer. Hillsdale, NJ: Erlbaum [Google Scholar]
- Blurton-Jones N. G.1987Tolerated theft, suggestions about the ecology and evolution of sharing, hoarding and scrounging. Soc. Sci. Inform. 26, 31–54 (doi:10.1177/053901887026001002) [Google Scholar]
- Boesch C.1992New elements of a theory of mind in wild chimpanzees. Behav. Brain Sci. 15, 149–150 [Google Scholar]
- Boesch C.1994Cooperative hunting in wild chimpanzees. Anim. Behav. 48, 653–667 (doi:10.1006/anbe.1994.1285) [Google Scholar]
- Bonnie K. E., de Waal F. B. M.2004Primate social reciprocity and the origin of gratitude. In The psychology of gratitude (eds Emmons R. A., McCullough M. E.), pp. 213–229 Oxford, UK: Oxford University Press [Google Scholar]
- Boyd R.2006The puzzle of human sociality. Science 314, 1555–1556 (doi:10.1126/science.1136841) [DOI] [PubMed] [Google Scholar]
- Bräuer J., Call J., Tomasello M.2009Are apes inequity averse? New data on the token-exchange paradigm. Am. J. Primatol. 71, 175–181 (doi:10.1002/ajp.20639) [DOI] [PubMed] [Google Scholar]
- Brosnan S. F., Beran M. J.2009Trading behavior between conspecifics in chimpanzees (Pan troglodytes). J. Comp. Psychol. 123, 181–194 (doi:10.1037/a0015092) [DOI] [PubMed] [Google Scholar]
- Brosnan S. F., de Waal F. B. M.2002A proximate perspective on reciprocal altruism. Hum. Nat. 13, 129–152 (doi:10.1007/s12110-002-1017-2) [DOI] [PubMed] [Google Scholar]
- Brosnan S. F., de Waal F. B. M.2003Monkeys reject unequal pay. Nature 425, 297–299 (doi:10.1038/nature01963) [DOI] [PubMed] [Google Scholar]
- Brosnan S. F., Schiff H., de Waal F. B. M.2005Tolerance for inequity increases with social closeness in chimpanzees. Proc. R. Soc. B 272, 253–258 (doi:10.1002/ajp.20261) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosnan S. F., Freeman C., de Waal F. B. M.2006Partner's behavior, not reward distribution, determines success in an unequal cooperative task in capuchin monkeys. Am. J. Primatol. 68, 713–724 (doi:10.1002/ajp.20261) [DOI] [PubMed] [Google Scholar]
- Brosnan S. F., Silk J. B., Heinrich J., Mareno M. C., Lambeth S. P., Schapiro S. J.2009Chimpanzees (Pan troglodytes) do not develop contingent reciprocity in an experimental task. Anim. Cogn. 12, 587–597 (doi:10.1007/s10071-009-218-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosnan S. F., Talbot C., Ahlgren M., Lambeth S. P., Schapiro S. J.2010aMechanisms underlying the response to inequity in chimpanzees, Pan troglodytes. Anim. Behav. 79, 1229–1237 (doi:10.1016/j.anbehav.2010.02.019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosnan S. F., Salwiczek L., Bshary R.2010bThe interplay of cognition and cooperation. Phil. Trans. R. Soc. B 365, 2699–2710 (doi:10.1098/rstb.2010.0154) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S. L., Brown R. M.2006Selective investment theory: recasting the functional significance of close relationships. Psychol. Inq. 17, 1–29 (doi:10.1207/s15327965pli1701_01) [Google Scholar]
- Burkart J. M., Fehr E., Efferson C., van Schaik C. P.2007Other-regarding preferences in a non-human primate: common marmosets provision food altruistically. Proc. Natl Acad. Sci. USA 104, 19 762–19 766 (doi:10.1073/pnas.0710310104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell M. C., Caldwell D. K.1966Epimeletic (care-giving) behavior in Cetacea. In Whales, dolphins, and porpoises (ed. Norris K. S.), pp. 755–789 Berkeley, CA: University California Press [Google Scholar]
- Chalmeau R., Visalberghi E., Gallo A.1997Capuchin monkeys, Cebus apella, fail to understand a cooperative task. Anim. Behav. 54, 1215–1225 (doi:10.1006/anbe.1997.0517) [DOI] [PubMed] [Google Scholar]
- Chen Q., Panksepp J. B., Lahvis G. P.2009Empathy is moderated by genetic background in mice. PLoS ONE 4, e4387 (doi:10.1371/journal.pone.0004387) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark M. S., Grote N. K.2003Close relationships. In Handbook of psychology: personality and social psychology (eds Millon T., Lerner M. J.), pp. 447–461 New York, NY: John Wiley [Google Scholar]
- Clements K. C., Stephens D. W.1995Testing models of non-kin cooperation: mutualism and the Prisoner's Dilemma. Anim. Behav. 50, 527–535 (doi:10.1006/anbe.1995.0267) [Google Scholar]
- Colman A. D., Liebold K. E., Boren J. J.1969A method for studying altruism in monkeys. Psychol. Rec. 19, 401–405 [Google Scholar]
- Connor R. C., Norris K. S.1982Are dolphins reciprocal altruists? Am. Nat. 119, 358–372 (doi:10.1086/283915) [Google Scholar]
- Crawford M.1937The cooperative solving of problems by young chimpanzees. Comp. Psychol. Monogr. 14, 1–88 [Google Scholar]
- Cronin K. A., Kurian A. V., Snowdon C. T.2005Cooperative problem solving in a cooperatively breeding primate (Saguinus oedipus). Anim. Behav. 69, 133–142 (doi:10.1016/j.anbehav.2004.02.024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronin K. A., Schroeder K. K. E., Rothwell E. S., Silk J. B., Snowdon C. T.2009Cooperatively breeding cotton-top tamarins (Saguinus oedipus) do not donate rewards to their long-term mates. J. Comp. Psychol. 123, 231–241 (doi:10.1037/a0015094) [DOI] [PubMed] [Google Scholar]
- Dawkins R.1976The selfish gene. Oxford, UK: Oxford University Press [Google Scholar]
- Decety J., Jackson P. L.2006A social-neuroscience perspective on empathy. Curr. Dir. Psychol. Sci. 15, 54–58 (doi:10.1111/j.0963-7214.2006.00406.x) [Google Scholar]
- de Waal F. B. M.1982Chimpanzee politics: power and sex among apes. Baltimore, MD: John Hopkins University Press; (reprinted 2007). [Google Scholar]
- de Waal F. B. M.1987Tension regulation and nonreproductive functions of sex in captive bonobos (Pan paniscus). Nat. Geogr. Res. 3, 318–335 [Google Scholar]
- de Waal F. B. M.1989Food sharing and reciprocal obligations among chimpanzees. J. Hum. Evol. 18, 433–459 (doi:10.1016/0047-2484(89)90074-2) [Google Scholar]
- de Waal F. B. M.1992Coalitions as part of reciprocal relations in the Arnhem chimpanzee colony. In Coalitions and alliances in humans and other animals (eds Harcourt A., de Waal F. B. M.), pp. 233–257 Oxford, UK: Oxford University Press [Google Scholar]
- de Waal F. B. M.1996Good natured: the origins of right and wrong in humans and other animals. Cambridge, MA: Harvard University Press [Google Scholar]
- de Waal F. B. M.1997aFood-transfers through mesh in brown capuchins. J. Comp. Psychol. 111, 370–378 (doi:10.1037/0735-7036.111.4.370) [DOI] [PubMed] [Google Scholar]
- de Waal F. B. M.1997bThe chimpanzee's service economy: food for grooming. Evol. Hum. Behav. 18, 375–386 (doi:10.1016/S1090-5138(97)00085-8) [Google Scholar]
- de Waal F. B. M.1997cBonobo: the forgotten ape. Berkeley, CA: University of California Press [Google Scholar]
- de Waal F. B. M.2000Attitudinal reciprocity in food sharing among brown capuchin monkeys. Anim. Behav. 60, 253–261 (doi:10.1006/anbe.2000.1471) [DOI] [PubMed] [Google Scholar]
- de Waal F. B. M.2008Putting the altruism back into altruism: the evolution of empathy. Ann. Rev. Psychol. 59, 279–300 (doi:10.1146/annurev.psych.59.103006.093625) [DOI] [PubMed] [Google Scholar]
- de Waal F. B. M., Aureli F.1996Consolation, reconciliation, and a possible cognitive difference between macaque and chimpanzee. In Reaching into thought: the minds of the great apes (eds Russon A. E., Bard K. A., Parker S. T.), pp. 80–110 Cambridge, UK: Cambridge University Press [Google Scholar]
- de Waal F. B. M., Berger M. L.2000Payment for labour in monkeys. Nature 404, 563 (doi:10.1038/35007138) [DOI] [PubMed] [Google Scholar]
- de Waal F. B. M., Brosnan S. F.2006Simple and complex reciprocity in primates. In Cooperation in primates and humans: mechanisms and evolution (eds Kappeler P. M., van Schaik C. P.), pp. 85–105 Berlin, Germany: Springer [Google Scholar]
- de Waal F. B. M., Davis J. M.2003Capuchin cognitive ecology: cooperation based on projected returns. Neuropsychologia 41, 221–228 (doi:10.1016/S0028-3932(02)00152-5) [DOI] [PubMed] [Google Scholar]
- de Waal F. B. M., Luttrell L. M.1988Mechanisms of social reciprocity in three primate species: symmetrical relationship characteristics or cognition? Ethol. Sociobiol. 9, 101–118 (doi:10.1016/0162-3095(88)90016-7) [Google Scholar]
- de Waal F. B. M., van Roosmalen A.1979Reconciliation and consolation among chimpanzees. Behav. Ecol. Sociobiol. 5, 55–66 (doi:10.1007/BF00302695) [Google Scholar]
- de Waal F. B. M., Leimgruber K., Greenberg A. R.2008Giving is self-rewarding for monkeys. Proc. Natl Acad. Sci. USA 105, 13 685–13 689 (doi:10.1073/pnas.0807060105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dindo M., de Waal F. B. M.2007Partner effects on food consumption in brown capuchin monkeys. Am. J. Primatol. 69, 1–6 (doi:10.1002/ajp.20362) [DOI] [PubMed] [Google Scholar]
- Drea C. M., Carter A. N.2009Cooperative problem solving in a social carnivore. Anim. Behav. 78, 967–977 (doi:10.1016/j.anbehav.2009.06.030) [Google Scholar]
- Dubreuil D., Gentile M. S., Visalberghi E.2006Are capuchin monkeys (Cebus apella) inequity averse? Proc. R. Soc. B 273, 1223–1228 (doi:10.1098/rspb.2005.3433) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufour V., Pelé M., Neumann M., Thierry B., Call J.2009Calculated reciprocity after all: computation behind token transfers in orangutans. Biol. Lett. 5, 172–175 (doi:10.1098/rsbl.2008.0644) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugatkin L. A.1997Cooperation among animals. Oxford, UK: Oxford University Press [Google Scholar]
- Fehr E., Fischbacher U.2003The nature of human altruism. Nature 425, 785–791 (doi:10.1098/rspb.2005.3433) [DOI] [PubMed] [Google Scholar]
- Fehr E., Schmidt K. M.1999A theory of fairness, competition, and cooperation. Q. J. Econ. 114, 817–868 (doi:10.1162/003355399556151) [Google Scholar]
- Feistner A. T. C., McGrew W. C.1989Food-sharing in primates: a critical review. In Perspectives in primate biology, vol. 3 (eds Seth P. K., Seth S.), pp. 21–36 New Delhi, India: Today & Tomorrow's [Google Scholar]
- Fletcher G. E.2008Attending to the outcome of others: disadvantageous inequity aversion in male capuchin monkeys. Am. J. Primatol. 70, 901–905 (doi:10.1002/ajp.20576) [DOI] [PubMed] [Google Scholar]
- Fontenot M. B., Watson S. L., Roberts K. A., Miller R. W.2007Effects of food preferences on token exchange and behavioural responses to inequality in tufted capuchin monkeys, Cebus apella. Anim. Behav. 74, 487–496 (doi:10.1016/j.anbehav.2007.01.015) [Google Scholar]
- Fouts R., Mills T.1997Next of kin. New York, NY: Morrow [Google Scholar]
- Fraser O., Stahl D., Aureli A.2008Stress reduction through consolation in chimpanzees. Proc. Natl Acad. Sci. USA 105, 8557–8562 (doi:10.1073/pnas.0804141105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuichi T.1997Agonistic interactions and matrifocal dominance rank of wild bonobos at Wamba. Int. J. Primatol. 18, 855–875 (doi:10.1023/A:1026327627943) [Google Scholar]
- Gilby I. C.2006Meat sharing among the Gombe chimpanzees: harassment and reciprocal exchange. Anim. Behav. 71, 953–963 (doi:10.1016/j.anbehav.2005.09.009) [Google Scholar]
- Gilby I. C., Eberly L. E., Pintea L., Pusey A. E.2006Ecological and social influences on the hunting behaviour of wild chimpanzees, Pan troglodytes schweinfurthii. Anim. Behav. 72, 169–180 (doi:10.1016/j.anbehav.2006.01.013) [Google Scholar]
- Gintis H., Bowles S., Boyd R., Fehr E.2003Explaining altruistic behavior in humans. Evol. Hum. Behav. 24, 153–172 (doi:10.1016/S1090-5138(02)00157-5) [Google Scholar]
- Gomes C. M., Boesch C.2009Wild chimpanzees exchange meat for sex on a long-term basis. PLoS ONE 4, e5116 (doi:10.1371/journal.pone.0005116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenier F., Lüthi E.2010Mouse brains wired for empathy? Nat. Neurosci. 13, 406–408 (doi:10.1038/nn0410-406) [DOI] [PubMed] [Google Scholar]
- Hamilton-Douglas I., Bhalla S., Wittemyer G., Vollrath F.2006Behavioural reactions of elephants towards a dying and deceased matriarch. Appl. Anim. Behav. Sci. 100, 87–102 (doi:10.1016/j.applanim.2006.04.014) [Google Scholar]
- Hammerstein P.2003Why is reciprocity so rare in social animals? A protestant appeal. In Genetic and cultural evolution of cooperation (ed. Hammerstein P.), pp. 83–93 Cambridge, MA: MIT Press [Google Scholar]
- Harbaugh W. T., Mayr U., Burghart D. R.2007Neural responses to taxation and voluntary giving reveal motives for charitable donations. Science 326, 1622–1625 (doi:10.1126/science.1140738) [DOI] [PubMed] [Google Scholar]
- Hare B., Melis A. P., Hastings S., Woods V., Wrangham R.2007Tolerance allows bonobos to outperform chimpanzees on a cooperative task. Curr. Biol. 17, 1–5 (doi:10.1016/j.cub.2007.02.040) [DOI] [PubMed] [Google Scholar]
- Hattori Y., Kuroshima H., Fujita K.2005Cooperative problem solving by tufted capuchin monkeys (Cebus apella): spontaneous division of labor, communication, and reciprocal altruism. J. Comp. Psych. 119, 335–342 (doi:10.1037/0735-7036.119.3.335) [DOI] [PubMed] [Google Scholar]
- Hockings K. J., Humle T., Anderson J. R., Biro D., Sousa C., Ohashi G., Matsuzawa T.2007Chimpanzees share forbidden fruit. PLoS ONE 2, e886 (doi:10.1371/journal.pone.0000886) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman M. L.1981Is altruism part of human nature? J. Pers. Soc. Psychol. 40, 121–137 (doi:10.1037/0022-3514.40.1.121) [Google Scholar]
- Jaeggi A. V., Burkart J. M., Van Schaik C. P.2010On the psychology of cooperation in humans and other primates: combining the natural history and experimental evidence of prosociality. Phil. Trans. R. Soc. B 365, 2723–2735 (doi:10.1098/rstb.2010.0118) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen K.2010Punishment and spite, the dark side of cooperation. Phil. Trans. R. Soc. B 365, 2635–2650 (doi:10.1098/rstb.2010.0146) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen K., Hare B., Call J., Tomasello M.2006What's in it for me? Self-regard precludes altruism and spite in chimpanzees. Proc. R. Soc. B 273, 1013–1021 (doi:10.1098/rspb.2005.3417) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan J.2000Human morality is distinctive. J. Conscious. Stud. 7, 46–48 [Google Scholar]
- Kano T.1992The last ape: pygmy chimpanzee behavior and ecology. Stanford, CA: Stanford University Press [Google Scholar]
- Koski S., Sterck E. H. M.2009Post-conflict third-party affiliation in chimpanzees: what's in it for the third party? Am. J. Primatol. 71, 409–418 (doi:10.1002/ajp.20668) [DOI] [PubMed] [Google Scholar]
- Koyama N. F., Caws C., Aureli F.2006Interchange of grooming and agonistic support in chimpanzees. Int. J. Primatol. 27, 1293–1309 (doi:10.1007/s10764-006-9074-8) [Google Scholar]
- Lakshminarayanan V. R., Santos L. R.2008Capuchin monkeys are sensitive to others' welfare. Curr. Biol. 18, 999–1000 (doi:10.1016/j.cub.2008.08.057) [DOI] [PubMed] [Google Scholar]
- Langergraber K. E., Mitani J. C., Vigilant L.2007The limited impact of kinship on cooperation in wild chimpanzees. Proc. Natl Acad. Sci. USA 104, 7786–7790 (doi:10.1073/pnas.0611449104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langford D. J., Crager S. E., Shehzad Z., Smith S. B., Sotocinal S. G., Levenstadt J. S., Chanda M. L., Levitin D. J., Mogil J. S.2006Social modulation of pain as evidence for empathy in mice. Science 312, 1967–1970 (doi:10.1126/science.1128322) [DOI] [PubMed] [Google Scholar]
- Melis A. P., Semmann D.2010How is human cooperation different? Phil. Trans. R. Soc. B 365, 2663–2674 (doi:10.1098/rstb.2010.0157) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis A. P., Hare B., Tomasello M.2006aChimpanzees recruit the best collaborators. Science 311, 1297–1300 (doi:10.1126/science.1123007) [DOI] [PubMed] [Google Scholar]
- Melis A. P., Hare B., Tomasello M.2006bEngineering cooperation in chimpanzees: tolerance constraints on cooperation. Anim. Behav. 72, 275–286 (doi:10.1016/j.anbehav.2005.09.018) [Google Scholar]
- Melis A. P., Hare B., Tomasello M.2008Do chimpanzees reciprocate received favours? Anim. Behav. 76, 951–962 (doi:10.1016/j.anbehav.2008.05.014) [Google Scholar]
- Mendres K. A., de Waal F. B. M.2000Capuchins do cooperate: the advantage of an intuitive task. Anim. Behav. 60, 523–529 (doi:10.1006/anbe.2000.1512) [DOI] [PubMed] [Google Scholar]
- Mitani J. C.2006Reciprocal exchange in chimpanzees and other primates. In Cooperation in primates: mechanisms and evolution (eds Kappeler P., van Schaik C.), pp. 101–113 Heidelberg, Germany: Springer-Verlag [Google Scholar]
- Mitani J. C., Watts D. P.2001Why do chimpanzees hunt and share meat? Anim. Behav. 61, 915–924 (doi:10.1006/anbe.2000.1681) [Google Scholar]
- Mulcahy N. J., Call J.2006Apes save tools for future use. Science 312, 1038–1040 (doi:10.1126/science.1125456) [DOI] [PubMed] [Google Scholar]
- Neiworth J. J., Johnson E. T., Whillock K., Greenberg J., Brown V.2009Is a sense of inequity an ancestral primate trait? Testing social inequity in cotton top tamarins (Saguinus oedipus). J. Comp. Psychol. 123, 10–17 (doi:10.1037/a0012662) [DOI] [PubMed] [Google Scholar]
- Nishida T., Hasegawa T., Hayaki H., Takahata Y., Uehara S.1992Meat-sharing as a coalition strategy by an alpha male chimpanzee? In Topics in primatology: vol. 1, human origins (eds Nishida T., McGrew W. C., Marler P., Pickford M., de Waal F. B. M.), pp. 159–174 Tokyo: University of Tokyo Press [Google Scholar]
- Nissen H., Crawford M.1932A preliminary study of food-sharing behaviour in young chimpanzees. J. Comp. Psychol. 22, 383–419 (doi:10.1037/h0062234) [Google Scholar]
- Noë R.2006Cooperation experiments: coordination through communication versus acting apart together. Anim. Behav. 71, 1–18 (doi:10.1016/j.anbehav.2005.03.037) [Google Scholar]
- Noë R., Hammerstein P.1994Biological markets: supply and demand determine the effect of partner choice in cooperation, mutualism, and mating. Behav. Ecol. Sociobiol. 35, 1–11 (doi:10.1007/BF00167053) [Google Scholar]
- Osvath M.2009Spontaneous planning for future stone throwing by a male chimpanzee. Curr. Biol. 19, R190–R191 (doi:10.1016/j.cub.2009.01.010) [DOI] [PubMed] [Google Scholar]
- Panksepp J.1996The psychobiology of prosocial behaviors: separation distress, play, and altruism. In Altruism and aggression: biological and social origins (eds Zahn-Waxler C., Cummings E. M., Lannotti R.), pp. 19–57 Cambridge, UK: Cambridge University Press [Google Scholar]
- Pelé M., Dufour V., Thierry B., Call J.2009Token transfers among great apes (Gorilla gorilla, Pongo pygmaeus, Pan paniscus, Pan troglodytes): species differences, gestural requests, and reciprocal exchange. J. Comp. Psychol. 123, 375–384 (doi:10.1037/a0017253) [DOI] [PubMed] [Google Scholar]
- Perry S., Rose L.1994Begging and transfer of coati meat by white-faced capuchin monkeys, Cebus capucinus. Primates 35, 409–415 (doi:10.1007/BF02381950) [Google Scholar]
- Preston S. D., de Waal F. B. M.2002Empathy: its ultimate and proximate bases. Behav. Brain Sci. 25, 1–72 (doi:10.1017/S0140525X02000018) [DOI] [PubMed] [Google Scholar]
- Range F., Horn L., Viranyi Z., Huber L.2008The absence of reward induces inequity aversion in dogs. Proc. Natl Acad. Sci. USA 106, 340–345 (doi:10.1073/pnas.0810957105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzolatti G., Fadiga L., Gallesa V., Fogassi L.1996Premotor cortex and the recognition of motor actions. Cogn. Brain Res. 3, 131–141 (doi:10.1016/0926-6410(95)00038-0) [DOI] [PubMed] [Google Scholar]
- Roberts G.1997Testing mutualism: a commentary on Clements & Stephens. Anim. Behav. 53, 1361–1362 (doi:10.1006/anbe.1996.0378) [DOI] [PubMed] [Google Scholar]
- Roma P. G., Silberberg A., Ruggiero A. M., Suomi S. J.2006Capuchin monkeys, inequity aversion, and the frustration effect. J. Comp. Psychol. 120, 67–73 (doi:10.1037/0735-7036.120.1.67) [DOI] [PubMed] [Google Scholar]
- Romero T., de Waal F. B. M.In press Chimpanzee (Pan troglodytes) consolation behavior: third party identity as a window on possible function. J. Comp. Psychol. [DOI] [PubMed] [Google Scholar]
- Rose L.1997Vertebrate predation and food-sharing in Cebus and Pan. Int. J. Primatol. 18, 727–765 (doi:10.1023/A:1026343812980) [Google Scholar]
- Rutte C., Taborsky M.2007Generalized reciprocity in rats. PLoS Biol. 5, e196 (doi:10.1371/journal.pbio.0050196) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberberg A., Crescimbene L., Addessi E., Anderson J. R., Visalberghi E.2009Does inequity aversion depend on a frustration effect? A test with capuchin monkeys (Cebus apella). Anim. Cogn. 12, 505–509 (doi:10.1007/s10071-009-0211-6) [DOI] [PubMed] [Google Scholar]
- Silk J. B., Brosnan S. F., Vonk J., Henrich J., Povinelli D., Lambeth S., Richardson A., Mascaro J., Shapiro S.2005Chimpanzees are indifferent to the welfare of unrelated group members. Nature 437, 1357–1359 (doi:10.1038/nature04243) [DOI] [PubMed] [Google Scholar]
- Soares M. C., Bshary R., Fusani L., Goymann W., Hau M., Hirschenhauser K., Oliveira R. F.2010Hormonal mechanisms of cooperative behaviour. Phil. Trans. R. Soc. B 365, 2737–2750 (doi:10.1098/rstb.2010.0151) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford C. B.1996The hunting ecology of wild chimpanzees: implications for the evolutionary ecology of Pliocene hominids. Am. Anthropol. 98, 96–113 [Google Scholar]
- Stanford C. B., Wallis J., Mpongo E., Goodall J.1994Hunting decisions in wild chimpanzees. Behaviour 131, 1–18 (doi:10.1163/156853994X00181) [Google Scholar]
- Stevens J. R., Hauser M. D.2004Why be nice? Psychological constraints on the evolution of cooperation. Trends Cogn. Sci. 8, 60–65 (doi:10.1016/j.tics.2003.12.003) [DOI] [PubMed] [Google Scholar]
- Stevens J. R., Stephens D. W.2002Food sharing: a model of manipulation by harassment. Behav. Ecol. 13, 393–400 (doi:10.1093/beheco/13.3.393) [Google Scholar]
- Takimoto A., Kuroshima H., Fujita K.2010Capuchin monkeys (Cebus apella) are sensitive to others' reward: an experimental analysis of food-choice for conspecifics. Anim. Cogn. 13, 249–261 (doi:10.1007/s10071-009-0262-8) [DOI] [PubMed] [Google Scholar]
- Trivers R. L.1971The evolution of reciprocal altruism. Q. Rev. Biol. 46, 35–57 (doi:10.1086/406755) [Google Scholar]
- van Lawick-Goodall J.1968The behaviour of free-living chimpanzees in the Gombe Stream Reserve. Anim. Behav. Monogr. 1, 161–311 [Google Scholar]
- van Wolkenten M., Brosnan S. F., de Waal F. B. M.2007Inequity responses of monkeys modified by effort. Proc. Natl Acad. Sci. USA 104, 18 854–18 859 (doi:10.1073/pnas.0707182104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visalberghi E., Quarantotti B. P., Tranchida F.2000Solving a cooperation task without taking into account the partner's behavior: the case of capuchin monkeys (Cebus apella). J. Comp. Psychol. 114, 297–301 (doi:10.1037/0735-7036.114.3.297) [DOI] [PubMed] [Google Scholar]
- Warneken F., Hare B., Melis A. P., Hanus D., Tomasello M.2007Spontaneous altruism by chimpanzees and young children. PLoS Biol. 5, e184 (doi:10.1371/journal.pbio.0050184) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson G. S.1984Reciprocal food sharing in the vampire bat. Nature 308, 181–184 (doi:10.1038/308181a0) [Google Scholar]
- Wrangham R. W.1977Feeding behavior of chimpanzees in Gombe National Park, Tanzania. In Primate ecology (ed. Clutton-Brock T. H.), pp. 503–538 London, UK: Academic Press [Google Scholar]
- Yamamoto S., Tanaka M.2009Do chimpanzees (Pan troglodytes) spontaneously take turns in a reciprocal cooperation task? J. Comp. Psychol. 123, 242–249 (doi:10.1037/a0015838) [DOI] [PubMed] [Google Scholar]
- Zak P. J., Stanton A. A., Ahmadi S.2007Oxytocin increases generosity in humans. PLoS ONE 2, e1128 (doi:10.1371/journal.pone.00001128) [DOI] [PMC free article] [PubMed] [Google Scholar]