Abstract
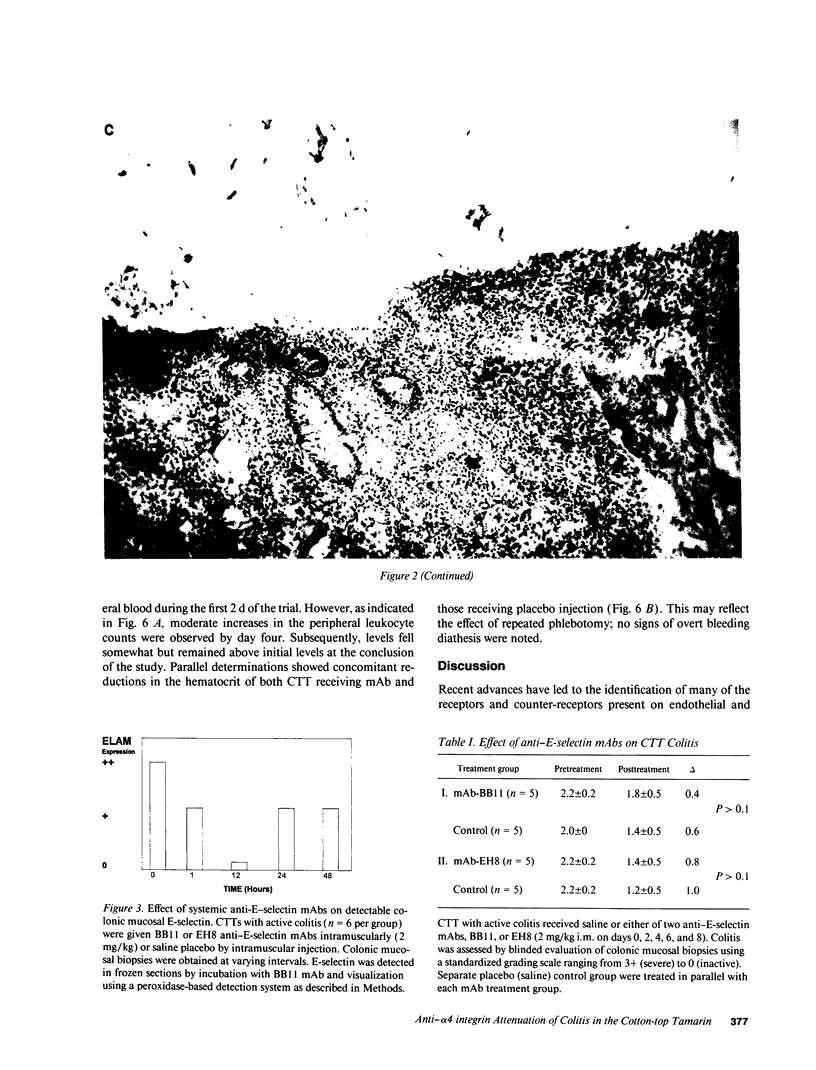
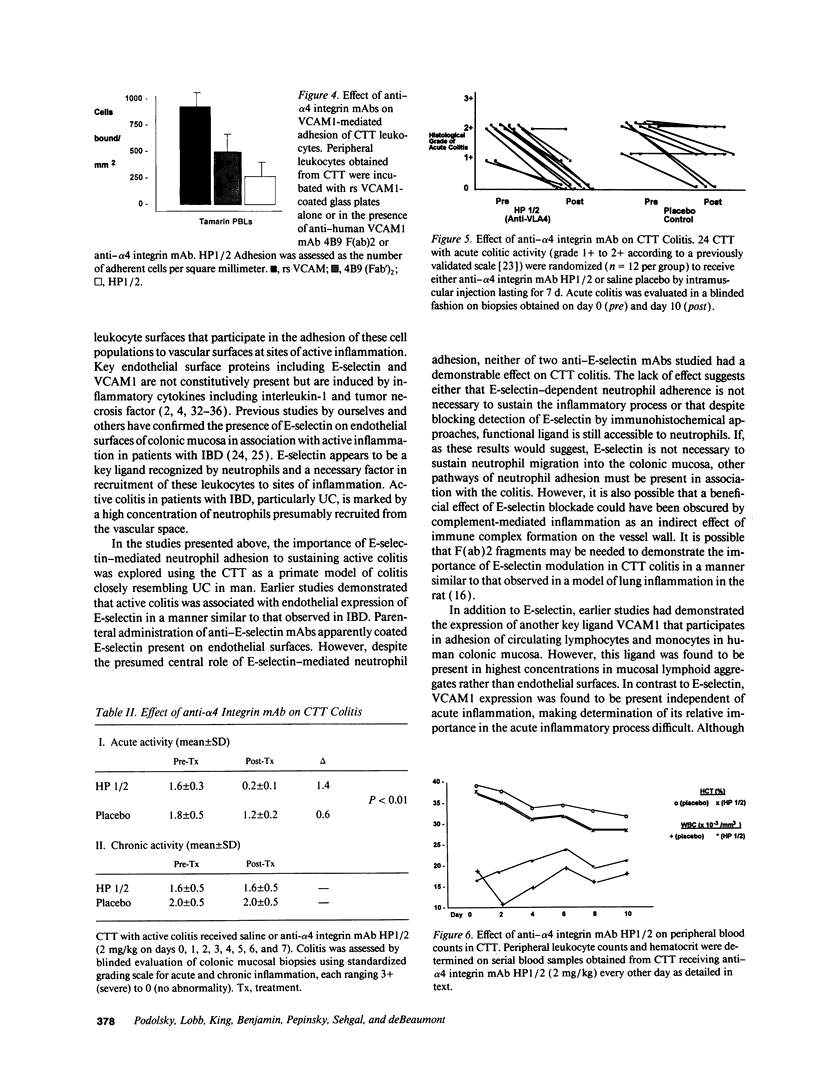
Recent studies have demonstrated the induced expression of endothelial adhesion molecules including E-selectin (also called endothelial leukocyte adhesion molecule-1), vascular cell adhesion molecule and intercellular adhesion molecule in actively involved mucosa of patients with ulcerative colitis and Crohn's disease. Similar induction has been demonstrated in the colon of the Cotton-top tamarin (CTT), a New World primate that experiences a spontaneous acute and chronic colitis resembling ulcerative colitis. To assess the potential importance of leukocyte adhesion as a necessary step in acute colitis, the effect of parenteral mAb directed against adhesion molecules on CTT colitis was evaluated in placebo-controlled blinded trials. Serial administration of either of two anti-E-selectin mAb designated BB11 and EH8 effectively coated endothelial surfaces expressing this vascular adhesion molecule. Although colitis activity was slightly diminished after the 10-d treatment period in CTT receiving either BB11 or EH8, this reduction was not significantly different than that seen in animals given a placebo control when assessed by a previously validated standardized scale of inflammatory activity: mean histologic activity grade 2.2 +/- 0.2 pretreatment vs 1.5 +/- 0.5 posttreatment in group receiving mAb and 2.1 +/- 0.1 pretreatment vs 1.3 +/- 0.5 posttreatment in the placebo group (P > 0.2). In contrast, administration of an anti-alpha 4 integrin mAb designated HP1/2 that binds VLA4 (alpha 4 beta 1) and presumably alpha 4 beta 7 integrins resulted in significant attenuation of acute colitis when compared to both pretreatment activity index (P = 0.005) and the placebo control group (P < 0.01): mean histologic activity grade 1.6 +/- 0.3 pretreatment vs 0.2 +/- 0.1 posttreatment in the group receiving HP1/2 and 1.8 +/- 0.5 pretreatment and 1.2 +/- 0.2 posttreatment in the placebo control group. These studies using a model of spontaneous colitis in the CTT demonstrate the feasibility of modulation of leukocyte-vascular adhesion and/or other integrin-mediated events possibly including T cell aggregation and T cell-stromal interactions, as well as lymphocyte homing. These results suggest both that these processes are important and possibly essential elements in sustaining acute colitis and that their disruption may result in therapeutic benefit.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benjamin C., Dougas I., Chi-Rosso G., Luhowskyj S., Rosa M., Newman B., Osborn L., Vassallo C., Hession C., Goelz S. A blocking monoclonal antibody to endothelial-leukocyte adhesion molecule-1 (ELAM1). Biochem Biophys Res Commun. 1990 Aug 31;171(1):348–353. doi: 10.1016/0006-291x(90)91400-m. [DOI] [PubMed] [Google Scholar]
- Berg E. L., Magnani J., Warnock R. A., Robinson M. K., Butcher E. C. Comparison of L-selectin and E-selectin ligand specificities: the L-selectin can bind the E-selectin ligands sialyl Le(x) and sialyl Le(a). Biochem Biophys Res Commun. 1992 Apr 30;184(2):1048–1055. doi: 10.1016/0006-291x(92)90697-j. [DOI] [PubMed] [Google Scholar]
- Berg E. L., Robinson M. K., Mansson O., Butcher E. C., Magnani J. L. A carbohydrate domain common to both sialyl Le(a) and sialyl Le(X) is recognized by the endothelial cell leukocyte adhesion molecule ELAM-1. J Biol Chem. 1991 Aug 15;266(23):14869–14872. [PubMed] [Google Scholar]
- Bevilacqua M. P., Stengelin S., Gimbrone M. A., Jr, Seed B. Endothelial leukocyte adhesion molecule 1: an inducible receptor for neutrophils related to complement regulatory proteins and lectins. Science. 1989 Mar 3;243(4895):1160–1165. doi: 10.1126/science.2466335. [DOI] [PubMed] [Google Scholar]
- Butcher E. C. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991 Dec 20;67(6):1033–1036. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- Carlos T. M., Schwartz B. R., Kovach N. L., Yee E., Rosa M., Osborn L., Chi-Rosso G., Newman B., Lobb R., Rosso M. Vascular cell adhesion molecule-1 mediates lymphocyte adherence to cytokine-activated cultured human endothelial cells. Blood. 1990 Sep 1;76(5):965–970. [PubMed] [Google Scholar]
- Carlos T. M., Schwartz B. R., Kovach N. L., Yee E., Rosa M., Osborn L., Chi-Rosso G., Newman B., Lobb R., Rosso M. Vascular cell adhesion molecule-1 mediates lymphocyte adherence to cytokine-activated cultured human endothelial cells. Blood. 1990 Sep 1;76(5):965–970. [PubMed] [Google Scholar]
- Elices M. J., Osborn L., Takada Y., Crouse C., Luhowskyj S., Hemler M. E., Lobb R. R. VCAM-1 on activated endothelium interacts with the leukocyte integrin VLA-4 at a site distinct from the VLA-4/fibronectin binding site. Cell. 1990 Feb 23;60(4):577–584. doi: 10.1016/0092-8674(90)90661-w. [DOI] [PubMed] [Google Scholar]
- Graber N., Gopal T. V., Wilson D., Beall L. D., Polte T., Newman W. T cells bind to cytokine-activated endothelial cells via a novel, inducible sialoglycoprotein and endothelial leukocyte adhesion molecule-1. J Immunol. 1990 Aug 1;145(3):819–830. [PubMed] [Google Scholar]
- Hession C., Osborn L., Goff D., Chi-Rosso G., Vassallo C., Pasek M., Pittack C., Tizard R., Goelz S., McCarthy K. Endothelial leukocyte adhesion molecule 1: direct expression cloning and functional interactions. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1673–1677. doi: 10.1073/pnas.87.5.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzmann B., Weissman I. L. Integrin molecules involved in lymphocyte homing to Peyer's patches. Immunol Rev. 1989 Apr;108:45–61. doi: 10.1111/j.1600-065x.1989.tb00012.x. [DOI] [PubMed] [Google Scholar]
- Imai Y., Singer M. S., Fennie C., Lasky L. A., Rosen S. D. Identification of a carbohydrate-based endothelial ligand for a lymphocyte homing receptor. J Cell Biol. 1991 Jun;113(5):1213–1221. doi: 10.1083/jcb.113.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issekutz T. B. Inhibition of in vivo lymphocyte migration to inflammation and homing to lymphoid tissues by the TA-2 monoclonal antibody. A likely role for VLA-4 in vivo. J Immunol. 1991 Dec 15;147(12):4178–4184. [PubMed] [Google Scholar]
- Jonjic N., Jílek P., Bernasconi S., Peri G., Martìn-Padura I., Cenzuales S., Dejana E., Mantovani A. Molecules involved in the adhesion and cytotoxicity of activated monocytes on endothelial cells. J Immunol. 1992 Apr 1;148(7):2080–2083. [PubMed] [Google Scholar]
- Kansas G. S., Spertini O., Stoolman L. M., Tedder T. F. Molecular mapping of functional domains of the leukocyte receptor for endothelium, LAM-1. J Cell Biol. 1991 Jul;114(2):351–358. doi: 10.1083/jcb.114.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
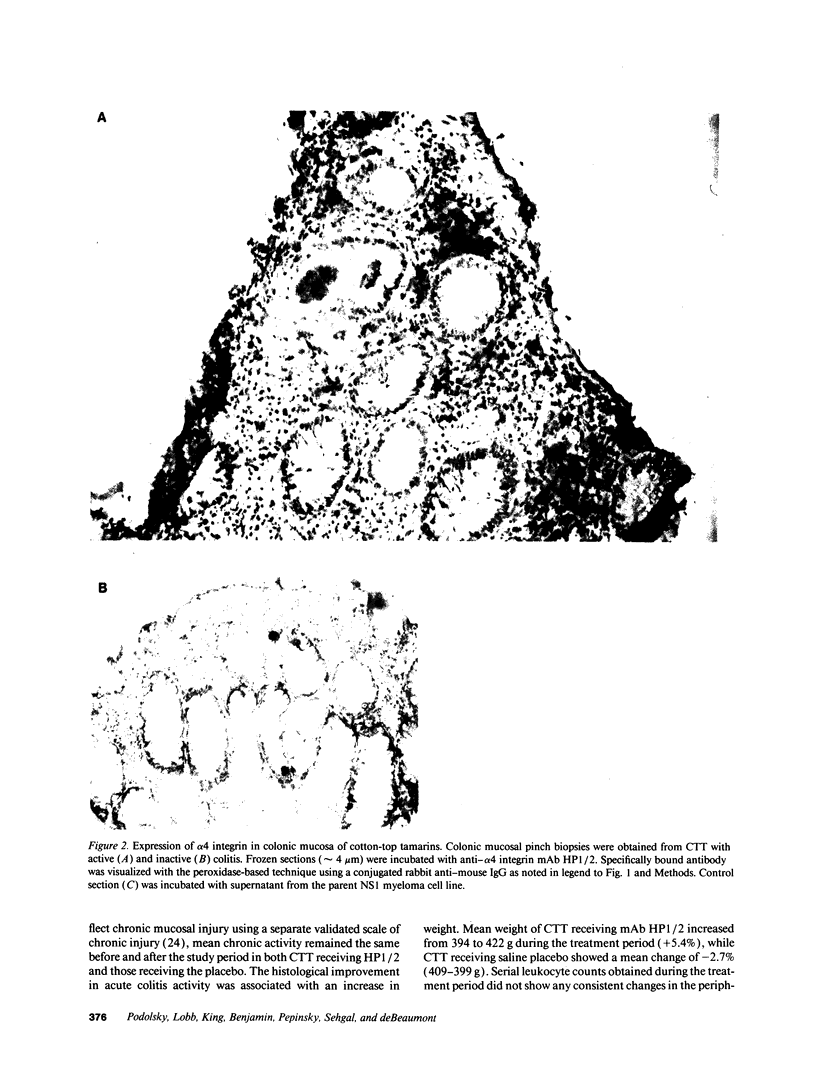
- Koizumi M., King N., Lobb R., Benjamin C., Podolsky D. K. Expression of vascular adhesion molecules in inflammatory bowel disease. Gastroenterology. 1992 Sep;103(3):840–847. doi: 10.1016/0016-5085(92)90015-q. [DOI] [PubMed] [Google Scholar]
- Kuijpers T. W., Hoogerwerf M., van der Laan L. J., Nagel G., van der Schoot C. E., Grunert F., Roos D. CD66 nonspecific cross-reacting antigens are involved in neutrophil adherence to cytokine-activated endothelial cells. J Cell Biol. 1992 Jul;118(2):457–466. doi: 10.1083/jcb.118.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobb R. R., Chi-Rosso G., Leone D. R., Rosa M. D., Bixler S., Newman B. M., Luhowskyj S., Benjamin C. D., Dougas I. G., Goelz S. E. Expression and functional characterization of a soluble form of endothelial-leukocyte adhesion molecule 1. J Immunol. 1991 Jul 1;147(1):124–129. [PubMed] [Google Scholar]
- Lobb R. R., Chi-Rosso G., Leone D. R., Rosa M. D., Bixler S., Newman B. M., Luhowskyj S., Benjamin C. D., Dougas I. G., Goelz S. E. Expression and functional characterization of a soluble form of endothelial-leukocyte adhesion molecule 1. J Immunol. 1991 Jul 1;147(1):124–129. [PubMed] [Google Scholar]
- Lobb R., Chi-Rosso G., Leone D., Rosa M., Newman B., Luhowskyj S., Osborn L., Schiffer S., Benjamin C., Dougas I. Expression and functional characterization of a soluble form of vascular cell adhesion molecule 1. Biochem Biophys Res Commun. 1991 Aug 15;178(3):1498–1504. doi: 10.1016/0006-291x(91)91063-i. [DOI] [PubMed] [Google Scholar]
- Madara J. L., Podolsky D. K., King N. W., Sehgal P. K., Moore R., Winter H. S. Characterization of spontaneous colitis in cotton-top tamarins (Saguinus oedipus) and its response to sulfasalazine. Gastroenterology. 1985 Jan;88(1 Pt 1):13–19. doi: 10.1016/s0016-5085(85)80126-8. [DOI] [PubMed] [Google Scholar]
- Malizia G., Calabrese A., Cottone M., Raimondo M., Trejdosiewicz L. K., Smart C. J., Oliva L., Pagliaro L. Expression of leukocyte adhesion molecules by mucosal mononuclear phagocytes in inflammatory bowel disease. Gastroenterology. 1991 Jan;100(1):150–159. doi: 10.1016/0016-5085(91)90595-c. [DOI] [PubMed] [Google Scholar]
- Montgomery K. F., Osborn L., Hession C., Tizard R., Goff D., Vassallo C., Tarr P. I., Bomsztyk K., Lobb R., Harlan J. M. Activation of endothelial-leukocyte adhesion molecule 1 (ELAM-1) gene transcription. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6523–6527. doi: 10.1073/pnas.88.15.6523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan M. S., Varani J., Dame M. K., Lane C. L., Smith C. W., Anderson D. C., Ward P. A. Role of endothelial-leukocyte adhesion molecule 1 (ELAM-1) in neutrophil-mediated lung injury in rats. J Clin Invest. 1991 Oct;88(4):1396–1406. doi: 10.1172/JCI115446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn L., Hession C., Tizard R., Vassallo C., Luhowskyj S., Chi-Rosso G., Lobb R. Direct expression cloning of vascular cell adhesion molecule 1, a cytokine-induced endothelial protein that binds to lymphocytes. Cell. 1989 Dec 22;59(6):1203–1211. doi: 10.1016/0092-8674(89)90775-7. [DOI] [PubMed] [Google Scholar]
- Phillips M. L., Nudelman E., Gaeta F. C., Perez M., Singhal A. K., Hakomori S., Paulson J. C. ELAM-1 mediates cell adhesion by recognition of a carbohydrate ligand, sialyl-Lex. Science. 1990 Nov 23;250(4984):1130–1132. doi: 10.1126/science.1701274. [DOI] [PubMed] [Google Scholar]
- Picker L. J., Kishimoto T. K., Smith C. W., Warnock R. A., Butcher E. C. ELAM-1 is an adhesion molecule for skin-homing T cells. Nature. 1991 Feb 28;349(6312):796–799. doi: 10.1038/349796a0. [DOI] [PubMed] [Google Scholar]
- Picker L. J., Warnock R. A., Burns A. R., Doerschuk C. M., Berg E. L., Butcher E. C. The neutrophil selectin LECAM-1 presents carbohydrate ligands to the vascular selectins ELAM-1 and GMP-140. Cell. 1991 Sep 6;66(5):921–933. doi: 10.1016/0092-8674(91)90438-5. [DOI] [PubMed] [Google Scholar]
- Podolsky D. K., Madara J. L., King N., Sehgal P., Moore R., Winter H. S. Colonic mucin composition in primates. Selective alterations associated with spontaneous colitis in the cotton-top tamarin. Gastroenterology. 1985 Jan;88(1 Pt 1):20–25. doi: 10.1016/s0016-5085(85)80127-x. [DOI] [PubMed] [Google Scholar]
- Postigo A. A., Garcia-Vicuña R., Diaz-Gonzalez F., Arroyo A. G., De Landázuri M. O., Chi-Rosso G., Lobb R. R., Laffon A., Sánchez-Madrid F. Increased binding of synovial T lymphocytes from rheumatoid arthritis to endothelial-leukocyte adhesion molecule-1 (ELAM-1) and vascular cell adhesion molecule-1 (VCAM-1). J Clin Invest. 1992 May;89(5):1445–1452. doi: 10.1172/JCI115734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulido R., Elices M. J., Campanero M. R., Osborn L., Schiffer S., García-Pardo A., Lobb R., Hemler M. E., Sánchez-Madrid F. Functional evidence for three distinct and independently inhibitable adhesion activities mediated by the human integrin VLA-4. Correlation with distinct alpha 4 epitopes. J Biol Chem. 1991 Jun 5;266(16):10241–10245. [PubMed] [Google Scholar]
- Pulido R., Elices M. J., Campanero M. R., Osborn L., Schiffer S., García-Pardo A., Lobb R., Hemler M. E., Sánchez-Madrid F. Functional evidence for three distinct and independently inhibitable adhesion activities mediated by the human integrin VLA-4. Correlation with distinct alpha 4 epitopes. J Biol Chem. 1991 Jun 5;266(16):10241–10245. [PubMed] [Google Scholar]
- Rüegg C., Postigo A. A., Sikorski E. E., Butcher E. C., Pytela R., Erle D. J. Role of integrin alpha 4 beta 7/alpha 4 beta P in lymphocyte adherence to fibronectin and VCAM-1 and in homotypic cell clustering. J Cell Biol. 1992 Apr;117(1):179–189. doi: 10.1083/jcb.117.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu Y., Newman W., Gopal T. V., Horgan K. J., Graber N., Beall L. D., van Seventer G. A., Shaw S. Four molecular pathways of T cell adhesion to endothelial cells: roles of LFA-1, VCAM-1, and ELAM-1 and changes in pathway hierarchy under different activation conditions. J Cell Biol. 1991 Jun;113(5):1203–1212. doi: 10.1083/jcb.113.5.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu Y., Shaw S., Graber N., Gopal T. V., Horgan K. J., Van Seventer G. A., Newman W. Activation-independent binding of human memory T cells to adhesion molecule ELAM-1. Nature. 1991 Feb 28;349(6312):799–802. doi: 10.1038/349799a0. [DOI] [PubMed] [Google Scholar]
- Tyrrell D., James P., Rao N., Foxall C., Abbas S., Dasgupta F., Nashed M., Hasegawa A., Kiso M., Asa D. Structural requirements for the carbohydrate ligand of E-selectin. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10372–10376. doi: 10.1073/pnas.88.22.10372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace J. L., Higa A., McKnight G. W., MacIntyre D. E. Prevention and reversal of experimental colitis by a monoclonal antibody which inhibits leukocyte adherence. Inflammation. 1992 Aug;16(4):343–354. doi: 10.1007/BF00917626. [DOI] [PubMed] [Google Scholar]
- Yamada K. M. Adhesive recognition sequences. J Biol Chem. 1991 Jul 15;266(20):12809–12812. [PubMed] [Google Scholar]
- van Dinther-Janssen A. C., Horst E., Koopman G., Newmann W., Scheper R. J., Meijer C. J., Pals S. T. The VLA-4/VCAM-1 pathway is involved in lymphocyte adhesion to endothelium in rheumatoid synovium. J Immunol. 1991 Dec 15;147(12):4207–4210. [PubMed] [Google Scholar]