Abstract
Conservation of water is critical to the ecological success of Drosophila species living in the drier montane localities of the Western Himalayas. We observed clinal variation in desiccation resistance for both sexes of Drosophila kikkawai from an altitudinal transect (512–2226 m above sea level). Since more than 90 per cent of body water is lost through cuticular transpiration, the target of selection may be cuticular lipids or cuticular melanization. We tested whether melanic females and non-melanic males of D. kikkawai have similar mechanisms of desiccation resistance. There is clinal variation in the amount of cuticular lipids per fly in males, but not in females. By contrast, for females, elevational increase in melanization is positively correlated with desiccation resistance and negatively with cuticular water loss, but there is no variation in the amount of cuticular lipids. Thus, sexual dimorphism for the mechanism of desiccation resistance in D. kikkawai matches the water proofing role of body melanization as well as cuticular lipids.
Keywords: Drosophila kikkawai, desiccation resistance, cuticular lipids, cuticular melanization, non-melanic males
1. Introduction
Drosophila species have invaded a wide range of terrestrial habitats and differ greatly in their desiccation resistance (Gibbs et al. 2003). Both laboratory and natural populations have adapted to desiccation stress through reduction in respiratory as well as cuticular water loss (Gibbs et al. 1997; Gibbs 2002). A strong negative correlation has been shown between respiratory water loss and desiccation resistance (Gibbs et al. 2003). However, cuticular impermeability has been associated with changes in the amount of cuticular lipids, e.g. correlative analyses (Hadley 1994), and by clinal variation in Melanoplus sanguinipes (Rourke 2000) and in Zaprionus indianus (Parkash et al. 2008a). By contrast, a similar quantity of surface lipids in seven Drosophila species from mesic versus arid habitats did not account for variable cuticular water loss (Gibbs et al. 2003). Further, lack of differences in the amount of cuticular lipids per fly cannot explain the desiccation cline in latitudinally varying populations of Drosophila melanogaster from India (Parkash et al. 2008a). Thus, there is lack of direct supporting evidence for the water proofing role of cuticular lipids in some Drosophila species (Gibbs 2002). However, in some insect taxa, the target of selection might be cuticular melanization; e.g. a negative correlation between body melanization and rate of cuticular water loss has been shown in D. melanogaster (Parkash et al. 2008b). Thus, there may be different modes of water-proofing mechanisms in ectothermic insects living under drier habitats.
The desiccation cline was earlier investigated only for males in the latitudinal populations of Drosophila kikkawai (Karan & Parkash 1998). Such geographical variation in desiccation resistance match climatic conditions (tropical humid versus drier subtropical) on the Indian subcontinent. However, this study did not consider the mechanistic basis as well as the evolutionary response of female body colour polymorphism to desiccation. In the montium species subgroup, females of some species including D. kikkawai exhibit body colour dimorphism (dark and light), while males are either consistently dark (Drosophila auraria, Drosophila baimaii, Drosophila burlai, Drosophila rufa and Drosophila jambulina) or light (D. kikkawai and Drosophila bicornuta; Ohnishi & Watanabe 1985). For both sexes, we found clinal variation in desiccation resistance but their underlying mechanistic bases were not similar. Desiccation resistance was positively correlated: (i) with changes in the amount of surface lipids in males; and (ii) with changes in body melanization in females. We present data for divergence in the modes of water-proofing mechanisms across sexes in montane populations of D. kikkawai.
2. Material and methods
Wild-living D. kikkawai flies (n = 150–180 per population) were collected by net sweeping in September 2007 from six altitudinal sites (table 1) and 30 isofemale lines per population were maintained for three generations at 21°C before experimental analyses. Drosophila kikkawai shows body colour dimorphism for the last two abdominal segments (sixth and seventh) in females but males are non-melanic. For each population, different isofemale lines that gave either all dark or all light progeny were considered as true breeding strains and these were checked for homozygosity for eight generations. We confirmed the genetic basis of colour dimorphism through crosses (F1 and F2) between dark versus light homozygous strains that resulted in a 1∶2∶1 genotypic ratio characteristic of monogenic control; in F1 the dark allele (for sixth and seventh segments) was dominant to light at 21°C. However, heterozygous phenotypes were identified on the basis of average melanization score (mid values between parental dark and light strains) of the four anterior abdominal segments.
Table 1.
Data on female dark allele frequency; per cent melanization per fly and desiccation-related traits (mean ± s.e.) in six montane populations of D. kikkawai. For all traits, altitudinal slope values for the two sexes were compared with a t-test.
| populations | sex | frequency of dark allele | melanization (%) | cuticular lipids (µg cm−2 fly−1) | desiccation (LT50 in hours) | dehydration tolerance (%) | cuticular water loss (µg h−1) |
|---|---|---|---|---|---|---|---|
| Pinjore (512 m) | female | 0.33 | 34.0 ± 2.42 | 12.2 ± 0.07 | 11.1 ± 1.02 | 44.0 ± 2.45 | 20.3 ± 0.54 |
| male | — | 1.06 ± 0.40 | 16.1 ± 0.08 | 9.20 ± 1.01 | 41.1 ± 2.30 | 24.1 ± 0.60 | |
| Kalka (600 m) | female | 0.39 | 35.2 ± 2.44 | 12.0 ± 0.05 | 12.8 ± 1.04 | 45.4 ± 2.48 | 19.4 ± 0.51 |
| male | — | 1.07 ± 0.42 | 20.7 ± 0.06 | 10.4 ± 1.01 | 42.2 ± 2.36 | 21.8 ± 0.53 | |
| Bhuntar (1096 m) | female | 0.50 | 43.4 ± 2.50 | 11.9 ± 0.06 | 14.0 ± 1.05 | 48.2 ± 2.80 | 18.5 ± 0.52 |
| male | — | 1.06 ± 0.50 | 24.1 ± 0.07 | 11.5 ± 1.03 | 43.6 ± 2.51 | 20.2 ± 0.50 | |
| Solan (1440 m) | female | 0.56 | 47.2 ± 2.54 | 12.1 ± 0.08 | 15.3 ± 1.06 | 49.8 ± 2.89 | 17.6 ± 0.48 |
| male | — | 1.07 ± 0.56 | 28.3 ± 0.09 | 12.8 ± 1.04 | 45.3 ± 2.60 | 19.7 ± 0.49 | |
| Kasauli (1951 m) | female | 0.69 | 54.6 ± 2.64 | 12.3 ± 0.07 | 18.6 ± 1.06 | 52.2 ± 3.06 | 16.4 ± 0.44 |
| male | — | 1.05 ± 0.81 | 30.4 ± 0.10 | 15.2 ± 1.05 | 47.6 ± 2.71 | 18.4 ± 0.41 | |
| Chail (2226 m) | female | 0.75 | 56.2 ± 2.70 | 12.1 ± 0.07 | 20.3 ± 1.07 | 52.9 ± 3.12 | 15.3 ± 0.40 |
| male | — | 1.07 ± 0.87 | 32.2 ± 0.12 | 16.1 ± 1.06 | 48.2 ± 2.75 | 17.2 ± 0.36 | |
| altitudinal slope (b) | female | 0.00025 | 0.014 | 0 | 0.0049 | 0.0051 | −0.0026 |
| male | — | 0 | 0.011 | 0.0041 | 0.0041 | −0.0033 | |
| t-test | — | *** | *** | *** | *** | *** |
***p < 0.001.
Melanization was estimated from a lateral view of the abdomen ranging from 0 (no melanization) to 10 (complete melanization) for each of the six abdominal segments (second to seventh) following Parkash et al. (2008b). The data on per cent melanization were calculated as ((Σ observed weighted melanization scores of abdominal segments per fly)/(Σ relative size of each abdominal segment × maximum melanization score of 10 for each segment)) × 100. Furthermore, desiccation resistance, cuticular water loss, total body water, dehydration tolerance and cuticular lipid mass per fly were analysed following Parkash et al. (2008a).
Clinal variation was analysed on the basis of population means (n = 30 isofemale lines) and data were used for illustrations. For colour dimorphism in females, we scored dark (60–75%), intermediate (40–50%) and light (25–35%) phenotypes that correspond with their respective genotypes. The allele frequency was calculated from wild-caught females (n = 80 per population) on the basis of the Hardy-Weinberg Law. For each population, data on five dark as well as five light isofemale lines were used for correlations and regression analyses. The significance of the slopes were tested by t-tests. For regression analysis with body melanization, the data were arcsine transformed. Statistical calculations and illustrations were made with Statistica 5.0.
3. Results
(a). Sex-specific trait variations
(i). Females
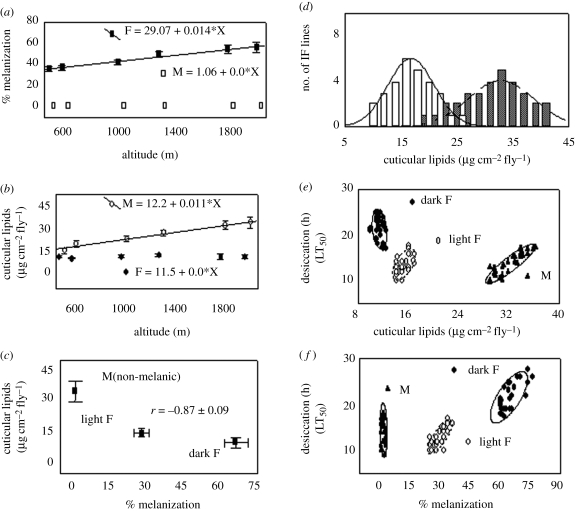
Data on discrete colour polymorphism in females (dark and light morphs) showed a clinal increase (2.27-fold) in the frequency of the dark allele (table 1). Altitudinal slope value analysis showed a 25 per cent increase in the frequency of the dark allele per 1000 m. Data on female body melanization and desiccation-related traits in altitudinal populations of D. kikkawai are shown in table 1. In females, a positive cline is evident for body melanization (figure 1a), desiccation resistance and dehydration tolerance but a negative cline for cuticular water loss (table 1). Another interesting observation is the lack of altitudinal changes in the amount of cuticular lipids per fly in females (figure 1b). However, desiccation resistance is correlated with body melanization and the data are shown in figure 1f.
Figure 1.
(a) Changes in per cent melanization; (b) cuticular lipids per fly along an altitudinal gradient in both the sexes; (c) lack of correlation between melanization and cuticular lipids; (d) isofemale line variability for changes in the amount of cuticular lipids in males from highland (checked bars) versus lowland (white bars) populations; (e) correlations of desiccation resistance with cuticular lipids; and (f) with per cent melanization in D. kikkawai. F, female; M, male.
In order to check whether desiccation-related traits differ between morphs as well as populations, we compared dark and light homozygous strains from high- versus lowland populations and the data are shown in table 2. At the intrapopulation level, there are significant differences between dark versus light morphs for desiccation-related traits in highland as well as lowland populations (table 2). However, a statistical comparison of trait values in dark versus dark as well as light versus light female homozygous strains from highland versus lowland populations showed non-significant differences (table 2). Thus, trait values do not vary in homozygous dark or light strains across an elevational gradient. However, the population mean values differ owing to changes in the morph frequency.
Table 2.
Data (mean ± s.d.) on per cent body melanization per fly and desiccation-related traits in homozygous strains (n = 5) for dark and light female morphs; and non-melanic males of D. kikkawai from lowland versus highland localities. n.s., non-significant.
| lowland (512 m) |
highland (2226 m) |
HL versus LL (t-test) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| traits | dark (D) female | light (L) female | D versus L t-test | non-melanic male | dark female | light female | D versus L t-test | non-melanic male | D versus D or L versus L female | male |
| % melanization | 66.2 ± 4.10 | 28.1 ± 1.20 | *** | 1.06 ± 0.40 | 67.6 ± 4.28 | 28.9 ± 1.80 | *** | 1.07 ± 0.87 | n.s. | n.s. |
| desiccation (LT50 in hours) | 20.2 ± 2.02 | 10.8 ± 1.80 | *** | 12.2 ± 1.01 | 21.0 ± 2.12 | 11.2 ± 1.46 | *** | 17.1 ± 1.03 | n.s. | *** |
| cuticular water loss | 15.1 ± 0.42 | 22.5±0.53 | *** | 24.3 ± 0.48 | 16.4 ± 0.42 | 21.3±0.51 | *** | 18.0 ± 0.46 | n.s. | *** |
| cuticular lipids | 10.3 ± 0.09 | 14.8±0.10 | *** | 16.1 ± 0.08 | 10.4 ± 0.07 | 14.6±0.09 | *** | 32.2 ± 0.12 | n.s. | *** |
| total body water | 65.2 ± 1.10 | 65.1 ± 1.20 | n.s. | 65.0 ± 1.04 | 65.8 ± 1.10 | 65.4 ± 1.20 | n.s. | 65.2 ± 1.08 | n.s. | n.s. |
| dehydration tolerance | 56.1 ± 3.20 | 44.2 ± 1.40 | *** | 43.1 ± 2.30 | 57.2 ± 3.40 | 45.0 ± 1.70 | *** | 46.8 ± 2.71 | n.s. | * |
*p < 0.05.
***p < 0.001.
For both the morphs, there are significant regression slope values as well as coefficients of determination (r2) for desiccation-related traits as a function of body melanization but regression coefficients are non-significant for cuticular lipids (table 3). However, consistent differences in the amount of cuticular lipids per fly were observed in dark versus light morphs (higher values in light morph; figure 1c), i.e. changes in cuticular lipids may be independent of changes in melanization.
Table 3.
Regression analyses of changes in desiccation resistance, cuticular water loss per fly and dehydration tolerance as a function of either changes in (i) melanization or (ii) cuticular lipids in six altitudinal populations of D. kikkawai. n.s., non-significant; a, intercept; b, slope; r2, coefficient of determination; s.e., standard error.
| (i) melanization |
(ii) cuticular lipids (µg cm−2 fly−1) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| traits | r | a ± s.e. | b ± s.e. | r2 | r | a ± s.e. | b ± s.e. | r2 | |
| desiccation (LT50 in hours) | dark female | 0.87 | −0.06 ± 0.006 | 0.36 ± 0.007*** | 0.76*** | −0.29 | 42.2 ± 15.3 | −1.07 ± 1.30 n.s. | 0.09 n.s. |
| light female | 0.72 | 0.13 ± 0.007 | 0.22 ± 0.001*** | 0.52 ** | 0.24 | 18.0 ± 4.40 | 0.38 ± 0.52 n.s. | 0.16 n.s. | |
| male | 0.21 | 0.11 ± 0.02 | −3.04 ± 3.23 n.s. | 0.10 n.s. | 0.90 | 4.75 ± 2.46 | 0.75 ± 0.13*** | 0.89*** | |
| cuticular water loss | dark female | −0.90 | 0.18 ± 0.017 | −0.12 ± 0.02*** | 0.82*** | 0.28 | 8.63 ± 8.43 | 2.28 ± 2.69 n.s. | 0.08 n.s. |
| light female | −0.60 | 0.13 ± 0.010 | −0.18 ± 0.012** | 0.69** | −0.20 | 23.3 ± 4.80 | −0.47 ± 0.56 n.s. | 0.06 n.s. | |
| male | −0.16 | 0.15 ± 0.013 | −0.80 ± 1.77 n.s. | 0.02 n.s. | −0.87 | 36.0 ± 2.87 | −1.92 ± 0.37*** | 0.76*** | |
| dehydration tolerance | dark female | 0.93 | 0.34 ± 0.04 | 0.37 ± 0.05*** | 0.86*** | −0.21 | 61.9 ± 14.0 | −0.69 ± 1.17 n.s. | 0.04 n.s. |
| light female | 0.80 | 0.49 ± 0.01 | 0.47 ± 0.06** | 0.64** | −0.18 | 43.6 ± 4.04 | −0.26 ± 0.51 n.s. | 0.07 n.s. | |
| male | −0.22 | 0.45 ± 0.01 | −1.22 ± 1.85 n.s. | 0.05 n.s. | 0.75 | 32.0 ± 2.85 | 1.21 ± 0.37*** | 0.87*** | |
**p < 0.01.
***p < 0.001.
(ii). Males
Males from all the populations are non-melanic (tables 1 and 2). Clinal variation is evident for desiccation-related traits in the males of six altitudinal populations, i.e. elevational slope values are similar and positive for desiccation resistance and dehydration tolerance but negative for cuticular water loss (table 1). Changes in the amount of cuticular lipids per fly reflect clinal variation (figure 1b) as well as for the quantitative variability in isofemale lines (figure 1d). Correlations between desiccation resistance and cuticular lipids are shown in figure 1e. However, cuticular lipids per fly, desiccation resistance and dehydration tolerance are higher in males from highland than lowland populations (tables 1 and 2). Accordingly, the cuticular water loss in males is negatively correlated with cuticular lipids as well as desiccation resistance (table 1). A regression analysis of changes in desiccation-related traits as a function of cuticular lipids per fly showed a significant slope as well as r2 values, while such values are non-significant with body melanization (table 3).
4. Discussion
The ecological success of different Drosophila species depends upon the diversity of adaptive mechanisms to cope with environmental stresses. Drosophila species from contrasting habitats (xeric versus mesic; tropical versus subtropical; and increasing aridity along elevational gradients) face strong selection pressure on their water balance mechanisms. In insects, the cuticle is the interface between physiological systems and environmental conditions; and changes in cuticular components are expected to confer desiccation resistance. Evidence in favour of cuticular lipids as barriers for cuticular water loss include: (i) negative correlation between melting temperature (Tm) and cuticular water loss based on correlative analyses (Hadley 1994); and (ii) changes in the amount of cuticular lipids (approx. 56–110 µg cm−2) in five altitudinal populations of M. sanguinipes (Rourke 2000); and latitudinal divergence in the amount of cuticular lipid mass per fly (9–25 µg fly−1) in Z. indianus (Parkash et al. 2008a). By contrast, some studies do not favour the water-proofing role of cuticular lipids in Drosophila species (Gibbs 2002; Gibbs et al. 2003). For example, there are no changes in the amount of cuticular lipids in: (i) laboratory-selected desiccation tolerant and control strains of D. melanogaster (Gibbs et al. 1997); (ii) seven Drosophila species from mesic versus arid habitats (Gibbs et al. 2003); and (iii) latitudinal populations of D. melanogaster showing a desiccation cline in India (Parkash et al. 2008a). However, in the present study, changes in the amount of cuticular lipids (16.1–32.2 µg cm−2 fly−1) in non-melanic males of D. kikkawai support the role of cuticular lipids in conferring desiccation resistance. Thus, a water-proofing role of cuticular lipids is evident in non-melanics, e.g. M. sanguinipes (Rourke 2000), Z. indianus (Parkash et al. 2008a); and in the males of D. kikkawai.
In D. kikkawai females, neither dark nor light morphs show geographical variation in the amount of cuticular lipids per fly, and thus cannot account for a reduction in cuticular water loss. Interestingly, there is a negative relationship between cuticular lipids and body melanization in the dark and light morphs (figure 1c). We may infer that changes in cuticular lipids (i.e. 50% more in light female morph) are independent of changes in body melanization in D. kikkawai. However, darker flies from the highland population result from more than twofold increase in the frequency of the dark allele in the females (table 1). The observed allele frequency changes for body colour morphs in females result in darker flies with a higher desiccation resistance but reduced cuticular water loss in highland populations, while the reverse is true for lowland populations. There is evidence in support of the melanism-desiccation hypothesis (based on a water-proofing role of cuticular melanization). For example (i) in latitudinal populations of D. melanogaster, body melanization changes are negatively correlated with cuticular water loss (Parkash et al. 2008a); and (ii) at within-population level, assorted darker and lighter flies are negatively correlated with changes in cuticular water loss (Parkash et al. 2008b). Thus, changes in the body melanization may provide a water-proofing role in the females of D. kikkawai. Further, in insects as well as Drosophila species, respiratory water loss accounts for less than 10 per cent of total body water loss; and there is a negative correlation between respiratory water loss rates and desiccation resistance (Gibbs et al. 2003). A possible explanation for the reduction in respiratory water loss could be a lower metabolic rate in melanic flies, leading to water conservation. However, this aspect has not been analysed in the present studies.
In conclusion, our results are novel in showing sexual dimorphism for water balance mechanisms in D. kikkawai. Our data have shown quantitative variation in cuticular lipids per fly in the males, while a discrete colour polymorphism results in varying body melanization levels in females. For desiccation-related traits, there are significant differences in the altitudinal slope values for both the sexes (table 1), i.e. there may be different responses to selection pressures for body melanization in females versus cuticular lipids in males. We suggest that in the melanic species, body melanization serves as a barrier for cuticular water loss while in non-melanics, cuticular lipids play a similar role.
Acknowledgements
We are grateful to the reviewers for their helpful comments. Financial assistance from UGC and CSIR are gratefully acknowledged.
References
- Gibbs A. G.2002Lipid melting and cuticular permeability: new insights into an old problem. J. Insect Physiol. 48, 391–400 (doi:10.1016/S0022-1910(02)00059-8) [DOI] [PubMed] [Google Scholar]
- Gibbs A. G., Chippendale A. K., Rose M. R.1997Physiological mechanisms of evolved desiccation resistance in Drosophila melanogaster. J. Exp. Biol. 200, 1821–1832 [DOI] [PubMed] [Google Scholar]
- Gibbs A. G., Fukuzato F., Matzkin L. M.2003Evolution of water conservation mechanism in Drosophila. J. Exp. Biol. 206, 1183–1192 (doi:10.1242/jeb.00233) [DOI] [PubMed] [Google Scholar]
- Hadley N. F.1994Water relations of terrestrial arthropods. San Diego, CA: Academic Press [Google Scholar]
- Karan D., Parkash R.1998Desiccation tolerance and starvation resistance exhibit opposite latitudinal clines in Indian geographical populations of Drosophila kikkawai. Ecol. Entomol. 23, 391–396 (doi:10.1046/j.1365-2311.1998.00157.x) [Google Scholar]
- Ohnishi S., Watanabe T. K.1985Genetic analysis of colour dimorphism in the Drosophila montium subgroup. Jpn J. Genet. 60, 355–358 (doi:10.1266/jjg.60.355) [Google Scholar]
- Parkash R., Kalra B., Sharma V.2008aChanges in cuticular lipids, water loss and desiccation resistance in a tropical drosophilid: analysis of within population variation. Fly 2, 189–197 [DOI] [PubMed] [Google Scholar]
- Parkash R., Rajpurohit S., Ramniwas S.2008bParallel changes in body melanisation and desiccation resistance in highland vs. lowland populations of D. melanogaster. J. Insect Physiol. 54, 1050–1056 (doi:10.1016/j.jinsphys.2008.04.008) [DOI] [PubMed] [Google Scholar]
- Rourke B. C.2000Geographical and altitudinal variation in water balance and metabolic rate in a California grasshopper, Melanoplus sanguinipes. J. Exp. Biol. 203, 2699–2712 [DOI] [PubMed] [Google Scholar]