Abstract
The response of bystanders to information available in their social environment can have a potent influence on the evolution of cooperation and signalling systems. In the presence of bystanders, individuals might be able to increase their payoff by exaggerating signals beyond their means (cheating) or investing to help others despite considerable costs. In doing so, animals can accrue immediate benefits by manipulating (or helping) individuals with whom they are currently interacting and delayed benefits by convincing bystanders that they are more fit or cooperative than perhaps is warranted. In this paper, I provide some illustrative examples of how bystanders could apply added positive selection pressure on both cooperative behaviour and dishonest signalling during courtship or conflict. I also discuss how the presence of bystanders might select for greater flexibility in behavioural strategies (e.g. conditional or condition dependence), which could maintain dishonesty at evolutionarily stable frequencies under some ecological conditions. By recognizing bystanders as a significant selection pressure, we might gain a more realistic approximation of what drives signalling and/or interaction dynamics in social animals.
Keywords: cooperation, cheating, dishonest signalling, aggression, communication network, social eavesdropping
1. Introduction
Why would a pair of pied flycatchers (Ficedula hypoleuca) opt to join forces with their neighbours to mob a predator (Krams et al. 2008)? Why would cleaner fish (Labroides dimidiatus) pass on their preferred food (fish mucus) to pick ectoparasites from clients (Bshary & Grutter 2006)? Why would hermit crabs (Pagurus bernhardus) signal aggression but fail to back it up with an attack when challenged (Laidre 2009)? Why would small male green tree frogs (Rana clamitans) alter the dominant frequency of their calls to sound like large territory holders (Bee et al. 2000)? Historically, these questions have been viewed in terms of the immediate payoffs received by the actor in the context of its current interaction. Doing so made it difficult to understand why animals would behave in an apparently altruistic manner towards non-kin (i.e. incurring an immediate cost to help others). Conversely, thinking about immediate payoffs made it rather easy to understand why animals might bluff aggressive signals—to gain an instant fitness benefit at the cost of one's opponent (Krebs & Dawkins 1984).
With regard to cooperation, the paradox of helping non-kin was partly resolved by recognizing that the immediate costs paid by an actor could be recouped if the recipient returned the favour at some later time (reciprocity; Trivers 1971). This, of course, requires that individuals interact repeatedly and that participants keep tabs on each other's prior strategies (e.g. cooperate, defect; Axelrod & Hamilton 1981). Although there is some evidence supporting reciprocity in social animals (e.g. Krams et al. 2008), there also is a renewed sense that alternative explanations for cooperation in non-kin should be explored both empirically and theoretically (e.g. Clutton-Brock 2009). With regard to dishonest signalling in mating or aggressive contests, it might seem paradoxical that effective communication systems persist through time (Johnstone 1998; table 1). If actors derive instant benefits from dishonest signalling and if recipients do best to disregard these signals, communication should ultimately break down. Nevertheless, honest signalling appears to be quite common (e.g. Bradbury & Vehrencamp 1998; Maynard Smith & Harper 2003; Searcy & Nowicki 2005; Laidre 2009). These honest signalling systems could represent a snapshot in evolutionary time where we are observing a phase of honesty amidst the constant flux between honest and dishonest strategies (Johnstone 1998). Alternatively, honesty could be maintained if signal production requires significant investment that low-quality individuals cannot afford (e.g. handicaps; Zahavi & Zahavi 1997). Signals of intent, which require lower production costs, might be more prone to dishonesty (Searcy & Nowicki 2005; Laidre 2009) but Maynard Smith & Harper (2003) highlight several ways in which honesty could be maintained for minimal-cost signals (e.g. common interest or repeated interaction between actor and recipient; punishment). There also are some signals that simply cannot be faked because they are inextricably linked to, for instance, parasite load, condition or body size (Maynard Smith & Harper 2003).
Table 1.
Definitions of key terms from the text.
| dishonest signalling: signalling in a way that is not reflective of actual quality or motivation; in terms of the immediate payoffs to actor and recipients (Brosnan & Bshary 2010) dishonest signalling mirrors cheating (actor +, recipient −) |
| bystander: individual within range to detect interactions or signalling interchanges that occur between others in its social environment |
| eavesdropper: bystander that extracts information from signalling interchanges; social eavesdroppers extract information about the relative quality of the signallers while interceptive eavesdroppers use signalling information to hone in on, and intercept, a receiver (e.g. mate, prey item; Peake 2005) |
| image scoring: mechanism that could promote cooperation through indirect reciprocity. Here, bystanders elevate the social reputation of individuals who help a needy recipient and decrease the social reputation of individuals who fail to donate help (Nowak & Sigmund 1998); the social standing of individuals in need of help is not considered |
| standing strategy: mechanism that could promote cooperation through indirect reciprocity and that outcompetes image scoring (Leimar & Hammerstein 2001). Here, bystanders decrease the social reputation only of individuals who fail to help a recipient in good social standing (e.g. a cooperator) |
At the core of current explanations for the evolution of apparently altruistic behaviours and for the dearth of dishonesty in signalling exchanges is the assumption that interactions between the actor and the recipient occur in a social vacuum (see concepts presented by Leimar & Hammerstein 2010). For instance, in a game of tit-for-tat, individuals monitor only their partner's prior move(s) when gauging whether to cooperate in the future. In an aggressive encounter, the decision to bluff depends only on an animal's own internal state and the identity of its opponent or the likelihood of opponent retaliation. This dyadic approach, however, is an unrealistic way to think about the dynamics of interactions among predominantly social animals, which likely occur in the context of a communication network (McGregor 2005; or in contexts with multiple individuals, see Connor 2010). There is a vast amount of information contained in pairwise interactions (e.g. predator inspection bouts) and signalling interchanges (e.g. conflict and courtship) and this information is by and large available to and used by bystanders. For example, Aquiloni & Gherardi (2010) demonstrated convincingly in crayfish (Procambarus clarkii) that females determine suitable mates by fusing information gathered from male–male aggressive interactions with individual recognition. Female crayfish bystanders were given visual and chemical access to contesting males and then were asked to choose between dominant and subordinate males that were either familiar (female witnessed the fight) or unfamiliar (males came from a separate fight witnessed by a different female). Females preferred dominant males only when they had access to information (visual/chemical) during the fight and encountered familiar males during the choice trials, indicating rather sophisticated means of social information processing and discrimination. The capacity of animals to exploit information available in their social environment cuts across invertebrate and vertebrate taxonomic groups (see supporting examples in the following sections). This strongly suggests that harvesting social information has deep evolutionary roots or perhaps reflects many episodes of convergence and that it does not require the complex neural machinery characteristic of higher vertebrate groups (Bshary et al. 2002).
The ways in which bystanders respond to information available in their social environment can have a potent influence on the evolution of cooperation (e.g. image scoring: Nowak & Sigmund 1998; standing strategy: Leimar & Hammerstein 2001; Roberts 2008) and aggressive behaviour (Johnstone 2001; Johnstone & Bshary 2004). Recognizing bystanders as a significant source of evolutionary pressure could bring us closer to a realistic approximation of what drives signalling and/or interaction dynamics in social animals. In this paper, I give a brief introduction to communication networks and a generalized conceptual model of the evolution of signalling within these networks. I then provide some illustrative examples of how bystanders could exert positive selection, above and beyond the immediate payoffs derived from a current interaction, on both cooperative behaviour and dishonest signalling. I end with a discussion of how the presence of bystanders might select for greater flexibility in behavioural strategies (e.g. condition dependence), which could maintain dishonest signalling at evolutionarily stable frequencies under some ecological conditions. Although this discussion will not be rooted mathematically, it extends from recent theories on the evolution of spite, deceptive communication and indirect reciprocity (e.g. Johnstone & Bshary 2004; Rowell et al. 2006; see Jensen 2010 for more on spite), and I hope that it will stimulate future theoretical treatment coupled with field and laboratory experimentation (Leimar & Hammerstein 2006).
2. Communication networks: general overview
McGregor (2005) proposed that social interactions occur within a communication network, where information emitted by a signaller is available to both the intended receiver and bystanders within the range to detect the signal. Bystanders that attend to, and use, information emitted by signallers are termed eavesdroppers. Interceptive eavesdroppers are bystanders that use signals as a means of, for instance, seizing females as they approach a calling male or estimating the spatial proximity of males to determine the likelihood of extrapair copulations (e.g. Tobias & Seddon 2002; Peake 2005; Crockford et al. 2007). Social eavesdroppers, on the other hand, are bystanders that extract information about the quality of the observed individuals using information contained within the signalling interchange (e.g. fighting ability, courtship vigour; Peake 2005; Bonnie & Earley 2007). Cues that provide additional information to the content of signalling interactions might also be available to bystanders as public information (e.g. individual identity or strategy played; Danchin et al. 2004; Valone 2007). Furthermore, bystanders can refine their social decisions by fusing personal information with that gained through eavesdropping (Leimar & Hammerstein 2001; Peake et al. 2002; Paz-y-Miño et al. 2004; Mery et al. 2009). In the context of cooperation and mutualism, bystanders can gauge an individual's reputation (e.g. cooperator, defector) by being attentive to the outcome of an observed interaction (e.g. mutually cooperative, exploitative or mutually defective; Bshary & Bergmüller 2008). Using image scores or standing strategies, bystanders can then discern future courses of action (e.g. cooperate or defect) based on the information gained (Milinski et al. 2001; Bshary & Grutter 2006; Melis & Semmann 2010).
Given their ability to extract information from the social environment, it stands to reason that bystanders constitute a significant selection pressure in the evolution of interaction dynamics (e.g. cooperation) and signalling interchanges (e.g. aggression and courtship). This is a reasonable proposal only if signallers also stay tuned to their social environment. Compelling evidence exists for the so-called audience effects (Matos & Schlupp 2005), where animals modulate their behaviour or signalling performance depending on the presence and, sometimes, the identity of bystanders. Chimpanzees who are being victimized in an aggressive dispute will emit exaggerated screams only when bystanders are present who outrank the assailant (Slocombe & Zuberbühler 2007). Ravens (Corvus corax) and eastern grey squirrels (Sciurus carolinensis) will adjust their caching (i.e. food storage) strategies in the presence of conspecifics that might pilfer the resource (Bugnyar & Kotrschal 2002; Steele et al. 2008). The presence and identity of a bystander also measurably impacts the vigor of agonistic and courtship displays in fishes and birds (Matos & Schlupp 2005; see §6). These examples reveal that individuals are intimately aware of their social surroundings and that bystanders can trigger immediate changes in the behaviour of those being watched (or heard). From an evolutionary perspective, then, it seems plausible that bystanders exert significant selection pressure on individual behaviour and the dynamics of cooperation, courtship and conflict interactions. Indeed, the influence of bystanders on the evolution of cooperation has attracted a good deal of theory (image scoring: Nowak & Sigmund 1998; standing strategies: Leimar & Hammerstein 2001; Roberts 2008), but their influence on the evolution of courtship and conflict signalling systems has received relatively little attention (Johnstone 2001; Johnstone & Bshary 2004).
3. Signalling in communication networks
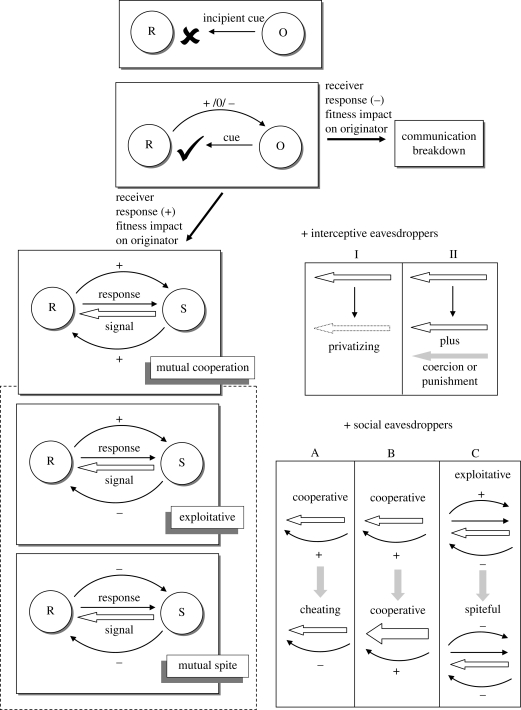
Wisenden & Stacey (2005) used an example of chemical communication to explore evolutionary transitional states between originators that release cues to signallers that emit signals; their basic framework can be applied to all signal modalities (figure 1). In the ancestral state, the population consists of originators and receivers that lack mechanisms to detect or respond to cues. This state then transitions into a situation where receivers evolve mechanisms to detect cues (e.g. olfactory systems become sensitive to chemicals) and can respond to these cues in ways that might benefit the originator. The system becomes communication when receiver detection and responses exert positive selection on cue specialization (e.g. for the purpose of conveying information to intended receivers), resulting in a switch from originator to signaller and cue to signal. This dyad-based system, in which the payoffs to signaller and receiver are based solely on their interaction partner, may not reflect the diversity of outcomes that could arise in a communication network. If bystanders intercept signals and respond in ways that negatively impact the fitness of the signaller, one might expect selection to favour the evolution of mechanisms to communicate along increasingly private channels (e.g. through changes in signal design or usage; Dabelsteen 2005; figure 1). For instance, subordinate male baboons (Papio hamadryas ursinus) will attend to temporal and spatial properties of female copulation calls and male grunts to gauge opportunities for extrapair mating (Crockford et al. 2007). Selection might thus favour male baboons that employ less conspicuous grunts that do not reveal his position relative to the female or, if it pays the female to publicize her location, perhaps selection would favour male coercion or punishment to prevent females from advertising (Clutton-Brock 2009). The pressure that bystanders exert upon signaller–receiver dynamics does not necessitate the evolution of a pure ‘private’ or ‘coercive’ strategy but perhaps flexibility in signal or strategy usage depending on social circumstance (e.g. probability of bystander interception).
Figure 1.
A general diagrammatic model for the evolution of signalling in a communication network (see Wisenden & Stacey 2005). The evolutionary trajectory begins with an originator (O) releasing an incipient cue that receivers (R) are insensitive to (×). If the receiver evolves a mechanism for signal detection and processing (✓), this could have a variety of fitness consequences for the originator. If receiver detection negatively impacts originator fitness, the cue will fall out of favour evolutionarily, leading to the breakdown of an incipient signalling system. If receiver detection positively impacts originator fitness, a signal that benefits receiver fitness may evolve; this signal is emitted by what is now a signaller (S). In this background, positive net payoffs foster the origins of a signalling system (Bradbury & Vehrencamp 1998) but this mutually cooperative system can morph into an exploitative or spiteful dynamic in certain cases (surrounded in a dashed box to indicate that this would not be the original face of the signalling system). The positive (+), negative (−) and neutral (0) symbols associated with each arrow indicate the impact of either the signal on receiver fitness (S → R) or the impact of receiver responses on signaller/originator fitness (R → S/O). The right portion of the figure shows how signalling dynamics might change in the presence of eavesdroppers; right-pointing arrow denotes R → S; left-pointing arrow denotes S → R and (+) or (−) symbols denote payoffs. In the presence of interceptive eavesdroppers, signal design might become less conspicuous (I; transition from solid arrow to dotted arrow) or alternative strategies to avoid interception might evolve (II; e.g. coercion; punishment; Clutton-Brock 2009). Social eavesdroppers might exert positive selection pressure on cheating (A), frequency of cooperative behaviour (indicated by a thicker arrow in B) or spiteful interactions (C). For a finer-scale analysis of the transition from cue to signal and alternatives to signal evolution trajectories, see Bradbury & Vehrencamp (1998, pp. 497–535). This diagrammatic model admittedly neglects the contribution of receiver biases (e.g. Garcia & Ramirez 2005).
Social eavesdroppers do not intercept receivers but rather extract and subsequently use information about the quality of both signaller and receiver. In the next sections, I build on a core idea of bystander–signaller–receiver dynamics to illustrate how social eavesdropping can exert a profound impact on the evolution of cooperation and perhaps serve as a social mechanism that promotes the coexistence of honest and dishonest strategies in courtship and conflict signalling (figures 1 and 2). I begin by assuming that signalling interchanges during conflict and courtship are mutually beneficial (figures 1 and 2) and that individuals who would receive a net negative payoff by signalling honestly (e.g. low quality) will simply opt not to interact. If cheating or deception (e.g. signalling dishonestly, defecting) infiltrated the system, the immediate payoff for the actor will increase and the immediate payoff for the recipient will decrease (Bshary & Bergmüller 2008).
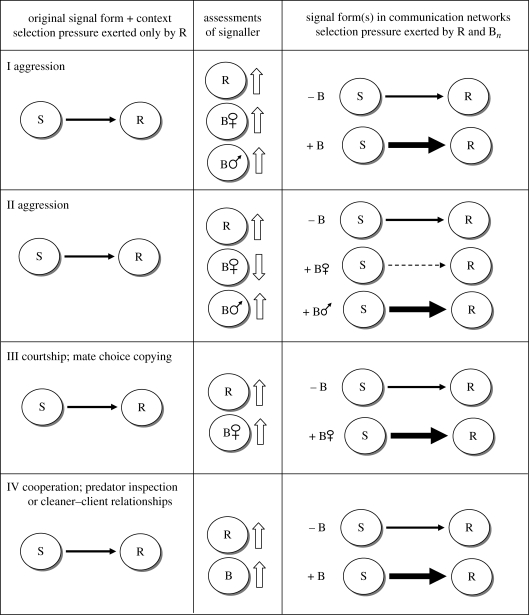
Figure 2.
The predicted evolutionary trajectories for signals in a variety of contexts (I–IV) in the presence (+) or absence (−) of bystanders (B; potential social eavesdroppers). The first column represents an abstraction of ‘original signal form’ that might have emerged if payoffs were dependent solely on signaller (S)–receiver (R) dynamics. Both receivers and bystanders gain information from a signaller and adjust their perception of the signaller accordingly (up or down arrows in the second column). In cases where bystanders are not present, signals should remain at the status quo (original signal form) because the only selection pressure driving signal form is that which is exerted by receiver responses. However, in the presence of bystanders, additional selection pressures emerge, which may drive the evolution of conditional strategies wherein signallers alter their behaviour depending on the constitution of their social environment. In context II, female/male bystanders (designated by B plus the male and female symbols) downgrade/upgrade their perception of an aggressive signaller. Thus, individuals might be selected to exhibit plasticity in aggressive signalling depending on which type of bystander is present; in the presence of females, they become less aggressive (dashed arrow) while in the presence of males, they become more aggressive (bold arrow). In contexts III and IV, bold arrows indicate that signallers are exhibiting more vigorous courtship displays or higher frequencies of cooperation, respectively.
4. The core concept: signallers can double their benefits
The dyadic paradigm assumes that a signaller's payoff is linked only to an intended receiver's response. In communication networks, however, a signaller could receive an immediate (or future) payoff from its intended receiver and an added, perhaps delayed, payoff from attentive bystanders. Given these added benefits, a signaller might invest more heavily in its signals and be willing to incur greater costs in its interaction with the receiver. This should be especially true when signal enhancement has the same impact on both the receiver's and the bystander's assessment of the signaller (figure 2). For instance, paying the cost to help a partner could increase the signaller's image score and yield future benefits in the form of direct reciprocity (receiver helps in return) and indirect reciprocity (more likely to receive help from a bystander). In the context of conflict and courtship, signallers might display increased motivation to fight, persist longer in a contest or perform more costly courtship displays. In these cases, the signaller might convince both the receiver and any bystanders that it is a force to be reckoned with or that it is a superior mate. Thus, the signaller might reap benefits in the form of securing a current mate or deterring a current opponent as well as future access to mates, future contest avoidance or greater sums of resource (e.g. if bystanders avoid a signaller observed to be aggressive).
Social eavesdropping thus will exert added positive selection pressure on signallers to invest more heavily in costly undertakings. If the extra payoff exceeds the investment (and any associated costs), it could drive individuals to cooperate with greater frequency than they would in a traditional pairwise interaction. In similar ways, a greater net payoff might also favour dishonest signalling during courtship and conflict. However, it is unlikely that social eavesdropping will drive pure cooperators or pure cheaters to fixation. The payoff for exhibiting cooperative behaviour or for signalling beyond one's means will be realized only if bystanders are present in sufficient numbers to ensure that added benefits are available to balance the extra investment (e.g. Nowak & Sigmund 1998). Individuals that cooperate or signal dishonestly all the time will suffer a reduction in lifetime fitness benefits relative to individuals who employ a conditional strategy (e.g. cooperate or signal dishonestly when bystanders are present or when the probability is high, otherwise cheat or signal honestly, respectively). To employ a conditional strategy, however, requires that signallers pay close attention to their social surroundings. Given that the social environment is inherently dynamic, with a constant flux of individuals with varying identities who are within the range to observe an interaction (or detect a signal), signallers also must be able to momentarily estimate the expected payoff structure and maintain flexibility in their strategy usage contingent upon these payoffs. Such social complexity could set in motion dramatic changes in neural architecture and cognitive abilities (Shumway 2008) required to process and respond appropriately to bystanders and an associated, ever-changing payoff structure (see Brosnan et al. 2010 for a discussion of the interplay between cognition and cooperation).
5. Tit-for-tat and image scoring in a network
The literature is rich with investigations of cooperative behaviour in animals, much of this stemming from early work by Trivers (1971) and Axelrod & Hamilton (1981) that forwarded reciprocity (and tit-for-tat) as solutions to the Prisoner's Dilemma (defectors receive the highest individual payoff but mutual cooperation trumps mutual defection). Although research has advanced in a prolific and insightful way, and although the literature is now brewing with alternatives to direct reciprocity (Clutton-Brock 2009), I use a classic example and a more recent body of work to illustrate the potential evolutionary impact of social eavesdroppers on cooperative behaviour (figure 2, IV).
Predator inspection, where two or more animals will break off from a social group to gain information about a lurking threat, is arguably one of the best non-primate examples of a situation in which tit-for-tat-like strategies could operate (Dugatkin 2008). Both sticklebacks (Gasterosteus aculeatus) and guppies (Poecilia reticulata) adopt this behaviour and in doing so assume significant costs in the form of increased predation risk (Dugatkin 1992). If the pair cooperates by swimming in lockstep towards the predator, the partners share the costs; if one partner defects by lagging behind, the leading animal assumes the brunt of the cost. For this reason, it makes sense for individuals engaged in predator inspection to pay attention to a partner's last move (cooperate versus defect) and, on that basis, modify their behaviour during future interactions with that individual (Milinski 1987; Milinski et al. 1990; Dugatkin 1991; Croft et al. 2006; but see Thomas et al. 2008). By observing predator inspection bouts, social eavesdroppers also could gain relevant information about individual tendencies towards cooperation and defection while avoiding the costs of predator approach (Brosnan et al. 2003). If social eavesdroppers use this information during future interactions with the observed inspectors in a tit-for-tat-like manner (which has not been demonstrated), then there should be added incentive for inspectors to cooperate. Add to this the possibility that females might pay attention to predator inspection bouts to gauge male attractiveness (bold males are preferred; Godin & Dugatkin 1996), and it becomes clear that the payoff for cooperating extends well beyond the inspection dyad. In this case, direct reciprocity, indirect reciprocity and ‘social prestige’ (Zahavi 2003) can all exert, perhaps synergistically, positive selection pressure on an individual's investment in the cooperative enterprise (figure 2, IV).
An equally intriguing system is the cleaner–client mutualism (Bshary & D'Souza 2005). Both predatory and non-predatory clients will visit cleaner wrasses (L. dimidiatus) to have ectoparasites and dead or infected tissue removed. Bshary (2001) described the ‘jolting’ behaviour of clients in response to cheating cleaners (i.e. those that bite instead of clean); non-predatory clients jolt significantly more often than predatory clients. From the cleaners' perspective, it makes sense to cheat strategically given that non-predatory clients have no means of retaliation whereas predatory clients could respond to a bite by eating the cleaner. Interestingly, in the Red Sea, cleaners often pass on their preferred meal (fish mucus and scales) and scour visiting non-predatory client fish for parasites. Why? It turns out that bystanding clients (social eavesdroppers) keep tabs on the cooperative behaviour of cleaner wrasses, perhaps by tallying jolts or remaining attentive to cleaners who are chased by resident fish retaliating a bite (Bshary & D'Souza 2005). Clients consequently invite the services of cooperative cleaners most often, cleaners with no record less often, and cheaters least often (Bshary 2002; Bshary & D'Souza 2005). Thus, the presence of image scoring clients and their punishment of cheaters drive positive selection on cleaners that cooperate indiscriminately when bystanders are present (figure 2, IV; Bshary & D'Souza 2005) because doing so would ensure the maintenance of a positive image score and an abundance of feeding opportunity. There is a twist to this story, however. Cleaners will cooperate with small, non-predatory clients (as above) and exploit the image scoring system to lure to the area larger, non-predatory clients whose mucus is more easily obtained (Bshary 2002). It is possible that these apparently deceptive cheaters bite only out of necessity; for instance, perhaps cleaners exploit image scoring only when starved or in poor condition (Bshary & D'Souza 2005).
6. Conflict and courtship in a network
Animal conflict remains an area of research where there is considerable interest in understanding whether, for what reasons and under which circumstances animals convey accurate information about their quality or motivation or, alternatively, become embroiled in a strategic game of manipulation and mind reading (e.g. social chess; Adams & Mesterton-Gibbons 1995; Johnstone 1998; Andrews 2001; Szalai & Számadó 2009). Most aggressive encounters move through a series of increasingly escalated phases that appear to provide progressively more accurate information about the fighting ability of a signaller to the receiver (Enquist & Leimar 1983). Although mutual opponent assessment during contests is hotly debated (Arnott & Elwood 2009), providing honest information about fighting ability to an opponent could reduce contest costs (Hurd 1997). In situations where signal exchange is mutually beneficial, aggressive contests qualify as cooperation. Theory predicts that cheaters should readily invade and perhaps dismantle cooperative signalling during contests (Bradbury & Vehrencamp 1998). Nevertheless, there is mounting evidence suggesting that cheaters, whose signals are discordant with their fighting ability or motivation, can exist stably at low frequencies (Rowell et al. 2006; Laidre 2009; see Számadó 2000 for high, stable cheater frequencies).
As an alternative to the hypothesis that these low levels of cheating simply reflect ‘the rise of the cheater’ in an evolutionary arms race between honesty and dishonesty (Krebs & Dawkins 1984), I propose that social eavesdropping can under certain circumstances select for stable, low frequency cheating (figure 2). In addition, I propose that social eavesdropping will select for individuals who invest in cheating; that is, instead of simply bluffing a signal of intent (a low-cost behaviour), cheaters might be expected to escalate beyond their means. This hypothesis relies on several conditions: (i) the signal itself can be graded (e.g. time spent displaying) or discrete (e.g. fins erect or flush with body) but all individuals in a population must be capable of performing the signal in question; (ii) there must be costs to signalling dishonestly; costs can take the form of receiver retaliation (punishment) or energy expenditure past some threshold; (iii) the benefit of deterring one's opponent (e.g. winning the resource at hand) is not sufficient to counter these costs; (iv) receiver and bystander assessment of the dishonest signal is concordant (i.e. both appraise the signaller as being better than she/he is); (v) the combined benefit of deterring both one's opponent and at least one bystander outweighs the cheating costs; and (vi) if bystanders are abundant, individuals that signal dishonestly may lose a current contest but still manage a net positive payoff.
The costs of escalated fighting are varied but significant, ranging from exhaustion and injury to fatality (Enquist & Leimar 1990; Briffa & Sneddon 2007). For purposes of illustrating conditions i and ii above, take the opercular threat display (i.e. gill flaring) that many fish, including Betta splendens, exhibit during aggressive interactions. All Bettas can perform this display, and they modulate display frequency and duration according to their physiological condition (e.g. hypoxia; Abrahams et al. 2005), suggesting that the signal is costly. Bettas do not, by default, display at their threshold physiological maximum (i.e. past which they would suffer serious fitness costs). Rather, the signal can be graded, with associated increases in cost, based on opponent characteristics and the presence/absence of an audience (Matos & Schlupp 2005). Because the dynamics of aggressive contests depend critically on both opponents, it is difficult to pinpoint the precise display intensity at which the signal would become dishonest. To avoid an extended discussion along these lines, it is reasonable to posit that the fish signals dishonestly when it displays to a cost threshold that exceeds what it would normally do against a given opponent type. The fish need not hit a physiological red zone, where displaying becomes perilous, for the signal to be dishonest; rather, the fish simply needs to bypass a threshold set by its own condition and by opponent characteristics. Individuals who signal dishonestly in contests will therefore incur considerable costs, probably higher net costs than honest signallers.
Given the diversity of resources over which individuals fight, it is difficult to estimate whether successfully deterring an opponent would outweigh the costs of dishonestly signalling. However, there is evidence that bystanders come to the same basic conclusion as receivers about a signaller's fighting ability. Individuals who signal aggressively and persistently during a contest deter both their opponent and any onlookers (Earley & Dugatkin 2002). Even eventual losers who escalated will discourage challenge from a bystander (Earley & Dugatkin 2002). Thus, investing in an inevitable loss by escalating could lead to future benefits in the form of dissuading confrontation and, as a consequence, securing higher social status or valuable resources (‘good loser hypothesis’; Peake & McGregor 2004). This example addresses an important caveat. Although punishment (when a bluff is called) is thought to stabilize honest signalling systems (Maynard Smith & Harper 2003), it may not be sufficient to do so in a social network teeming with attentive bystanders. If enough bystanders tune in to the contest in which the eventual loser fought hard, and if these bystanders elevate their perception of the loser's fighting ability, then cheating can pay fitness dividends in the form of cumulative deterrence of many bystanders.
In the presence of bystanders, selection should favour individuals that exaggerate aggressive signals (Johnstone 2001; Johnstone & Bshary 2004) perhaps to the point where they become dishonest (not conveying accurate information about quality), even in the face of potential retaliation and loss. This might explain why aggressive contests between males become markedly more intense in the presence of male audiences (Dzieweckzyski et al. 2005). If female bystanders prefer to mate with more aggressive or dominant males (Doutrelant & McGregor 2000; van Breukelen & Draud 2006), positive selection for dishonest aggressive signalling in the presence of audiences could be further intensified (figure 2, I aggression). However, if female bystanders' assessment of highly aggressive males conflicts with that of male bystanders (figure 2, II aggression), then selection should favour individuals that curtail escalated signals in the presence of females and exaggerate in the presence of males. This might be particularly relevant for species such as Japanese quail (Coturnix japonica), where females prefer to affiliate with contest losers to avoid possible damage inflicted by highly aggressive males during courtship/mating (Ophir & Galef 2003). Either of these situations—withholding information or elaborating signals beyond what one's quality substantiates—meet the requirements for dishonest signalling (Ducoing & Thierry 2003).
In the context of mate attractiveness, it is clear that animals cannot transform ornaments and armaments on a moment-to-moment basis to accommodate changes in the payoff structure of their social environment; even if it would benefit a male to suddenly become more colorful or more ornate, it simply cannot be done (but see Candolin 2000 for a rapid colour reduction in sticklebacks). However, behavioural displays such as the spectacular courtship rituals of male golden-collared manakins (Manacus vitellinus; Fusani et al. 2007) could be adapted quickly to social conditions and may even be more telling to a female (Shamble et al. 2009). Given the prevalence of non-independent mate choice, where males that have successfully mated have a greater probability of being selected by female observers (Westneat et al. 2000), it might pay males to increase courtship vigour in the presence of a female audience. The logic behind this argument is essentially the same as made for aggressive signalling. In situations where bystanders and receivers will both elevate their assessment of a courting male, and where the costs of increased investment in courtship can be balanced by the sum of current and future returns, social eavesdropping might exert positive selection on dishonest courtship signalling. Few studies have been conducted in this area, but there is some evidence that animals modulate their courtship intensity and/or mate preferences in the presence of an audience (Dzieweczynski et al. 2009). A fascinating example of deception in the context of mate choice copying comes from the Atlantic mollies (Poecilia mexicana; Plath et al. 2005). Atlantic mollies coexist with a sexual parasite, the gynogenetic Amazon molly (P. formosa), whose females use the sperm of Atlantic molly males to initiate embryogenesis. Males will copy the choice of other males who have successfully mated, and sperm competition reduces the probability that the ‘copied’ male's sperm will successfully fertilize the eggs of female conspecifics. In the absence of an audience, males show an overwhelming tendency to initiate sexual behaviour with large conspecific females rather than small conspecifics or heterospecifics. However, in the presence of a male audience, males initiate sexual behaviour with the less preferred females (small or heterospecific). Thus, it is possible in this system that males have evolved deceptive means of courtship signalling to avoid the fitness detriment of sperm competition.
7. Conditional and condition-dependent strategies
Examples in the previous sections illustrate that individuals are attentive to the presence of prospective eavesdroppers and that the behavioural strategies they employ are malleable in response to changes in their social environment (i.e. payoffs associated with interacting and/or signalling). These examples strongly suggest that eavesdroppers apply considerable evolutionary pressure to signalling dynamics and cooperative exchanges. At this point, there is plenty of theoretical evidence pointing to the possibility that eavesdroppers can drive extreme aggression (Johnstone 2001). But when animals show marked increases in aggression or courtship in response to bystander presence, does this necessarily mean they are being dishonest? I have purposefully maintained that eavesdroppers ‘could’ be responsible for wholesale changes in communication systems but I think it would be suspect to envision that social eavesdroppers will favour uniformly dishonest signalling. Regardless of whether cheats creep into a signalling system that is wholly dyadic or one that is rich with opportunities to eavesdrop, their success should be negatively frequency dependent (but see Számadó 2000).
Low frequencies of dishonesty could be maintained if cheating (e.g. elevating aggression or courtship beyond their means; exhibiting displays that are inconsistent with actual motivational state) occurs only when bystanders are present. In most social animals, however, eavesdroppers are likely ubiquitous so conditional cheating may render the strategy obsolete in a matter of generations. If cheating were both condition dependent (e.g. weak versus strong; Számadó 2000) and conditional on bystander presence, cheaters could be held at an evolutionarily stable frequency. Signalling is a game of diminishing returns: once an animal has reached a certain signal intensity or quality, there is little added benefit to elaborating further. Given that high-quality individuals are likely to have reached a payoff asymptote, cheating should make evolutionary sense only for the low-quality sector of the population. Several studies on dishonest aggressive signalling and cooperation support this prediction. Hungry female cleaner wrasses cheat their clients more often than males (Bshary & D'Souza 2005); vulnerable, newly molted stomatopods (Gonodactylus bredini) flaunt aggressive intent despite being unable to fight (Steger & Caldwell 1983); small hermit crabs and those facing well-endowed opponents dishonestly signal aggressive intent (cheliped presentation; Laidre 2009; Arnott & Elwood 2010) and small male green tree frogs will invest in emitting lower frequency calls in response to intrusions by large males (Bee et al. 2000). Communication networks may thus be one source that selects for inter-individual variation in levels of cooperation and deception, a topic that has received increasing attention (Bergmüller et al. 2010; McNamara & Leimar 2010).
If low quality is the factor that favours cheating, then it comes as no surprise that dishonest signalling during aggression and courtship is difficult to document empirically. However, the hypothesis that social eavesdropping and condition dependence interact to favour cheating gives rise to a number of testable predictions provided evolutionary pressures have already set the process in motion. In the laboratory, it should be relatively straightforward to manipulate both the social environment and the condition of the animal (e.g. starvation, stress) before conducting studies on courtship or aggression. If all else were equal (e.g. body size, opponent type), low-quality but not high-quality animals would signal beyond their means only in the presence of social eavesdroppers; ‘signalling beyond their means’ could be quantified using a residual technique similar to that of Arnott & Elwood (2010). Furthermore, ecologically relevant population-level studies could be conducted to test the hypothesis that the prevalence of cheating will be a function of the number of low-quality individuals occupying a particular area. After monitoring such things as habitat productivity and food availability, one could generate a distribution of individual qualities (e.g. body condition index) for each population. Performing a field experiment would be feasible with a tractable animal model in which fights could be staged on site, bystander presence and identity could be either documented or manipulated, and honesty objectively evaluated (see Laidre 2009). One would predict again that low-quality individuals would be more prone to dishonest signalling, particularly in the presence of bystanders. Furthermore, dishonest signalling should be more prevalent in populations derived from marginal habitats where a greater proportion of individuals fall on the low-quality end of the condition distribution.
8. Caveats and considerations
The previous discussion has assumed that, although bystanders actively gather and use information available in signalling exchanges, they take this information at face value. Male or female bystanders that attend to an aggressive contest therefore do not discriminate between individuals who won (or lost) the contest using honest versus dishonest signalling tactics. There is some weak support for this assumption. Bystanders respond quite predictably to individuals whose fights or courtship rituals they witness (McGregor 2005) and some, such as swordtail bystanders, even avoid eventual losers that escalated in the watched contest (Earley & Dugatkin 2002). Although these studies on social eavesdropping indicate that there is some truth to bystanders taking what they see at face value, none addressed signal honesty. Thus, it is tenuous at this point to claim, for instance, that all eventual losers who fought intensely were cheating and that bystanders were misled about their fighting ability. Searcy & Nowicki (2005) provide a contrasting view about how bystanders influence the evolution of communication systems. They propose that bystanders can evaluate signal reliability while watching signalling exchanges (‘third-party skepticism’). Bystanders would be expected to respond to dishonest signallers as they would to unfamiliar individuals, disregarding false information conveyed during the watched interaction. Only when the signaller is deemed honest would a bystander heed what was observed. Searcy & Nowicki (2005) thus hypothesize that eavesdropping will stabilize honest signalling systems, a significant departure from the hypotheses that I derived above.
Fortunately, these two alternative hypotheses are testable, both empirically and theoretically. One rather simple experimental approach in the context of aggression could involve manipulating animals such that their behaviour is patently discordant with their condition and/or ability. For instance, one could: (i) establish pairs of contestants that differ in size, weaponry, or some other index of fighting ability; (ii) in one treatment manipulate the weaker/smaller of the two (e.g. testosterone injections) to trigger aggression levels that are discordant with actual fighting ability; in a second treatment, inject with a control solution (e.g. saline); (iii) allow the animals to engage in the presence (or absence) of a bystander; (iv) once the contest has settled (perhaps in favour of the weaker), and after a short period of recovery, allow bystanders to engage with the weaker/smaller animals that were injected with testosterone (dishonest) or saline (honest). If bystanders take information at face value, they might avoid testosterone-treated, highly aggressive animals significantly more than saline-treated animals, and in situations when they observed versus did not observe fights involving the testosterone-treated individuals. If bystanders recognize discordance between aggression and fighting ability, they would respond the same to testsoterone-treated (seen and unseen) and saline-treated animals.
Searcy & Nowicki's (2005) third-party skepticism is one of many potential mechanisms that could favour honest communication systems, or at least retention of the evolutionarily stable status quo, over a system riddled with cheating. Cryptic eavesdropping, where bystanders might position themselves out of view of the signallers, could evolve as a strategy to mitigate cheating. Indeed, one might expect the fitness of bystanders, and the persistence of eavesdropping strategies, to hinge on signal reliability (Bonnie & Earley 2007), thereby promoting innovative ways to keep signallers in check. Similar to manipulator-mind reader games (Krebs & Dawkins 1984), this type of social dynamic could explode into an evolutionary arms race involving eavesdroppers and signallers. More subtly, cryptic eavesdropping certainly would alter a signaller's perception of bystander abundance. With fewer perceived bystanders in the vicinity, the payoff structure (see §6) would be altered dramatically in favour of the maintenance of honesty. That is, the net benefit of cheating would be perceived as low because the signaller would accrue costs during the signalling exchange and, owing to few bystanders, would not be able to recoup this cost. This scenario, and probably many others, emerges as a consequence of thinking about signalling interactions in the context of communication networks. We may find that social eavesdropping has negligible effects on the evolution of cooperation, courtship and conflict. However, at least for cooperation, a bourgeoning body of theory and empirical work strongly suggests otherwise. There is a growing need to bolster empirical and theoretical treatments that explore the influence of social networks on courtship and conflict signalling, and the goal of this review was to provide some ammunition for future research in this area.
9. Conclusion
The objective of this paper was, in part, to stimulate additional research in the area of social eavesdropping and communication networks. Social eavesdropping burst onto the scene in the early 2000s but interest has tailed off significantly since then. We know comparatively little about the impact of bystanders on courtship and aggression relative to signaller–receiver dynamics in a dyadic setting. There are still major empirical voids including how female bystanders might impact male courtship vigour, how bystander responses (and thus, payoffs to the signaller) change with its state (e.g. larger or smaller than the signaller; prior winner or loser) or sex, how social eavesdropping can be applied to other types of signalling interactions and whether what is known about communication networks in birds and fishes can be applied cross-taxonomically (McGregor 2005). I attempted to highlight the potentially potent evolutionary pressures that social eavesdroppers can apply to signalling dynamics. The impetus for doing so was to generate some experimental fodder for theoreticians and empiricists alike so that we might understand signalling in contexts that better approximate the social complexities encountered by animals on a moment-to-moment basis.
Acknowledgements
I wish to thank Sarah Brosnan and Redouan Bshary for organizing this issue and for their patience (particularly with me) as the issue developed. I am grateful to Shu-Ping Huang, Ximena Bernal, Boopathy Sivaraman, Amanda Hanninen and Mark Garcia for discussions. Mark Laidre provided exceptionally insightful feedback on earlier versions of the manuscript, and I would like to credit an anonymous reviewer for some fantastic ideas—for instance, cryptic eavesdropping—that added dimension to this manuscript.
Footnotes
One contribution of 14 to a Theme Issue ‘Cooperation and deception: from evolution to mechanisms’.
References
- Abrahams M. V., Robb T. L., Hare J. F.2005Effect of hypoxia on opercular displays: evidence for an honest signal? Anim. Behav. 70, 427–432 (doi:10.1016/j.anbehav.2004.12.2007) [Google Scholar]
- Adams E. S., Mesterton-Gibbons M.1995The cost of threat displays and the stability of deceptive communication. J. Theor. Biol. 175, 405–421 (doi:10.1006/jtbi.1995.0151) [Google Scholar]
- Andrews P. W.2001The psychology of social chess and the evolution of attribution mechanisms: explaining the fundamental attribution error. Evol. Human Behav. 22, 11–29 (doi:10.1016/S1090-5138(00)00059-3) [DOI] [PubMed] [Google Scholar]
- Aquiloni L., Gherardi F.2010Crayfish females eavesdrop on fighting males and use smell and sight to recognize the identity of the winner. Anim. Behav. 79, 265–269 (doi:10.1016/j.anbehav.2009.09.024) [Google Scholar]
- Arnott G., Elwood R. W.2009Assessment of fighting ability in animal contests. Anim. Behav. 77, 991–1004 (doi:10.1016/j.anbehav.2009.02.010) [Google Scholar]
- Arnott G., Elwood R. W.2010Signal residuals and hermit crab displays: flaunt it if you have it! Anim. Behav. 79, 137–143 (doi:10.1016/j.anbehav.2009.10.011) [Google Scholar]
- Axelrod R., Hamilton W. D.1981The evolution of cooperation. Science 211, 1390–1396 (doi:10.1126/science.7466396) [DOI] [PubMed] [Google Scholar]
- Bee M. A., Perrill S. A., Owen P. C.2000Male green frogs lower the pitch of acoustic signals in defense of territories: a possible dishonest signal of size? Behav. Ecol. 11, 169–177 (doi:10.1093/beheco/11.2.169) [Google Scholar]
- Bergmüller R., Schürch R., Hamilton I. M.2010Evolutionary causes and consequences of consistent individual variation in cooperative behaviour. Phil. Trans. R. Soc. B 365, 2751–2764 (doi:10.1098/rstb.2010.0124) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnie K. E., Earley R. L.2007Expanding the scope for social information use. Anim. Behav. 74, 171–181 (doi:10.1016/j.anbehav.2006.12.009) [Google Scholar]
- Bradbury J. W., Vehrencamp S. L.1998Principles of animal communication. Sunderland, MA: Sinauer Associates, Inc [Google Scholar]
- Briffa M., Sneddon L. U.2007Physiological constraints on contest behaviour. Funct. Ecol. 21, 627–637 (doi:10.1111/j.1365-2435.2006.01188.x) [Google Scholar]
- Brosnan S. F., Bshary R.2010Cooperation and deception: from evolution to mechanisms. Phil. Trans. R. Soc. B 365, 2593–2598 (doi:10.1098/rstb.2010.0155) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosnan S. F., Earley R. L., Dugatkin L. A.2003Observational learning and predator inspection in guppies (Poecilia reticulata). Ethology 109, 823–833 (doi:10.1046/j.0179-1613.2003.00928.x) [Google Scholar]
- Brosnan S. F., Salwiczek L., Bshary R.2010The interplay of cognition and cooperation. Phil. Trans. R. Soc. B 365, 2699–2710 (doi:10.1098/rstb.2010.0154) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bshary R.2001The cleaner fish market. In Economics in nature (eds Noë R., van Hoof J. A. R. A. M., Hammerstein P.), pp. 146–172 Cambridge, UK: Cambridge University Press [Google Scholar]
- Bshary R.2002Biting cleaner fish use altruism to deceive image-scoring client reef fish. Proc. R. Soc. Lond. B 269, 2087–2093 (doi:10.1098/rspb.2002.2084) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bshary R., Bergmüller R.2008Distinguishing four fundamental approaches to the evolution of helping. J. Evol. Biol. 21, 405–420 (doi:10.111/j.1420-9101.2007.01482.x) [DOI] [PubMed] [Google Scholar]
- Bshary R., D'Souza A.2005Cooperation in communication networks: indirect reciprocity in interactions between cleaner fish and client reef fish. In Animal communication networks (ed. McGregor P. K.), pp. 521–539 Cambridge, UK: Cambridge University Press [Google Scholar]
- Bshary R., Grutter A. S.2006Image scoring and cooperation in a cleaner fish mutualism. Nature 441, 975–978 (doi:10.1038/nature04755) [DOI] [PubMed] [Google Scholar]
- Bshary R., Wickler W., Fricke H.2002Fish cognition: a primate's eye view. Anim. Cogn. 5, 1–13 [DOI] [PubMed] [Google Scholar]
- Bugnyar T., Kotrschal K.2002Observational learning and the raiding of food caches in ravens, Corvus corax: is it ‘tactical’ deception? Anim. Behav. 64, 185–195 (doi:10.1006/anbe.2002.3056) [Google Scholar]
- Candolin U.2000Male–male competition ensures honest signaling of male parental ability in the three-spined stickleback (Gasterosteus aculeatus). Behav. Ecol. Sociobiol. 49, 57–61 (doi:10.1007/s002650000267) [Google Scholar]
- Clutton-Brock T.2009Cooperation between non-kin in animal societies. Nature 462, 51–57 (doi:10.1038/nature08366) [DOI] [PubMed] [Google Scholar]
- Connor R. C.2010Cooperation beyond the dyad: on simple models and a complex society. Phil. Trans. R. Soc. B 365, 2687–2697 (doi:10.1098/rstb.2010.0150) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crockford C., Wittig R. M., Seyfarth R. M., Cheney D. L.2007Baboons eavesdrop to deduce mating opportunities. Anim. Behav. 73, 885–890 (doi:10.1016/j.anbehav.2006.10.016) [Google Scholar]
- Croft D. P., James R., Thomas P. O. R., Hathaway C., Mawdsley D., Laland K. N., Krause J.2006Social structure and co-operative interactions in a wild population of guppies (Poecilia reticulata). Behav. Ecol. Sociobiol. 59, 644–650 (doi:10.1007/s00265-005-0091-y) [Google Scholar]
- Dabelsteen T.2005Public, private or anonymous? Facilitating and countering eavesdropping. In Animal communication networks (ed. McGregor P. K.), pp. 63–83 Cambridge, UK: Cambridge University Press [Google Scholar]
- Danchin E., Giraldeau L. A., Valone T. J., Wagner R. H.2004Public information: from nosy neighbors to cultural evolution. Science 305, 487–491 (doi:10.1126/science.1098254) [DOI] [PubMed] [Google Scholar]
- Doutrelant C., McGregor P. K.2000Eavesdropping and mate choice in female fighting fish. Behaviour 137, 1655–1669 (doi:10.1163/156853900502763) [Google Scholar]
- Ducoing A. M., Thierry B.2003Withholding information in semifree-ranging Tonkean macaques (Macaca tonkeana). J. Comp. Psychol. 117, 67–75 (doi:10.1037/0735-7036.117.1.67) [DOI] [PubMed] [Google Scholar]
- Dugatkin L. A.1991Dynamics of the TIT FOR TAT strategy during predator inspection in the guppy (Poecilia reticulata). Behav. Ecol. Sociobiol. 29, 127–132 (doi:10.1007/BF00166487) [Google Scholar]
- Dugatkin L. A.1992Tendency to inspect predators predicts mortality risk in the guppy (Poecilia reticulata). Behav. Ecol. 2, 124–127 [Google Scholar]
- Dugatkin L. A.2008Principles of animal behaviour. New York, NY: W.W. Norton & Company, Inc [Google Scholar]
- Dzieweczynski T. L., Earley R. L., Green T. M., Rowland W. J.2005Audience effect is context dependent in Siamese fighting fish, Betta splendens. Behav. Ecol. 16, 1025–1030 (doi:10.1093/beheco/ari088) [Google Scholar]
- Dzieweczynski T. L., Lyman S., Poor E. A.2009Male Siamese fighting fish, Betta splendens, increase rather than conceal courtship behaviour when a rival is present. Ethology 115, 186–195 (doi:10.1111/j.1439-0310.2008.01602.x) [Google Scholar]
- Earley R. L., Dugatkin L. A.2002Eavesdropping on visual cues in green swordtail (Xiphophorus helleri) fights: a case for networking. Proc. R. Soc. Lond. B 269, 943–952 (doi:10.1098/rspb.2002.1973) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enquist M., Leimar O.1983Evolution of fighting behaviour: decision rules and assessment of relative strength. J. Theor. Biol. 102, 387–410 (doi:10.1016/0022-5193(83)90376-4) [Google Scholar]
- Enquist M., Leimar O.1990The evolution of fatal fighting. Anim. Behav. 39, 1–9 (doi:10.1016/S0003-3472(05)80721-3) [Google Scholar]
- Fusani L., Giordana M., Day L. B., Schlinger B. A.2007High-speed video analysis reveals individual variation in courtship displays of male golden-collared manakins. Ethology 113, 964–972 (doi:10.1111/j.1439-0310.2007.01395.x) [Google Scholar]
- Garcia C. M., Ramirez E.2005Evidence that sensory traps can evolve into honest signals. Nature 434, 501–505 (doi:10.1038/nature03363) [DOI] [PubMed] [Google Scholar]
- Godin J.-G. J., Dugatkin L. A.1996Female mating preference for bold males in the guppy, Poecilia reticulata. Proc. Natl Acad. Sci. USA 93, 10 262–10 267 (doi:10.1073/pnas.93.19.10262) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurd P. L.1997Cooperative signaling between opponents in fish fights. Anim. Behav. 54, 1209–1315 [DOI] [PubMed] [Google Scholar]
- Jensen K.2010Punishment and spite, the dark side of cooperation. Phil. Trans. R. Soc. B 365, 2635–2650 (doi:10.1098/rstb.2010.0146) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone R. A.1998Game theory and communication. In Game theory and animal behaviour (eds Dugatkin L. A., Reeve H. K.). New York, NY: Oxford University Press [Google Scholar]
- Johnstone R. A.2001Eavesdropping and animal conflict. Proc. Natl Acad. Sci. USA 98, 9177–9180 (doi:10.1073/pnas.161058798) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone R. A., Bshary R.2004Evolution of spite through indirect reciprocity. Proc. R. Soc. Lond. B 271, 1917–1922 (doi:10.1098/rspb.2003.2581) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krams I., Krama T., Igaune K., Mänd R.2008Experimental evidence of reciprocal altruism in the pied flycatcher. Behav. Ecol. Sociobiol. 62, 599–605 (doi:10.1007/s00265-007-0484-1) [Google Scholar]
- Krebs J. R., Dawkins R.1984Animal signals: mind-reading and manipulation. In Behavioural ecology: an evolutionary approach (eds Krebs J. R., Davies N. B.), 2nd edn Sunderland, MA: Sinauer Associates, Inc [Google Scholar]
- Laidre M. E.2009How often do animals lie about their intentions? An experimental test. Am. Nat. 173, 337–346 (doi:10.1086/596530) [DOI] [PubMed] [Google Scholar]
- Leimar O., Hammerstein P.2001Evolution of cooperation through indirect reciprocity. Proc. R. Soc. Lond. B 268, 745–753 (doi:10.1098/rspb.2000.1573) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leimar O., Hammerstein P.2006Facing the facts. J. Evol. Biol. 19, 1403–1405 (doi:10.1111/j.1420-9101.2006.01156.x) [DOI] [PubMed] [Google Scholar]
- Leimar O., Hammerstein P.2010Cooperation for direct fitness benefits. Phil. Trans. R. Soc. B 365, 2619–2626 (doi:10.1098/rstb.2010.0116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matos R. J., Schlupp I.2005Performing in front of an audience: signalers and the social environment. In Animal communication networks (ed. McGregor P. K.), pp. 63–83 Cambridge, UK: Cambridge University Press [Google Scholar]
- Maynard Smith J., Harper D.2003Animal signals. Oxford, UK: Oxford University Press [Google Scholar]
- McGregor P. K.2005Animal communication networks. Cambridge, UK: Cambridge University Press [Google Scholar]
- McNamara J. M., Leimar O.2010Variation and the response to variation as a basis for successful cooperation. Phil. Trans. R. Soc. B 365, 2627–2633 (doi:10.1098/rstb.2010.0159) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis A. P., Semmann D.2010How is human cooperation different? Phil. Trans. R. Soc. B 365, 2663–2674 (doi:10.1098/rstb.2010.0157) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mery F., Varela S. A. M., Danchin E., Blanchet S., Parejo D., Coolen I., Wagner R. H.2009Public versus personal information for mate copying in an invertebrate. Curr. Biol. 19, 730–734 (doi:10.1016/j.cub.2009.02.064) [DOI] [PubMed] [Google Scholar]
- Milinski M.1987Tit-for-tat in sticklebacks and the evolution of cooperation. Nature 325, 433–435 (doi:10.1038/325433a0) [DOI] [PubMed] [Google Scholar]
- Milinski M., Külling D., Kettler R.1990Tit for tat: sticklebacks (Gasterosteus aculeatus) ‘trusting’ a cooperating partner. Behav. Ecol. 1, 7–11 (doi:10.1093/beheco/1.1.7) [Google Scholar]
- Milinski M., Semmann D., Bakker T. C. M., Krambeck J.2001Cooperation through indirect reciprocity: image scoring or standing strategy? Proc. R. Soc. Lond. B 268, 2495–2501 (doi:10.1098/rspb.2001.1809) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak M. A., Sigmund K.1998Evolution of indirect reciprocity by image scoring. Nature 393, 573–576 (doi:10.1038/31225) [DOI] [PubMed] [Google Scholar]
- Ophir A. G., Galef B. G., Jr2003Female Japanese quail that ‘eavesdrop’ on fighting males prefer losers to winners. Anim. Behav. 66, 399–407 (doi:10.1006/anbe.2003.2230) [Google Scholar]
- Paz-y-Miño G., Bond A. B., Kamil A. C., Balda R. P.2004Pinyon jays use transitive inference to predict social dominance. Nature 430, 778–781 (doi:10.1038/nature02723) [DOI] [PubMed] [Google Scholar]
- Peake T. M.2005Eavesdropping in communication networks. In Animal communication networks (ed. McGregor P. K.), pp. 13–37 Cambridge, UK: Cambridge University Press [Google Scholar]
- Peake T. M., McGregor P. K.2004Information and aggression in fishes. Learn. Behav. 32, 114–121 [DOI] [PubMed] [Google Scholar]
- Peake T. M., Terry A. M. R., McGregor P. K., Dabelsteen T.2002Do great tits assess rivals by combining direct experience with information gathered by eavesdropping? Proc. R. Soc. Lond. B 269, 1925–1929 (doi:10.1098/rspb.2002.2112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plath M., Richter S., Tiedemann R., Schlupp I.2005Male fish deceive competitors about mating preferences. Curr. Biol. 18, 1138–1141 (doi:10.1016/j.cub.2008.06.067) [DOI] [PubMed] [Google Scholar]
- Roberts G.2008Evolution of direct and indirect reciprocity. Proc. R. Soc. B 275, 173–179 (doi:10.1098/rspb.2007.1134) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowell J. T., Ellner S. P., Reeve H. K.2006Why animals lie: how dishonesty and belief can coexist in a signaling system. Am. Nat. 168, E180–E204 (doi:10.1086/508809) [DOI] [PubMed] [Google Scholar]
- Searcy W. A., Nowicki S.2005The evolution of animal communication: reliability and deception in signaling systems. Princeton, NJ: Princeton University Press [Google Scholar]
- Shamble P. S., Wilgers D. J., Swoboda K. A., Hebets E. A.2009Courtship effort is a better predictor of mating success than ornamentation for male wolf spiders. Behav. Ecol. 20, 1242–1251 (doi:10.1093/beheco/arp116) [Google Scholar]
- Shumway C. A.2008Habitat complexity, brain, and behaviour. Brain Behav. Evol. 72, 123–134 (doi:10.1159/000151472) [DOI] [PubMed] [Google Scholar]
- Slocombe K. E., Zuberbühler K.2007Chimpanzees modify recruitment screams as a function of audience composition. Proc. Natl Acad. Sci. USA 104, 17 228–17 233 (doi:10.1073/pnas.0706741104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele M. A., Halkin S. L., Smallwood P. D., McKenna T. J., Mitsopoulos K., Beam M.2008Cache protection strategies of a scatter-hoarding rodent: do tree squirrels engage in behavioural deception? Anim. Behav. 75, 705–714 (doi:10.1016/j.anbehav.2007.07.026) [Google Scholar]
- Steger R., Caldwell R. L.1983Intraspecific deception by bluffing: a defense strategy of newly molted stomatopods (Arthropoda: Crustacea). Science 221, 558–560 (doi:10.1126/science.221.4610.558) [DOI] [PubMed] [Google Scholar]
- Szalai F., Számadó S.2009Honest and cheating strategies in a simple model of aggressive communication. Anim. Behav. 78, 949–959 (doi:10.1016/j.anbehav.2009.06.025) [DOI] [PubMed] [Google Scholar]
- Számadó S.2000Cheating as a mixed strategy in a simple model of aggressive communication. Anim. Behav. 59, 221–230 (doi:10.1006/anbe.1999.1293) [DOI] [PubMed] [Google Scholar]
- Thomas P. O. R., et al. 2008Does defection during predator inspection affect social structure in wild shoals of guppies? Anim. Behav. 75, 43–53 (doi:10.1016/j.anbehav.2007.06.004) [Google Scholar]
- Tobias J. A., Seddon N.2002Female begging in European robins: do neighbors eavesdrop for extrapair copulations? Behav. Ecol. 13, 637–642 (doi:10.1093/beheco/13.5.637) [Google Scholar]
- Trivers R. L.1971The evolution of reciprocal altruism. Q. Rev. Biol. 46, 35–57 (doi:10.1086/406755) [Google Scholar]
- Valone T. J.2007From eavesdropping on performance to copying the behaviour of others: a review of public information use. Behav. Ecol. Sociobiol. 62, 1–14 (doi:10.1007/s00265-007-0439-6) [Google Scholar]
- Van Breukelen N. A., Draud M.2006The roles of male size and female eavesdropping in divorce in the monogamous convict cichlid (Archocentrus nigrofasciatus, Cichlidae). Behaviour 142, 1029–1041 [Google Scholar]
- Westneat D. F., Walters A., McCarthy T. M., Hatch M. I., Hein W. K.2000Alternative mechanisms of nonindependent mate choice. Anim. Behav. 59, 467–476 (doi:10.1006/anbe.1999.1341) [DOI] [PubMed] [Google Scholar]
- Wisenden B. D., Stacey N. E.2005Fish semiochemicals and the evolution of communication networks. In Animal communication networks (ed. McGregor P. K.), pp. 540–567 Cambridge, UK: Cambridge University Press [Google Scholar]
- Zahavi A.2003Indirect selection and individual selection in sociobiology: my personal views on theories of social behaviour. Anim. Behav. 65, 859–863 (doi:10.1006/anbe.2003.2109) [Google Scholar]
- Zahavi A., Zahavi A.1997The handicap principle: a missing piece of Darwin's puzzle. New York, NY: Oxford University Press [Google Scholar]