Abstract
No-take zones may protect populations of targeted marine species and restore the integrity of marine ecosystems, but it is unclear whether they benefit top predators that rely on mobile pelagic fishes. In South Africa, foraging effort of breeding African penguins decreased by 30 per cent within three months of closing a 20 km zone to the competing purse-seine fisheries around their largest colony. After the fishing ban, most of the penguins from this island had shifted their feeding effort inside the closed area. Birds breeding at another colony situated 50 km away, whose fishing grounds remained open to fishing, increased their foraging effort during the same period. This demonstrates the immediate benefit of a relatively small no-take zone for a marine top predator relying on pelagic prey. Selecting such small protected areas may be an important first conservation step, minimizing stakeholder conflicts and easing compliance, while ensuring benefit for the ecosystems within these habitats.
Keywords: African penguins, marine protected areas, offshore reserves, umbrella species, seabirds, sustainable fishing
1. Introduction
Oceans have been over-exploited for decades, mainly through industrial-scale fishing (Pauly et al. 2002; Worm et al. 2009), and to date 80 per cent of the world's fish stocks are either fully or over-exploited (Food and Agriculture Organization 2009). Since 1992, the Convention on Biodiversity required the protection of 10 per cent of all marine areas, to reach the same target as for terrestrial environments. Although numbers of marine protected areas (MPAs) have increased, less than 0.7 per cent of the world's seas are as yet under protection (Wood et al. 2008), and most protected areas are coastal (Game et al. 2009). Sedentary fish populations and benthic organisms benefit from MPAs (Lester et al. 2009), as to some extent do more mobile fishes (Apostolaki et al. 2002). Furthermore, export effects of MPAs benefit biodiversity and fisheries in adjoining areas (White et al. 2008). Many top predators are threatened through direct exploitation, mortality on fishing gear and competition with fisheries. As these predators play pivotal roles in the stability and resilience of ecosystems, their loss can trigger cascading effects that disrupt entire ecosystems (Baum & Worm 2009). MPAs are often promoted as beneficial to top predators (Louzao et al. 2006; Frederiksen et al. 2008), but to date their effectiveness in this regard remains unclear. Moreover, such predators often rely on highly mobile prey, undergoing large-scale movements. In these situations, it is predicted that only very large MPAs may benefit them (Frederiksen et al. 2008; White et al. 2008).
African penguins (Spheniscus demersus), endemic to Southern Africa, have decreased by 90 per cent during the twentieth century, and between 2004 and 2008, the population halved to less than 26 000 pairs, the lowest value yet recorded (Crawford et al. 2008). Their survival and breeding success are closely tied to the availability of pelagic sardines (Sardinops sagax) and anchovies (Engraulis encrasicolus) within 20–30 km of their breeding sites (Pichegru et al. 2009). During their life, these fish can cover large distances (over hundreds of kilometres, Barange et al. 1999). Off South Africa's west coast, where many seabird colonies are located, these fish stocks have decreased markedly during the last decade, owing to changing environmental conditions and a lack of spatial management of the competing purse-seine fishery. Heavy fishing pressure persists in areas with low fish abundance because of the distribution of land-based processing plants (Pichegru et al. 2009).
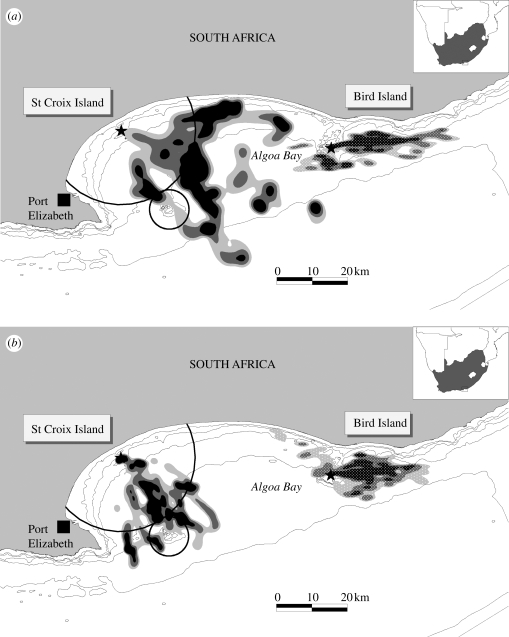
In January 2009, a 20 km radius area was closed to purse-seine fishing around the world's largest African penguin colony at St Croix Island, Algoa Bay (the ‘treatment’, figure 1). The waters around Bird Island, another penguin colony 50 km away within the same bay, remained open to fishing (the ‘control’). Historically, most pelagic fish catches by the industry in Algoa Bay occurred around St Croix Island (Pichegru et al. 2009). By studying the foraging behaviour of adult penguins raising chicks at both sites before and after the closure to fishing, we tested whether a relatively small no-take zone could benefit breeding penguins relying on pelagic prey.
Figure 1.
Foraging areas (density of feeding dives) of African penguins S. demersus breeding on St Croix Island and Bird Island (stars), in South Africa, (a) before (2008) and (b) after (2009) closure to purse-seine fishing within 20 km of St Croix Island and an adjacent area surrounding an offshore bank (circled). Foraging range: black, 50%; dark grey, 50–75%; and light grey, 75–90%.
2. Material and methods
The foraging behaviour of adult penguins raising chicks of one to three weeks old was studied at St Croix Island (33°48′ S, 25°46′ E, the ‘treatment colony’) and at Bird Island (33°50′ S, 26°17′ E, the ‘control colony’), before and after closure to fishing, in May–June 2008 and April–May 2009. The positions of purse-seine vessels were monitored constantly via satellite telemetry, ensuring compliance within the experimental closure. African penguins share the care of their brood of one or two chicks between March and August, with typically one adult attending the nest when the partner is at sea. Birds were equipped with GPS-TD loggers (a global positioning system recorder combined with a time-depth recorder; GPS-TD 96 × 39 × 26.5 mm; earth&OCEAN Technologies, Germany), which record latitude and longitude at 1 min intervals to an accuracy of less than 10 m, and depth at 1 s intervals to the nearest 0.1 m. The devices weighed less than 2.5 per cent of adult body mass and were housed in streamlined fibre-composite containers. They were attached to the penguins' lower back feathers with waterproof tape, causing no damage to the plumage. Handling lasted less than 6 min from capture to release, and these methods were approved by University of Cape Town's animal ethics committee. After deployment, nest sites of instrumented birds were monitored until the adult carrying the GPS returned, allowing it to be recaptured and the logger removed. Previous studies showed no significant difference in the foraging behaviour of instrumented versus control African penguins (Petersen et al. 2006). This was confirmed in 2008 on St Croix Island, where adults at control nests with chicks of similar age were marked without being handled, using bio-compatible dye, and had their foraging trip durations recorded. Handling and instrumentation did not affect the duration of foraging trips (control birds: 22.7 ± 3.1 h, n = 9, H = 0.76, p = 0.383).
On retrieval of the devices, trip duration, path length, maximum distance from the colony and diving effort were calculated in order to compare foraging effort at each island between years (before and after closure) and between islands (treatment versus control) using general linear models (GLMs). Each parameter (trip duration, path length, maximum distance from the colony and diving behaviour) was tested as an explanatory variable, while year or colony were taken as dependent factors. Data were only recorded for a single foraging trip per bird to avoid pseudo-replication. There was no difference in brood size between nests across years or colonies (GLM with brood size as the explanatory variable, year and colony as dependent factors, colony: F1,90 = 0.61, p = 0.54; year: F1,90 = 0.08, p = 0.78), so the data from nests of different brood size were pooled.
A GPS position was associated with each feeding dive (>3 m and diurnal, as defined by Wilson & Wilson 1990). Technical failures of the devices (no GPS positions or no dives recorded) decreased the sample of complete foraging trips where a GPS position could be attributed to each feeding dive. However, all other data were used for the calculation of foraging and diving effort. Adaptive kernel analyses were conducted using Arcview GIS 3.1 with the smoothing factor chosen according to the least-squares-cross-validation method (Worton 1989) to estimate contour levels covering 50–75–90% of the foraging locations.
3. Results
During the 2008 and 2009 breeding seasons (March–June), 91 complete foraging tracks were collected from penguins breeding on the two islands. None of the diving parameters for African penguins differed between years or colonies (table 1), but there were marked changes in horizontal foraging effort. In 2008, the average foraging path length travelled for birds from the treatment island was 70 ± 28 km (maximum 150 km), at 18–45 km away from the colony, for 22.5 ± 7 h (table 1). After the fishery closure in 2009, penguins reduced their effort by 25–30%, travelling 50 ± 17 km (maximum 80 km) to forage for 17.1 h on average within 5–30 km of the island (table 1). By contrast, from 2008 to 2009, penguins from the control island increased their time spent foraging (from 15.6 to 17.8 h on average), potentially as a result of reduced marine productivity and/or increased fishing pressure around the island in 2009. This inference is supported by a significant decrease in adult body mass in 2009 for the control colony (table 1). Treatment birds shifted their core feeding zones, from located mostly (75% of their feeding dives) outside the closure area in 2008, to concentrated within this area in 2009 (>70% of their feeding dives) (figure 1). The main feeding locations of birds from the control island were similar between years (figure 1).
Table 1.
Foraging behaviour of African penguins from St Croix and Bird Islands, Algoa Bay, before (2008) and after (2009) closure to purse-seine industrial fishing within 20 km of St Croix Island and a smaller adjacent area surrounding an offshore bank. (Values are mean ± s.d. (range). Level of significance (sig) is noted as follows: n.s. p > 0.05, *p < 0.05, **p < 0.01, ***p < 0.001.)
| St Croix Island |
year effect | Bird Island |
year effect | colony effect | |||
|---|---|---|---|---|---|---|---|
| 2008 | 2009 | sig | 2008 | 2009 | sig | sig | |
| n (GPS tracks) | 18 | 14 | 30 | 29 | |||
| body mass (g) | 3500 ± 550 (1950–4250) | 3300 ± 530 (2675–4750) | n.s. | 3780 ± 410 (2900–4400) | 3230 ± 350 (2525–4000) | *** | n.s. |
| trip duration (h) | 22.5 ± 7.1 (13.9–47.8) | 17.1 ± 4 (7.8–23) | * | 15.6 ± 4 (9.7–24) | 18 ± 5 (7.2–30) | * | * |
| foraging path length (km) | 69.3 ± 28.6 (25.9–152.3) | 50.2 ± 17 (11.2–77.5) | * | 39.2 ± 10.4 (25.6–66.7) | 41.5 ± 11.9 (10.9–59.8) | n.s. | *** |
| maximum distance from colony (km) | 32.3 ± 8 (18.7–44.5) | 19.7 ± 7.2 (4.7–30.7) | ** | 14.5 ± 6.8 (6.3–30.3) | 14 ± 4.9 (4.1–24.8) | n.s. | *** |
| average dive duration (s) | 79.6 ± 12.1 (max: 163) | 72.5 ± 27 (max: 153) | n.s. | 75.3 ± 11.7 (max: 154) | 76.1 ± 12.8 (max: 275) | n.s. | n.s. |
| average dive depth (m) | 26.4 ± 11.5 (max: 84.9) | 23 ± 16.1 (max: 76.7) | n.s. | 25 ± 10.9 (max: 77.2) | 26.8 ± 6.8 (max: 91) | n.s. | n.s. |
Breeding African penguins expend 207 kJ h−1 while foraging (Nagy et al. 1984) and 52 kJ h−1 sitting on the nest or resting on land. Therefore, a reduction of 5.4 h at sea represents a potential approximate energy saving of (207 − 52) × 5.4 = 837 kJ per foraging trip, i.e. 43 per cent of their daily energy expenditure (1945 kJ, Nagy et al. 1984).
4. Discussion
Our findings strongly suggest that even relatively small no-take zones can benefit penguins. Within three months of closure, breeding African penguins decreased their foraging effort by 25–30% and their daily energy expenditure by approximately 43 per cent, shifting their core foraging areas from outside to within the area closed to fishing (figure 1 and table 1). At the same time, foraging effort at an adjacent colony increased, possibly linked to displacement of effort from the MPA area. Even if other factors may have influenced these changes in the penguin's behaviour (e.g. natural changes in productivity or currents), our data suggest that purse-seine fishing had a significant negative effect on penguin foraging behaviour. Therefore, stopping the local harvest of mobile small pelagic fishes, even over a small zone, may benefit African penguins, which responded rapidly to such a change in their environment. Penguin species generally show flexible foraging behaviour across sites and seasons (e.g. Deagles et al. 2008). African penguins have evolved to cope with the highly dynamic Benguela upwelling ecosystem and associated variability in the spatial-temporal occurrence of pelagic prey at the end of the last glaciation. Although none of the diving parameters for African penguins differed between years or colonies in our study (table 1), they demonstrated flexibility in time spent at sea and distance travelled over their foraging trip (table 1 and figure 1). Such behavioural plasticity allowed them to respond rapidly and positively to the consequences of released fishing pressure on pelagic fish stocks, confirming their potential value as marine sentinels (Boersma 2008).
Conservation benefits from MPAs can be controversial because MPAs vary greatly in their levels of protection and compliance, leading to occasional ‘paper parks’ (e.g. Guidetti et al. 2008). This could be especially true in the offshore environment where reserves are challenging to design, because of the mobility of the pelagic species (Game et al. 2009), and it is often thought that these reserves should be large to ensure efficiency, consequently being expensive to monitor and to maintain (Sumaila et al. 2007). Here, we show that appropriately designed MPAs can benefit threatened top predators, even those that rely on mobile prey over a small area. That this value can be demonstrated is a major conservation step because designing MPAs to protect the habitat of predators with relatively small foraging ranges allows limiting the areas closed to fisheries, minimizing stakeholder conflicts and easing compliance. In this prospect, penguins could be an adequate model species to protect, thanks to their general charisma and restricted foraging range during the breeding season (Pichegru et al. 2009). Protecting their habitat would nevertheless act as an umbrella and protect entire ecosystems within these foraging ranges (Roberge & Angelstram 2004), while possibly benefiting the human communities close to the colony (e.g. Skewgar et al. 2009). Despite their preliminary nature, our results strongly encourage this strategy, and support the creation of further MPAs for oceanic top predators.
Acknowledgements
All procedures were approved by the University of Cape Town's animal ethics committee.
This study was financially and logistically supported by the Percy FitzPatrick Institute, DST/NRF Centre of Excellence, the Centre National de la Recherche Scientifique, RaggyCharters and South African National Parks. We thank P. Hockey, C. Attwood and Y. Ropert-Coudert for valuable comments on previous drafts of this manuscript.
References
- Apostolaki P., Milner-Gulland E. J., McAllister M. K., Kirkwood G. P.2002Modelling the effect of establishing a marine reserve for mobile fish species. Can. J. Zool. 59, 405–415 [Google Scholar]
- Barange M., Hamptom I., Roel B. A.1999Trends in the abundance and distribution of anchovy and sardine on the South African continental shelf in the 1990s, deduced from acoustic surveys. S. Afr. J. Mar. Sci. 21, 367–391 [Google Scholar]
- Baum J. K., Worm B.2009Cascading top-down effects of changing oceanic predator abundances. J. Anim. Ecol. 78, 699–714 (doi:10.1111/j.1365-2656.2009.01531.x) [DOI] [PubMed] [Google Scholar]
- Boersma P.2008Penguins as marine sentinels. BioScience 58, 597–607 (doi:10.1641/B580707) [Google Scholar]
- Crawford R. J. M., Underhill L. G., Coetzee J. C., Fairweather T., Shannon L. J., Wolfaardt A. C.2008Influences of the abundance and distribution of prey on African penguins Spheniscus demersus off western South Africa. Afr. J. Mar. Sci. 30, 167–175 (doi:10.2989/AJMS.2008.30.1.17.467) [Google Scholar]
- Deagles B. E., Gales N. J., Hindell M. A.2008Variability in foraging behaviour of chick-rearing macaroni penguins Eudyptes chrysolophus and its relation to diet. Mar. Ecol. Prog. Ser. 359, 295–309 (doi:10.3354/meps07307) [Google Scholar]
- Food and Agriculture Organization 2009FAO 2003–2009 Fisheries topics: statistics. Fisheries statistics and information. Rome, Italy: Fisheries & Aquaculture Department; See http://fao.org/ [Google Scholar]
- Frederiksen M., Jensen H., Daunt F., Mavor R. A., Wanless S.2008Differential effects of a local industrial sand lance fishery on seabird breeding performance. Ecol. Appl. 18, 701–710 (doi:10.1890/07-0797.1) [DOI] [PubMed] [Google Scholar]
- Game E. T., et al. 2009Pelagic protected areas: the missing dimension in ocean conservation. Trends Ecol. Evol. 24, 360–369 (doi:10.1016/j.tree.2009.01.011) [DOI] [PubMed] [Google Scholar]
- Guidetti P., et al. 2008Italian marine reserve effectiveness: does enforcement matter? Biol. Conserv. 141, 699–709 (doi:10.1016/j.biocon.2007.12.013) [Google Scholar]
- Lester S. E., Halpern B. S., Grorud-Colvert K., Lubchenco J., Ruttenberg B. I., Gaines S. D., Airamé S., Warner R.2009Biological effect within no-take reserves: a global synthesis. Mar. Ecol. Prog. Ser. 384, 33–46 (doi:10.3354/meps08029) [Google Scholar]
- Louzao M., Hyrenbach K. D., Arcos M. J., Abello P., de Sola L. G., Oro D.2006Oceanographic habitat of an endangered Mediterranean procellariiform: implications for marine protected areas. Ecol. Appl. 16, 683–1695 (doi:10.1890/1051-0761(2006)016[1683:OHOAEM]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- Nagy K. A., Siegfried W. R., Wilson R. P.1984Energy utilization by free-ranging jackazz penguins, Spehniscus demersus. Ecology 65, 1648–1655 (doi:10.2307/1939143) [Google Scholar]
- Pauly D., Christensen V., Guénette S., Pitcher T. J., Sumaila U. R., Walters C. J., Watson R., Zeller D.2002Towards sustainability in world fisheries. Nature 418, 689–695 (doi:10.1038/nature01017) [DOI] [PubMed] [Google Scholar]
- Petersen S. L., Ryan P. G., Grémillet D.2006Is food availability limiting African penguins at Boulders? A comparison of foraging effort at mainland and island colonies. Ibis 148, 14–26 (doi:10.1111/j.1474-919X.2006.00459.x) [Google Scholar]
- Pichegru L., Ryan P. G., Le Bohec C., Van der Lingen C. D., Navarro R., Petersen S., Lewis S., Van der Westhuizen J., Grémillet D.2009Overlap between vulnerable top predators and fisheries in the Benguela upwelling system: implications for marine protected areas. Mar. Ecol. Prog. Ser. 391, 199–208 (doi:10.3354/meps08283) [Google Scholar]
- Roberge J.-M., Angelstam P.2004Usefulness of the umbrella species concept as a conservation tool. Conserv. Biol. 18, 76–85 (doi:10.1111/j.1523-1739.2004.00450.x) [Google Scholar]
- Skewgar E., Simeone A., Boersma P.2009Marine reserve in Chile would benefit penguins and ecotourism. Ocean Coast. Manag. 52, 487–491 (doi:10.1016/j.ocecoaman.2009.07.003) [Google Scholar]
- Sumaila U. R., Zeller D., Watson R., Alder J., Pauly D.2007Potential costs and benefits of marine reserves in the high seas. Mar. Ecol. Prog. Ser. 345, 305–310 (doi:10.3354/meps07065) [Google Scholar]
- White C., Kendall B. E., Gaines S., Siegel D. A., Costello C.2008Marine reserves effects on fishery profit. Ecol. Lett. 11, 370–379 (doi:10.1111/j.1461-0248.2007.01151.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R. P., Wilson P. T.1990Foraging ecology of breeding Spheniscus penguins. In Penguin biology (eds Davis L. S., Darby J. T.), pp. 244–265 San Diego, CA: Academic Press [Google Scholar]
- Wood L. J., Fish L., Laughren J., Pauly D.2008Assessing progress towards global marine protection targets: shortfalls in information and action. Oryx 42, 340–351 (doi:10.1017/S003060530800046X) [Google Scholar]
- Worm B., et al. 2009Rebuilding global fisheries. Science 235, 578–585 (doi:10.1126/science.1173146) [DOI] [PubMed] [Google Scholar]
- Worton B. J.1989Kernel methods for estimating the utilization distribution in home-range studies. Ecology 70, 164–168 (doi:10.2307/1938423) [Google Scholar]